Abstract
Capillarization, lack of liver sinusoidal endothelial cell (LSEC) fenestration and formation of an organized basement membrane, not only precedes fibrosis, but is also permissive for hepatic stellate cell activation and fibrosis. Thus dysregulation of the LSEC phenotype is a critical step in the fibrotic process. Both a VEGF-stimulated, NO-independent pathway and a VEGF-stimulated NO-dependent pathway are necessary to maintain the differentiated LSEC phenotype. The NO-dependent pathway is impaired in capillarization and activation of this pathway downstream from NO restores LSEC differentiation in vivo. Restoration of LSEC differentiation in vivo promotes HSC quiescence, enhances regression of fibrosis, and prevents progression of cirrhosis.
Introduction
Capillarization, which is when liver sinusoidal endothelial cells (LSECs) lack fenestration and develop an organized basement membrane (1), is a prelude to fibrosis. This brief review will describe that capillarization not only precedes fibrosis, but that changes that occur in LSECs with the morphological features of capillarization are an integral part of the fibrotic process. This lends importance to our understanding of how the differentiated LSEC phenotype is maintained, which will be discussed in the first section. The second section will examine the differing effects of the differentiated and “capillarized” LSEC on hepatic stellate cell (HSC) quiescence and activation. The final section will examine in vivo studies that confirm that reversing capillarization accelerates regression of fibrosis and can prevent progression of cirrhosis.
The review is focused predominantly on the role of crosstalk between liver cells in general and LSECs more specifically in the mechanisms leading to fibrosis. Angiogenesis, the formation of new blood vessels from existing blood vessels, occurs in fibrosis. It has not yet been determined whether angiogenesis takes place in parallel with the development of fibrosis or plays a causal role in fibrosis. Given that it has not been established that LSECs promote fibrosis through angiogenesis, angiogenesis will not be discussed.
Regulation of the LSEC Phenotype
LSECs are a highly specialized endothelial cell with morphological and functional features characteristic of this particular endothelial cell.
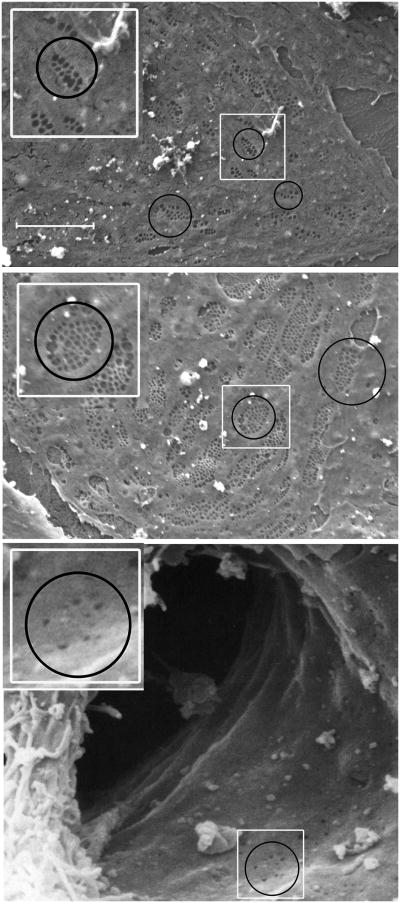
Morphologically, LSECs contain fenestrae, non-diaphragmed pores that transverse the cytoplasm. The fenestrae are 100-150 nm in size and are clustered in groups that have been termed sieve plates. Fenestration patterns vary across the liver lobule, with larger but fewer fenestrae per sieve plate in the periportal region and smaller but more numerous fenestrae per sieve plate in the centrilobular region (Figure 1). The endothelial cells that line the transition from the sinusoid to the central vein have occasional, scattered fenestrae without well-developed sieve plates (Figure 1, bottom panel). Fenestration is not unique to LSECs, but is seen in various endothelial cells in other organs. However in mammals only the glomerular endothelial cell and the LSEC have open (non-diaphragmed) fenestrae, and the glomerular endothelial cell differs from the LSEC in that it resides upon an organized basement membrane. Thus the LSEC has a morphologic phenotype that is unique in the mammal with the combination of open fenestrae and lack of a basement membrane. This creates open access for solutes between the sinusoidal blood and the space of Disse. Porosity, the amount of LSEC surface occupied by fenestrae is twice as high in the centrilobular region as in the periportal region (2), allowing increased exchange of oxygen as the pO2 drops across the lobule.
Figure 1. Scanning electron microscopy of liver sinusoidal endothelial cells.
Top panel, photomicrograph of a periportal LSEC isolated from rat liver (magnification 5000×). Middle panel, photomicrograph of a centrilobular LSEC isolated form rat liver (magnification 5000×). Note that periportal LSECs (top panel) have fewer fenestrae per sieve plate (average 8 fenestrae per sieve plate), but larger fenestrae than centrilobular LSECs (average 24 fenestrae per sieve plate; middle panel) (2). Smaller, non-clustered holes are endocytic vesicles. Bottom panel, scanning electron microscopic photograph of the lumen of a sinusoid opening into the central vein taken from human liver (magnification 8500×). The sinusoid is bisected by a Kupffer cell. Fenestrae are rare in the endothelial cells that line this transitional zone. Circles surround examples of sieve plates (top two panels) and one of the few areas of the transitional endothelial cell that still has fenestrae (bottom panel). The white rectangles in each panel are enlarged and reproduced in the top left of each panel for better visualization. Bottom panel reproduced from Die Leber des Menschen / The Human Liver Rasterelektronenmikroskopischer Atlas / A Scanning Electron Microscopic Atlas by Franz Vonnahme (ISBN: 978-3-8055-5585-2) with kind permission from S. Karger AG, Basel.
A characteristic functional phenotypic feature of the LSEC is its high endocytic capacity (see recent review (3)). Endocytic vesicles can be seen on scanning electron microscopic exam of LSECs and are smaller, non-clustered holes that need to be distinguished from fenestrae. Three endocytosis receptors have been identified on LSECs: the collagen-α-chain/mannose receptor, the hyaluronan/scavenger receptor, and the FcγIIb2 receptor. The collagen- α-chain/mannose receptor (CD206) clears circulating collagen alpha chains, i.e. denatured collagen of several types of collagen, and glycoconjugates with terminal mannose, such as lysosomal enzymes, procollagen type I carboxyterminal propeptides, and tissue type plasminogen activator (4, 5). The hyaluronan/scavenger receptor, SR-H (stabilin-1 and stabilin-2), is the main functional scavenger receptor on the LSEC (6-8). The hyaluronan/scavenger receptor clears hyaluronan, chondroitin sulphate, formaldehyde treated serum albumin (FSA, used as a test ligand for scavenger receptor-mediated endocytosis (9-11)), procollagen type I and III N-terminal peptides, nidogen, acetylated and oxidized low density lipoprotein (12-14), plasma coagulation products, and advanced glycation end-products (15). The LSEC Fc-receptor, FcγIIb2 (CD32b or SE-1 (16)), clears immune complexes formed with Ig G (17).
The presence of fenestrae in sieve plates on electron microscopy is the gold standard for confirming the identity of LSECs and the ability to take up fluorescently labeled FSA or collagen α-chains is the current standard for assessing purity of an isolated population of LSECs. Markers that have been used to identify LSECs include the expression of FcγIIb2 (17) (CD32b or anti-SE-1 (16)), stabilin-2 (3, 18), liver/lymph node-specific ICAM-3-grabbing integrin [L-SIGN; aka CD209L or DC-SIGN-related (DC SIGNR)] (19), and LYVE-1 (20, 21). Uptake of di-acetylated-low density lipoprotein (di-Ac-LDL) is best avoided as a method to assess LSEC identity or purity. Di-Ac-LDL is taken up by endothelial cells in general and also by Kupffer cells. The high endocytotic activity of LSECs makes it theoretically possible to use di-Ac-LDL to distinguish LSECs from other liver endothelial cells, but requires careful attention to the low concentration of di-Ac-LDL and early time point of assessment needed to distinguish LSECs from other endothelial cells. Other LSEC markers have been proposed but require careful positive correlation with either fenestration or uptake of specific LSEC ligands, and negative correlation with other liver endothelial cells, e.g. the poorly fenestrated endothelial cells that transition to the central vein (see figure 1, bottom panel).
In this review, LSECs with normal fenestration and function will be referred to as differentiated LSECs, whereas LSECs that are defenestrated and lack functions present in differentiated LSECs will be termed capillarized LSECs. As described below, the ability to promote hepatic stellate cell quiescence is one of the functions of differentiated LSECs that is lost in capillarization.
In vivo, loss of the unique fenestrated phenotype has been shown to precede onset of fibrosis in alcoholic liver injury (22), non-alcoholic fatty liver disease (23), toxic liver injury (24), and the intragastric alcohol infusion model (DeLeve LD et al, unpublished observation). Endocytosis is impaired in capillarization (25), but there is not yet published evidence that the decline in endocytotic capacity precedes fibrosis or a comprehensive description of which endocytotic receptors are affected in capillarization. In vitro, fenestration is lost within 2-3 days in culture. In contrast, some degree of endocytosis persists longer than fenestration in culture. Thus fenestration, at least in cultured LSECs, may be a more accurate marker for the fully differentiated LSEC phenotype than endocytosis. Both fenestration and endocytosis persist longer in vitro in the right microenvironment (26, 27).
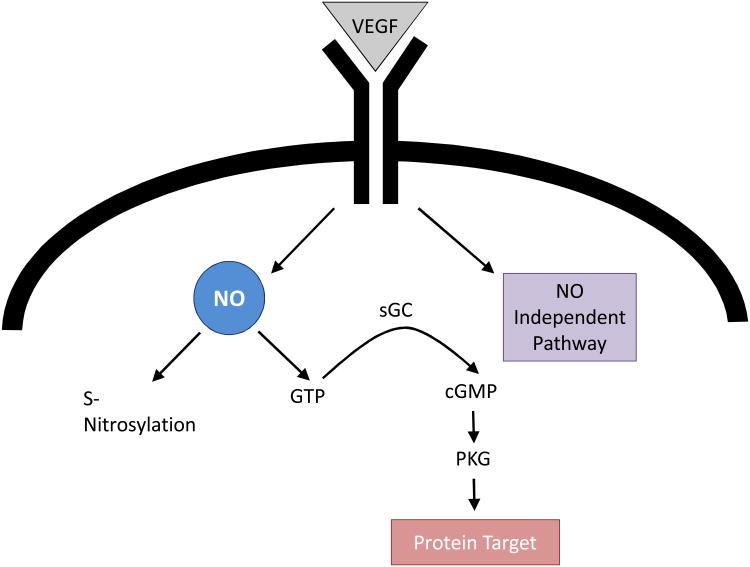
VEGF, derived from hepatocytes and hepatic stellate cells, is a key regulator of the LSEC phenotype, as demonstrated both in vitro and in vivo (26, 28-30). In vitro studies have demonstrated that maintenance of the fenestrated LSEC phenotype requires VEGF working through two pathways, a nitric oxide (NO) dependent and an NO-independent pathway (Figure 2) (26, 28). VEGF secreted by either hepatocytes or stellate cells stimulates NO release from endogenous nitric oxide synthase (eNOS) in LSEC. NO in turn acts through soluble guanylate cyclase (sGC), conversion of GTP to cGMP, and stimulation of protein kinase G (PKG), which can then phosphorylate protein targets (28). An alternate possibility, i.e. that NO acts through protein S-nitrosylation, was found not to be necessary to maintain the fenestrated LSEC phenotype (28). In addition to the VEGF-stimulated NO pathway, maintenance of the LSEC phenotype also requires an NO-independent pathway, which remains to be characterized (28).
Figure 2. VEGF pathways in LSEC.
Maintenance of the LSEC phenotype requires both the VEGF-stimulated NO pathway working through soluble guanylate cyclase, cGMP and protein kinase G and a VEGF pathway working independent of NO. The protein S-nitrosylation pathway is not necessary to maintain the LSEC phenotype. Abbreviations: LSEC, liver sinusoidal endothelial cell; NO, nitric oxide; sGC, soluble guanylate cyclase; VEGF, vascular endothelial growth factor;
In capillarization, eNOS protein is unchanged, but eNOS activity is diminished with low production of NO (31) due to increased binding to caveolin (32). Thus the VEGF-stimulated, NO-dependent pathway is impaired. VEGF is upregulated in cirrhosis (33, 34), so that total VEGF is not rate limiting in this process. Indeed, as will be discussed later in this review, activation of the NO-dependent pathway is sufficient to restore LSEC differentiation in cirrhotic liver, which demonstrates that the VEGF-stimulated NO-independent pathway is not impaired.
Hedgehog signaling is increased in LSECs that have capillarized in vitro and inhibition of hedgehog signaling blocks culture-induced capillarization (35). It remains to be determined experimentally how the two pathways linked to capillarization, i.e. decline in the VEGF-stimulated NO pathway and increased hedgehog signaling, are linked to one another.
Differing Effects of Differentiated and Capillarized LSECS on Hepatic Stellate Cell Activation (Figure 3)
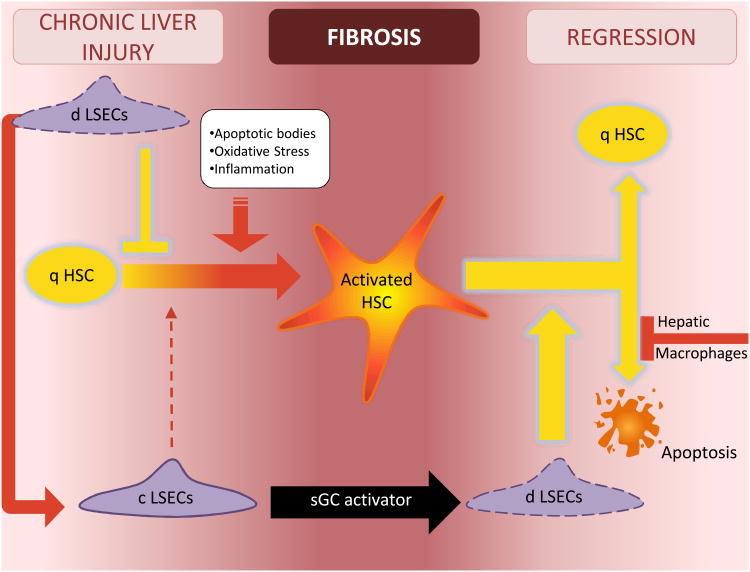
Figure 3. The crosstalk of liver cells in regulating HSC quiescence and activation.
In chronic liver injury, formation of apoptotic bodies, oxidative stress and cytokines promote HSC activation. Differentiated LSECs function as a gatekeeper, preventing activation of the HSC. However once the LSEC capillarizes, it no longer prevents HSC activation and permits or perhaps promotes activation of the HSC. LSEC capillarization can be reversed with an sGC activator that works downstream in the VEGF-NO-sGC pathway. Reversal of capillarized LSECs to differentiated LSECs promotes reversion of activated HSC to quiescence and induces some apoptosis of activated HSC. Hepatic macrophages in turn inhibit apoptosis of activated HSC. Abbreviations: c LSEC, capillarized liver sinusoidal endothelial cell; d LSEC, differentiated liver sinusoidal endothelial cell; q HSC, quiescent hepatic stellate cell; NO, nitric oxide; sGC, soluble guanylate cyclase.
Prevention of hepatic stellate cell activation
Hepatic stellate cells (HSCs) cultured alone on plastic become activated. When HSCs are placed in co-culture with differentiated LSECs, the presence of LSECs maintains HSC quiescence (36). This is true whether co-culture is performed with either heterotypic contact (that is with LSECs and HSCs in physical contact) or with physical separation of the cells and sharing of the culture medium. Thus the ability of LSECs to maintain HSC quiescence is through a paracrine factor. When quiescent HSC are co-cultured with capillarized LSECs, the rate of HSC activation is the same as for HSCs cultured alone (36). Cardiac microvascular endothelial cells have no effect on HSC activation, indicating that this is not a universal effect of microvascular endothelial cells (28).
Hepatocytes are unable to maintain HSC quiescence unless the culture medium is supplemented with an NO-donor, but it remains to be determined whether NO contributes to the paracrine effect of LSECs on HSC quiescence or whether other paracrine factors are necessary (36). Our group and others have reported that NO produced by LSECs maintains HSC quiescence: in co-culture of LSECs with HSCs, inhibition of eNOS by L-NAME blocks the ability of LSECs to maintain HSC quiescence (36, 37). However the flaw in the interpretation of these studies is that LSECs cultured in the presence of L-NAME capillarize and capillarized LSECs do not maintain HSC quiescence (36). At this point it has not been demonstrated that LSEC NO contributes to maintaining HSC quiescence. As described below, NO does not promote reversion of activated HSC to quiescence.
In cirrhosis, decreased production of NO by LSECs (31) and increased production of endothelin-1 by HSCs promotes contractility of HSCs (38) and increased production of prostanoids by LSECs increases portal pressure (39).
Reversion of activated HSC to quiescence
When differentiated LSECs are added to culture-activated HSCs and LSEC differentiation is maintained with a sGC activator, activated HSCs revert to quiescence (28). The paracrine mediator for the paracrine effect of LSECs on HSC activation has not been identified, but NO does not promote reversion of activated HSCs to quiescence (28). When differentiated LSECs are added to activated HSCs and no measures are taken to preserve LSEC differentiation, LSECs capillarize and HSCs remain activated (28). Thus differentiated LSECs promote reversion of activated HSC to quiescence, but capillarized LSECs do not.
In cirrhotic liver, statins increase hepatic eNOS activity and cGMP levels (40). In the bile duct ligation model, statins increase hepatic eNOS and PKG (protein kinase G) activity (41), decrease HSC Rho-kinase activity, and reduce portal pressure with decreased intrahepatic resistance (41). Statin-induced upregulation of the transcription factor KLF2 in hepatic endothelial cells increases eNOS expression, presumably contributing to the decreased intrahepatic resistance, and promotes hepatic endothelial cell-dependent reversion of activated HSCs to quiescence (37).
To summarize, differentiated LSECs prevent HSC activation and promote reversion to quiescence, but capillarized LSECs do not. The mediator for this has not been identified. Taken together with the observation that capillarization precedes fibrosis, these findings suggest that capillarization is at least permissive for fibrosis.
There are data that suggest that capillarized LSEC actively promote HSC activation. LSECs in a bile duct ligation model make increased quantities of EIIIA-fibronectin (42). HSCs cultured on matrix conditioned by LSECs isolated from the bile duct ligation model show increased activation that is blocked by antibodies to EIIIA fibronectin. This suggests that injured LSECs produce EIIIA-fibronectin that promotes HSC activation. However recent studies suggest that EIIIA fibronectin does not play an essential role in HSC activation in vitro or in vivo, but does contribute to HSC motility (43). The effect on HSC may contribute to a modest pro-fibrotic effect of EIIIA in a toxin-induced model of parenchymal fibrosis, but EIIIA does not appear to effect fibrosis in a bile duct ligation model (43).
Another potential mechanism by which capillarized LSECs might promote HSC activation is through acquired immunogenicity. In normal liver, LSECs are tolerogenic (44, 45). However in fibrosis, LSECs isolated from fibrotic liver secrete proinflammatory cytokines and chemokines and induce immunogenic T cells ex vivo (46). If confirmed in vivo, this would suggest that capillarized LSECs might promote HSC activation.
In vivo studies have suggested that activated HSC do not revert to full quiescence upon regression of fibrosis, but that HSC that develop a quiescent phenotype after regression of fibrosis are poised to rapidly re-activate when challenged with a fibrotic stimulus (47). These HSC have been termed inactivated HSC. It is not known whether HSC that revert to a quiescent-appearing phenotype in co-culture with differentiated LSECs are fully quiescent or so-called inactivated HSCs.
In Vivo Studies of The Role of LSECS in Fibrosis (Figure 3)
LSEC fenestration in normal animals is lost after knockdown of VEGF with anti-sense oligonucleotides, demonstrating that VEGF is essential to the LSEC phenotype (28). Similarly, conditional induction of a VEGF decoy receptor that sequesters VEGF and precludes VEGF signaling leads to loss of LSEC fenestration (29).
After early cirrhosis is induced in an experimental model of toxin-induced parenchymal fibrosis, the thioacetamide model, a one-week treatment with sGC activator normalizes LSEC fenestration, but does not alter HSC activation in vivo or modify degree of fibrosis (28). After the sGC activator is discontinued, the differentiated LSECs subsequently reduce the number of activated HSCs and accelerate regression of fibrosis. The decline in activated HSCs is predominantly due to reversion to quiescence, but the total number of activated plus quiescent HSCs declines indicating that LSECs also promote HSC apoptosis.
This particular sGC activator prevents HSC activation in vitro (28) and prevents hepatic fibrosis if given simultaneously with the fibrotic stimulus (48). Thus at first glance it might be surprising that treatment with the sGC activator did not lead to reversion of activated HSCs to quiescence. However activated HSCs lack the α1β1 heterodimeric sGC subunit needed to generate cGMP (49) and would therefore not be expected to respond to an sGC activator.
The effect of normalizing LSEC differentiation was also examined in a model in which early cirrhosis (3 weeks of thioacetamide) was induced, followed by treatment with an additional 3 weeks of thioacetamide plus an sGC activator (28). Treatment with the sGC activator completely normalized LSEC fenestration, which then promoted reversal of HSC activation to quiescence and some degree of apoptosis, and completely prevented any further progression of cirrhosis despite the ongoing treatment with thioacetamide (28).
Taken together these in vivo studies demonstrate that pharmacologic therapy that stimulates downstream in the VEGF-NO-sGC-cGMP-protein kinase G pathway restores LSEC fenestration in cirrhosis. Restoration of LSEC differentiation accelerates regression of fibrosis after discontinuing a fibrotic stimulus and prevents progression of cirrhosis despite an ongoing fibrotic stimulus.
Conclusions
The studies described above support the concept that differentiated LSECs function as a gatekeeper in the fibrotic process. As long as VEGF from hepatocytes and HSC maintain the phenotype of LSECs, the LSEC prevents HSCs from activating. However LSEC differentiation is lost prior to fibrosis, so-called capillarization, and this permits or promotes HSC activation and fibrosis. Pharmacological intervention to reverse LSEC capillarization promotes quiescence of HSC.
During resolution of fibrosis, the decline in activated HSC is due to both reversion to quiescence and apoptosis (28, 50). During chronic liver disease, Kupffer cells prevent HSC apoptosis and thereby sustain the fibrotic process (51). Thus there is an intricate interplay amongst the non-parenchymal cells that either prevents or sustains HSC activation and thereby determines fibrosis (Figure 3). Ultimately most chronic liver diseases that result in fibrosis are primarily diseases of the hepatocyte, not the non-parenchymal cells. Chronic injury to hepatocytes leading to formation of apoptotic bodies, reactive oxidative stress and inflammation are linked to activation of HSC and fibrosis. These changes, and perhaps additional links yet to be uncovered, connect sustained parenchymal injury with the non-parenchymal cell response that leads to fibrosis.
Acknowledgments
The author would like to gratefully acknowledge Lily Dara for her help with the figures.
Research described in this review was supported by NIH DK66423
References
- 1.Schaffner F, Popper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. [PubMed] [Google Scholar]
- 2.Xie G, L W, W X, W L, DeLeve LD. Isolation of periportal, mid-lobular and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am J Physiol-Gastrointest Liver Physiol. 2010;299:G1204–1210. doi: 10.1152/ajpgi.00302.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K, Smedsrod B. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1217–1230. doi: 10.1152/ajpregu.00686.2011. [DOI] [PubMed] [Google Scholar]
- 4.Malovic I, Sorensen KK, Elvevold KH, Nedredal GI, Paulsen S, Erofeev AV, Smedsrod BH, et al. The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology. 2007;45:1454–1461. doi: 10.1002/hep.21639. [DOI] [PubMed] [Google Scholar]
- 5.Martens JH, Kzhyshkowska J, Falkowski-Hansen M, Schledzewski K, Gratchev A, Mansmann U, Schmuttermaier C, et al. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. Journal of Pathology. 2006;208:574–589. doi: 10.1002/path.1921. [DOI] [PubMed] [Google Scholar]
- 6.Hansen B, Longati P, Elvevold K, Nedredal GI, Schledzewski K, Olsen R, Falkowski M, et al. Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Experimental Cell Research. 2005;303:160–173. doi: 10.1016/j.yexcr.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 7.McCourt PA, Smedsrod BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology. 1999;30:1276–1286. doi: 10.1002/hep.510300521. [DOI] [PubMed] [Google Scholar]
- 8.Politz O, Gratchev A, McCourt PAG, Schledzewski K, Guillot P, Johansson S, Svineng G, et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochemical Journal. 2002;362:155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomhoff R, Eskild W, Berg T. Endocytosis of formaldehyde-treated serum albumin via scavenger pathway in liver endothelial cells. Biochemical Journal. 1984;218:81–86. doi: 10.1042/bj2180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomhoff R, Smedsrød B, Eskild W, Granum PE, Berg T. Preparation of isolated liver endothelial cells and Kupffer cells in high yield by means of an enterotoxin. Experimental Cell Research. 1984;150:194–204. doi: 10.1016/0014-4827(84)90714-6. [DOI] [PubMed] [Google Scholar]
- 11.Eskild W, Kindberg GM, Smedsrod B, Blomhoff R, Norum KR, Berg T. Intracellular transport of formaldehyde-treated serum albumin in liver endothelial cells after uptake via scavenger receptors. Biochemical Journal. 1989;258:511–520. doi: 10.1042/bj2580511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blomhoff R, Drevon CA, Eskild W, Helgerud P, Norum KR, Berg T. Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. Journal of Biological Chemistry. 1984;259:8898–8903. [PubMed] [Google Scholar]
- 13.Nagelkerke JF, Barto KP, van Berkel TJ. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. Journal of Biological Chemistry. 1983;258:12221–12227. [PubMed] [Google Scholar]
- 14.Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. Journal of Biological Chemistry. 1991;266:2282–2289. [PubMed] [Google Scholar]
- 15.Smedsrød B, Melkko J, Araki N, Sano H, Horiuchi S. Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochemical Journal. 1997;322:567–573. doi: 10.1042/bj3220567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology. 2009;50:920–928. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousavi SA, Sporstøl M, Fladeby C, Kjeken R, Barois N, Berg T. Receptor-mediated endocytosis of immune complexes in rat liver sinusoidal endothelial cells is mediated by FcγRIIb2. Hepatology. 2007;46:871–884. doi: 10.1002/hep.21748. [DOI] [PubMed] [Google Scholar]
- 18.Falkowski M, Schledzewski K, Hansen B, Goerdt S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochemistry and Cell Biology. 2003;120:361–369. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- 19.Bashirova AA, Geijtenbeek TB, van DGC, van VSJ, Eilering JB, Martin MP, Wu L, et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. The Journal of Experimental Medicine. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouta CC, Nasser SM, di TE, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Research. 2001;61:8079–8084. [PubMed] [Google Scholar]
- 21.Nonaka H, Tanaka M, Suzuki K, Miyajima A. Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:2258–2267. doi: 10.1002/dvdy.21227. [DOI] [PubMed] [Google Scholar]
- 22.Horn T, Christoffersen P, Henriksen JH. Alcoholic liver injury: defenestration in noncirrhotic livers-a scanning electron microscopic study. Hepatology. 1987;7:77–82. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 23.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS. Prevention of Hepatic Fibrosis in a Murine Model of Metabolic Syndrome with Non-Alcoholic Steatohepatitis. American Journal of Pathology. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser R, Rogers GWT, Bowler LM, Day WA, Dobbs BR. Defenestration and vitamin A status in a rat model of cirrhosis. In: Wisse E, Knook DL, McCuskey RS, editors. Cells of the hepatic sinusoid. Vol. 3. Leiden: Kupffer Cell Foundation; 1991. pp. 195–198. [Google Scholar]
- 25.Tamaki S, Ueno T, Torimura T, Sata M, Tanikawa K. Evaluation of hyaluronic acid binding ability of hepatic sinusoidal endothelial cells in rats with liver cirrhosis. Gastroenterology. 1996;111:1049–1057. doi: 10.1016/s0016-5085(96)70074-4. [DOI] [PubMed] [Google Scholar]
- 26.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is under paracrine and autocrine control. Am J Physiol-Gastrointest Liver Physiol. 2004;287:G757–763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 27.Géraud C, Schiedzewski K, Demory A, Klein D, Kaus M, Peyre F, Sticht C, et al. Liver Sinusoidal Endothelium: A Microenvironment-Dependent Differentiation Program in Rat Including the Novel Junctional Protein Liver Endothelial Differentiation-Associated Protein-1. Hepatology. 2010;52:313–326. doi: 10.1002/hep.23618. [DOI] [PubMed] [Google Scholar]
- 28.Xie G, Wang X, Wang L, Atkinson RD, Kanel GC, Gaarde WA, DeLeve LD. Role of liver sinusoidal endothelial cell differentiation in progression and regression of rat hepatic fibrosis. Gastroenterology. 2012;142:918–927. doi: 10.1053/j.gastro.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May D, Djonov V, Zamir G, Bala M, Safadi R, Sklair-Levy M, Keshet E. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations. PloS one. 2011;6:e21478. doi: 10.1371/journal.pone.0021478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1) Oncogene. 1994;9:2683–2690. [PubMed] [Google Scholar]
- 31.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 32.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 33.Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 34.Kwon SH, Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Kim YS, et al. Cyclooxygenase-2 and vascular endothelial growth factor in chronic hepatitis, cirrhosis and hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:287–294. doi: 10.3350/cmh.2012.18.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, Chan IS, et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2013;62:299–309. doi: 10.1136/gutjnl-2011-301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLeve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrone G, Russo L, Rosado E, Hide D, Garcia-Cardena G, Garcia-Pagan JC, Bosch J, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. Journal of Hepatology. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Shao R, Yan W, Rockey DC. Regulation of endothelin-1 synthesis by endothelin-converting enzyme-1 during wound healing. Journal of Biological Chemistry. 1999;274:3228–3234. doi: 10.1074/jbc.274.5.3228. [DOI] [PubMed] [Google Scholar]
- 39.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. Journal of Hepatology. 2007;47:220–227. doi: 10.1016/j.jhep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Abraldes JG, Rodriguez-Vilarrupla A, Graupera M, Zafra C, Garcia-Caldero H, Garcia-Pagan JC, Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. Journal of Hepatology. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 42.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. Journal of Cell Biology. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen AL, Sackey BK, Marcinkiewicz C, Boettiger D, Wells RG. Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts. Gastroenterology. 2012;142:928–937 e923. doi: 10.1053/j.gastro.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, et al. Induction of cytokine production in naive CD4+ T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428–1440. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 45.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature Medicine. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 46.Connolly MK, Bedrosian AS, Malhotra A, Henning JR, Ibrahim J, Vera V, Cieza-Rubio NE, et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol. 2010;185:2200–2208. doi: 10.4049/jimmunol.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knorr A, Hirth-Dietrich C, Alonso-Alija C, Hárter M, Hahn M, Keim Y, Wunder F, et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittel-Forschung. 2008;58:71–80. doi: 10.1055/s-0031-1296471. [DOI] [PubMed] [Google Scholar]
- 49.Failli P, Defranco RMS, Caligiuri A, Gentilini A, Romanelli RG, Marra F, Batignani G, et al. Nitrovasodilators Inhibit Platelet-Derived Growth Factor-Induced Proliferation and Migration of Activated Human Hepatic Stellate Cells. Gastroenterology. 2000;119:479–492. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- 50.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Laboratory investigation; a journal of technical methods and pathology. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 51.Pradere JP, Kluwe J, De MS, Jiao JJ, Gwak GY, Dapito DH, Jang MK, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]