Abstract
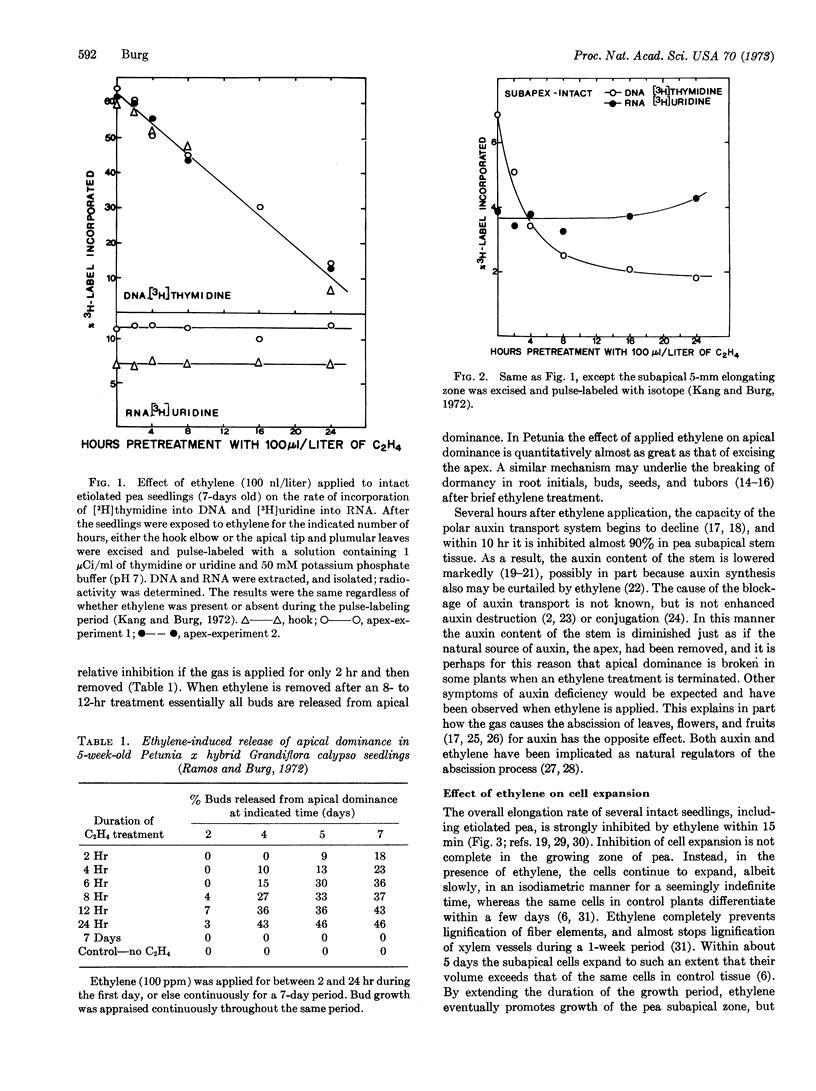
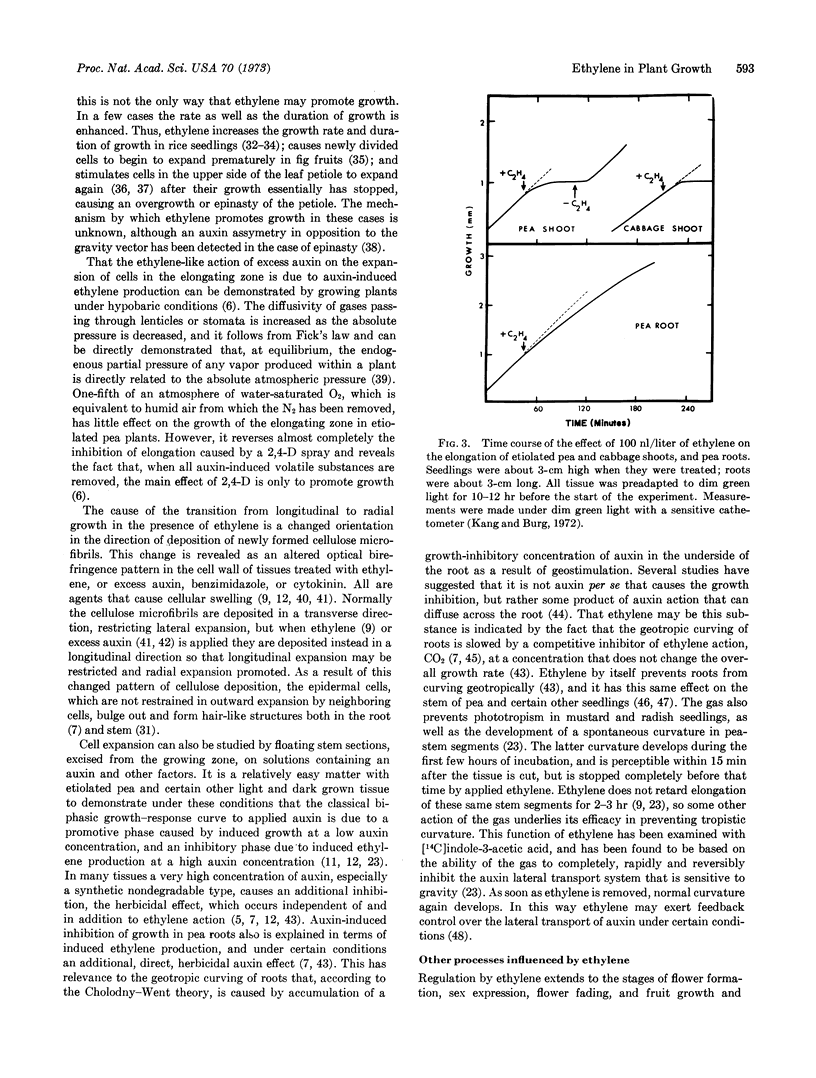
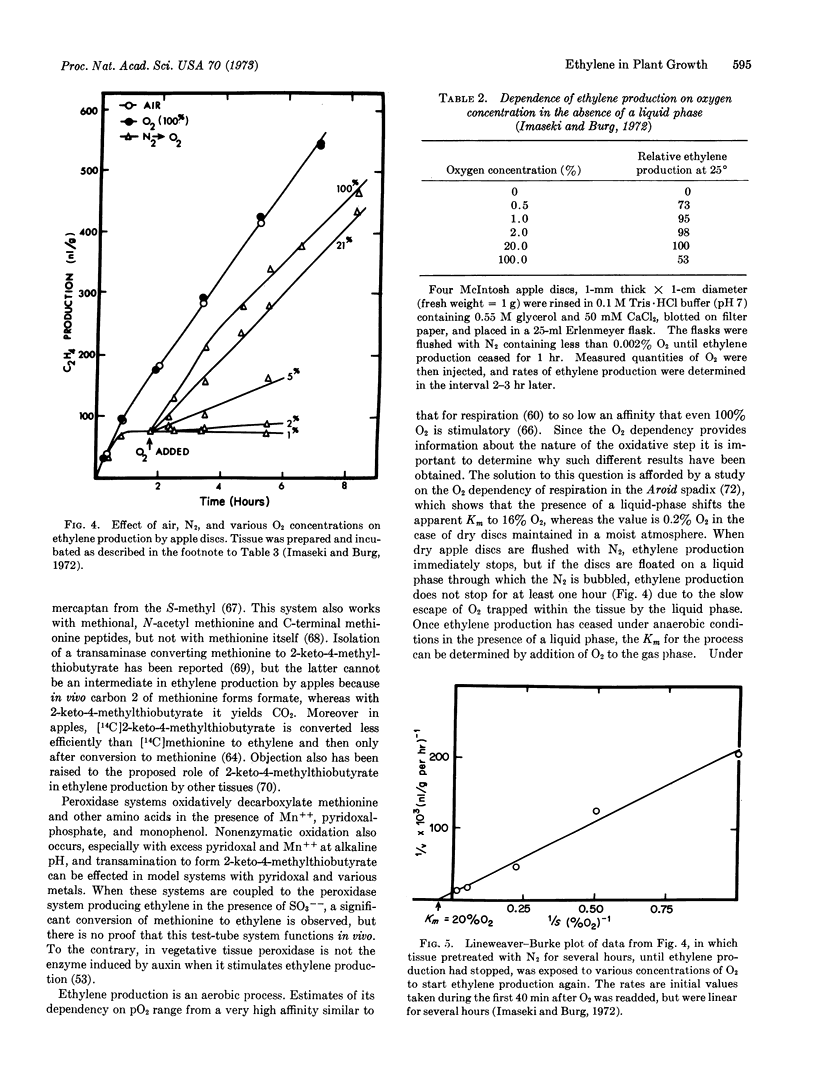
Ethylene inhibits cell division, DNA synthesis, and growth in the meristems of roots, shoots, and axillary buds, without influencing RNA synthesis. Apical dominance often is broken when ethylene is removed, apparently because the gas inhibits polar auxin transport irreversibly, thereby reducing the shoot's auxin content just as if the apex had been removed. A similar mechanism may underly ethylene-induced release from dormancy of buds, tubers, root initials, and seeds. Often ethylene inhibits cell expansion within 15 min, but delays differentiation so that previously expanding cells eventually grow to enormous size. These cells grow isodiametrically rather than longitudinally because their newly deposited cellulose microfibrils are laid down longitudinally rather than radially. Tropistic responses are inhibited when ethylene reversibly and rapidly prevents lateral auxin transport. In most of these cases, as well as certain other instances, ethylene action is mimicked by application of an auxin, since auxins induce ethylene formation. Regulation by ethylene extends to abscission, to flower formation and fading, and to fruit growth and ripening. Production of ethylene is controlled by auxin and by red light, auxin acting to induce a labile enzyme needed for ethylene synthesis and red light to repress ethylene production. Numerous cases in which a response to red light requires an intervening step dependent upon inhibition of ethylene production have been identified. Ethylene action requires noncovalent binding of the gas to a metal-containing receptor having limited access, and produces no lasting product. The action is competitively inhibited by CO2, and requires O2. Ethylene is biosynthesized from carbons 3 and 4 of methionine, apparently by a copper-containing enzyme in a reaction dependent upon an oxygen-requiring step with a Km = 0.2% O2. The oxidative step appears to be preceded by an energy-requiring step subsequent to methionine formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Ruth J. M. Mechanisms of hormone action: use of deuterated ethylene to measure isotopic exchange with plant material and the biological effects of deuterated ethylene. Plant Physiol. 1972 May;49(5):669–671. doi: 10.1104/pp.49.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burg S. P. Effect of Ethylene on Cell Division and Deoxyribonucleic Acid Synthesis in Pisum sativum. Plant Physiol. 1972 Jul;50(1):117–124. doi: 10.1104/pp.50.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burg S. P. Effects of Ethylene and 2,4-Dichlorophenoxyacetic Acid on Cellular Expansion in Pisum sativum. Plant Physiol. 1972 Jul;50(1):125–131. doi: 10.1104/pp.50.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur A. H., Yang S. F., Pratt H. K. Ethylene biosynthesis in fruit tissues. Plant Physiol. 1971 May;47(5):696–699. doi: 10.1104/pp.47.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur A. H., Yang S. F. Precursors of ethylene. Plant Physiol. 1969 Sep;44(9):1347–1349. doi: 10.1104/pp.44.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Mechanism of Ethylene Action: Biological Activity of Deuterated Ethylene and Evidence against Isotopic Exchange and cis-trans-Isomerization. Plant Physiol. 1972 May;49(5):672–675. doi: 10.1104/pp.49.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971 Aug;48(2):208–212. doi: 10.1104/pp.48.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Auxin-induced ethylene formation: its relation to flowering in the pineapple. Science. 1966 May 27;152(3726):1269–1269. doi: 10.1126/science.152.3726.1269. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indole-3-acetic Acid. Plant Physiol. 1968 Jul;43(7):1069–1074. doi: 10.1104/pp.43.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Fruit storage at subatmospheric pressures. Science. 1966 Jul 15;153(3733):314–315. doi: 10.1126/science.153.3733.314. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Inhibition of polar auxin transport by ethylene. Plant Physiol. 1967 Sep;42(9):1224–1228. doi: 10.1104/pp.42.9.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Dijkman M. J. Ethylene and auxin participation in pollen induced fading of vanda orchid blossoms. Plant Physiol. 1967 Nov;42(11):1648–1650. doi: 10.1104/pp.42.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene, plant senescence and abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1503–1511. [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Thimann K. V. THE PHYSIOLOGY OF ETHYLENE FORMATION IN APPLES. Proc Natl Acad Sci U S A. 1959 Mar;45(3):335–344. doi: 10.1073/pnas.45.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. An explanation of the inhibition of root growth caused by indole-3-acetic Acid. Plant Physiol. 1967 Mar;42(3):415–420. doi: 10.1104/pp.42.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. Regulation of root growth by auxin-ethylene interaction. Plant Physiol. 1970 Feb;45(2):192–200. doi: 10.1104/pp.45.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demorest D. M., Stahmann M. A. Ethylene production from peptides and protein containing methionine. Plant Physiol. 1971 Mar;47(3):450–451. doi: 10.1104/pp.47.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger W. R., Burg S. P. Ethylene-induced Pea Internode Swelling: Its Relation to Ribonucleic Acid Metabolism, Wall Protein Synthesis, and Cell Wall Structure. Plant Physiol. 1972 Oct;50(4):510–517. doi: 10.1104/pp.50.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest L. C., Valdovinos J. G. Regulation of Auxin Levels in Coleus blumei by Ethylene. Plant Physiol. 1971 Oct;48(4):402–406. doi: 10.1104/pp.48.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H., Pjon C. J., Furuya M. Phytochrome Action in Oryza sativa L: IV. Red and Far Red Reversible Effect on the Production of Ethylene in Excised Coleoptiles. Plant Physiol. 1971 Sep;48(3):241–244. doi: 10.1104/pp.48.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Kang B. G., Burg S. P. Involvement of Ethylene in Phytochrome-mediated Carotenoid Synthesis. Plant Physiol. 1972 Apr;49(4):631–633. doi: 10.1104/pp.49.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Burg S. P. Relation of Phytochrome-enhanced Geotropic Sensitivity to Ethylene Production. Plant Physiol. 1972 Jul;50(1):132–135. doi: 10.1104/pp.50.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Yocum C. S., Burg S. P., Ray P. M. Ethylene and carbon dioxide: mediation of hypocotyl hook-opening response. Science. 1967 May 19;156(3777):958–959. doi: 10.1126/science.156.3777.958. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. An evaluation of 4-s-methyl-2-keto-butyric Acid as an intermediate in the biosynthesis of ethylene. Plant Physiol. 1971 Apr;47(4):576–580. doi: 10.1104/pp.47.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. Ethylene production from methionine. Biochem J. 1965 Nov;97(2):449–459. doi: 10.1042/bj0970449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon C. J. Ethylene inhibition of auxin transport by gravity in leaves. Plant Physiol. 1970 May;45(5):644–646. doi: 10.1104/pp.45.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener H. D. EFFECTS OF ETHYLENE ON PLANT GROWTH HORMONE. Science. 1935 Dec 6;82(2136):551–552. doi: 10.1126/science.82.2136.551. [DOI] [PubMed] [Google Scholar]
- Vacha G. A., Harvey R. B. THE USE OF ETHYLENE, PROPYLENE, AND SIMILAR COMPOUNDS IN BREAKING THE REST PERIOD OF TUBERS, BULBS, CUTTINGS, AND SEEDS. Plant Physiol. 1927 Apr;2(2):187–193. doi: 10.1104/pp.2.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos J. G., Ernest L. C., Henry E. W. Effect of ethylene and gibberellic Acid on auxin synthesis in plant tissues. Plant Physiol. 1967 Dec;42(12):1803–1806. doi: 10.1104/pp.42.12.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum C. S., Hackett D. P. Participation of Cytochromes in the Respiration of the Aroid Spadix. Plant Physiol. 1957 May;32(3):186–191. doi: 10.1104/pp.32.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]


