Abstract
Background
Although meningitis is the most severe form of infection caused by Mycobacterium tuberculosis, the immunopathogenesis of this disease is poorly understood. We tested the hypothesis that polymorphisms in Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP), an adaptor protein that mediates signals from Toll-like receptors activated by mycobacteria, are associated with susceptibility to tuberculosis (TB).
Methods
We used a case-population study design in Vietnam with cord-blood control samples (n = 392) and case patients (n = 358) who had either pulmonary (n = 183) or meningeal (n = 175) TB.
Results
The TIRAP single-nucleotide polymorphism (SNP) C558T was associated with increased susceptibility to TB, with a 558T allele frequency of 0.035 in control samples versus 0.074 in case patients (odds ratio [OR], 2.25; P < .001). Subgroup analysis revealed that SNP 558T was more strongly associated with susceptibility to meningeal TB (OR, 3.02; P < .001) than to pulmonary TB (OR, 1.55; P = .22). In comparison to the 558CC genotype, the 558TT genotype was associated with decreased whole-blood interleukin-6 production, which suggests that TIRAP influence disease susceptibility by modulating the inflammatory response.
Conclusions
These results provide the firs evidence of an association of a TIRAP SNP with the risk of any disease and also suggest that the Toll-like receptor pathway influence susceptibility to meningeal and pulmonary TB by different immune mechanisms.
Despite the discovery of the tuberculosis bacillus >100 years ago and the availability of effective drugs for >50 years, there remain a number of formidable challenges for controlling Mycobacterium tuberculosis (Mtb) infection [1]. The development of a more-effective vaccine is a high worldwide priority and depends on a thorough understanding of the host response to infection. Furthermore, tuberculosis (TB) causes different types of human disease, including localized pulmonary disease, extrapulmonary disease—such as meningitis—and miliary or disseminated disease. It is not known at present which host genetic, environmental, and bacterial virulence factors influence the different clinical presentations of TB. Tuberculous meningitis (TBM) is an especially devastating form of TB that causes death or severe neurological defici in more than one-half of persons affected [2]. Advances in genomic technology and immunology are creating new opportunities to understand the influence of human genetic variation on the pathophysiological characteristics of TB.
The molecular mechanisms that influence the development of a protective immune response to Mtb are only beginning to be elucidated. A series of studies with different methodologies over the course of the past 50 years have indicated that host genetics strongly influence susceptibility to tuberculosis [3-11]. Major susceptibility loci from whole-genome methods have not yet been conclusively identified which suggests that the genetic control of susceptibility to TB is polygenic. Candidate gene-association studies of critical host response genes have successfully identified some of the genes involved in susceptibility to TB. Using candidate gene approaches, we and others have recently discovered mutations and polymorphisms in Toll-like receptor (TLR)–pathway genes that affect host susceptibility to infection [12-15]. The discovery of TLRs precipitated a major advance in understanding the molecular mechanisms of both pathogen recognition and inflammation by the innate immune system [16-18]. Human TLRs are a family of 10 proteins that differentially recognize pathogen-associated molecular patterns (PAMPs) and initiate signaling pathways that lead to the activation of the innate immune response, cytokine production, and formation of the adaptive immune response. Mycobacteria are initially recognized by TLR1, −2, −4, and −6, which, in turn, interact with the adaptor proteins MyD88 and Toll-interleukin 1 receptor (TIR) domain containing adaptor protein (TIRAP; also known as Mal) to activate macrophages and dendritic cells [19-25]. TIRAP mediates signals from these TLRs through its TIR domain, which forms a homotypic interaction with the TIR domain of the TLR [26-29]. It is not presently known whether polymorphisms in TIRAP influence susceptibility to any infection.
Because of the central role that adaptor proteins play in mediating signals from TLRs that recognize Mtb, we hypothesized that polymorphisms in TIRAP are associated with susceptibility to TB. In the present article, we report the association of a TIRAP single-nucleotide polymorphism (SNP) with susceptibility to TBM and demonstrate that this polymorphism is associated with a decreased whole-blood cytokine response. To our knowledge, this is the firs reported association of a TIRAP SNP with any infection, which suggests that variants of TIRAP influence important differences in host susceptibility to TB.
SUBJECTS, MATERIALS, AND METHODS
Human subjects and study design
Study subjects with TBM were recruited from 2 centers in Ho Chi Minh City, Vietnam: Pham Ngoc Thach (PNT) Hospital for Tuberculosis and the Hospital for Tropical Diseases (HTD). These 500-bed hospitals serve the local community and act as tertiary referral centers for severe TB (PNT) and infectious diseases (HTD) for the whole of southern Vietnam (population, ~35 million). Adults (>14 years old) admitted consecutively to these centers between September 2000 and April 2003 with clinical meningitis (define as nuchal rigidity and abnormal cerebrospinal flui [CSF] parameters), a negative HIV test, and either acid-fast bacilli seen or Mtb isolated from the CSF were eligible to enter the study. Of the 175 subjects in the study, 158 had Mtb isolated from their CSF culture, and 17 had a positive stain for acid-fast bacilli. Between April 2001 and April 2003, all patients were recruited to a randomized, placebo-controlled trial of dexamethasone for the treatment of TBM; details of the methods of recruitment, treatment, and outcome assessments of these adults have been reported elsewhere [30]. Adults admitted before April 2001 did not receive adjunctive corticosteroids, and their clinical data were recorded as part of a different investigation [31].
Participants with pulmonary TB were recruited between April 2003 and December 2004 from a network of district TB control units within Ho Chi Minh City and surrounding provinces that exist to implement the directly observed therapy program. Inclusion criteria included age >14 years, no previous history of treatment for pulmonary TB, no evidence of miliary or extrapulmonary TB, chest x-ray results consistent with active disease (but not miliary disease), negative HIV test results, and Mtb cultured from sputum. Patients with pulmonary TB were initially matched to the patients with TBM by age (±5 years) and by residence in a TB control program administrative district. Matching by sex was attempted but was not achieved because of the greater availability of men with pulmonary TB in the district control units. Additional patients with pulmonary TB were also enrolled after matched control subjects were found.
Control subjects were enrolled at Hung Vuong Hospital in Ho Chi Minh City, where blood was collected from the umbilical cord of babies after birth. All participants were of Vietnamese Kinh ethnicity as assessed by questionnaire.
All protocols were approved by human-subject review committees at HTD, PNT, Health Services of Ho Chi Minh City, Hung Vuong Hospital, Oxford Tropical Research Ethics Committee, the University of Washington, and the Western Institutional Review Board (Olympia, WA). Written, informed consent was obtained from all patients or from their relatives if the patient could not provide consent. For cord-blood controls, parents provided informed consent.
Genomic techniques
Genomic DNA was purified from peripheral blood using the QIAamp DNA blood kit (Qiagen). To generate sequencing templates, 2 exons that make up the coding region of TIRAP were amplified from 25 ng of genomic DNA with primers 4 (gaatgagagcagggtaagtgcagcctttgtg) and 21 (gcgtctctctgagtttggacc) or with primers 3 (gtggagcaacaggtctctgagaataagatg) and 23 (ccaaggcacagagcgggtgagtaacttgg). The polymerase chain reaction (PCR) products were sequenced with Big Dye Terminator v3.0 and analyzed on an ABI PRISM 3730 capillary sequencer (Applied Biosystems). Sequences were aligned and analyzed using the programs PHRED/PHRAP (versions 0.020425.c/ 0.990319) and CONSED (version 14.0; all University of Washington) [32]. Genotyping was performed using a MassARRAY technique (Sequenom), as described elsewhere [33, 34].
Ex vivo whole-blood cytokine assay
Whole-blood cytokine assays were performed as described elsewhere [35]. Stimuli included 10 ng/mL ultrapure lipopolysaccharide (LPS) from Salmonella minnesota R595 (List Biological Labs), synthetic bacterial lipopeptides PAM2Cys-SKKK (PAM2) and PAM3Cys-SKKK (PAM3; EMC Microcollections), and M. tuberculosis H37Rv whole-cell lysate (TB Vaccine Testing and Research Materials Program, Colorado State University).
Statistical analysis
Univariate analysis was performed for categorical variables with a χ2 test and for continuous variables using a Mann-Whitney U test with 2-sided testing (SPSS version 11.5 [SPSS] and Prism version 4.03 [GraphPad] software). Haplotypes were constructed with an expectation/maximization algorithm implemented using the HAPIPF function in Stata software (Intercooled version 8.1; StataCorp) [36]. To correct for multiple comparisons, we used a conservative Bonferroni approach and multiplied the calculated P value by the number of polymorphisms that were genotyped (n = 4).
RESULTS
Common TIRAP polymorphisms in individuals with TBM
We hypothesized that TIRAP polymorphisms are associated with susceptibility to TB and/or to different clinical types of TB. We enrolled 175 HIV-negative adults with TBM in the study. The subjects were a mean of 37.0 years old, 50.3% were female, and they had a range of severity of neurological impairments, including cranial nerve palsies (39.1%) and hemiplegia (14.2%). Of the subjects, 18.8% died, and 59.8% made a complete recovery.
To discover polymorphisms, we PCR amplified and sequenced the 235-aa TIRAP coding region in 45 subjects with TBM. Four polymorphisms were present in the TIRAP coding region in Vietnamese subjects with TBM. Two of these SNPs were synonymous (G303A [Q101Q] and C558T [A186A]), and 2 were nonsynonymous (G164A [S55N] and C539T [S180L]). The minor allele frequencies of the 4 SNPs (G164A, G303A, C539T, and C558T) in Vietnam are 0.156, 0.011, 0.011, and 0.089. These 4 polymorphisms are also present in the National Center for Biotechnology Information database (available at: http://www.ncbi.nlm.nih.gov/), which contains an additional 4 SNPs that are not present in the Vietnamese cohort (P9A, W13R, N96D, and I197V).
Association of TIRAP SNP C558T with susceptibility to TBM
We next examined whether these SNPs were associated with susceptibility to TB using a case-population study design. In addition to the subjects with TBM, we enrolled 183 subjects with pulmonary TB (mean age, 40.0 years; 37.4% female). All of these individuals were HIV-negative and had localized pulmonary TB with no evidence of miliary or extrapulmonary involvement. For population controls, we obtained 392 cord-blood samples from a local maternity hospital. SNP C558T was associated with susceptibility to TB with a higher allele frequency in case subjects (0.074), compared with the control group (0.035) (odds ratio [OR], 2.25 [95% confidence interval {CI}, 1.40–3.62]; P < .001) (table 1). We next examined whether SNP C558T had stronger associations with different clinical forms of TB. SNP C558T was associated with susceptibility to TBM at a higher frequency in the TBM group (0.10) than in the controls (0.035) (OR, 3.02; P < .001) (table 1). The frequency was slightly higher in the group with pulmonary TB (0.05), but this difference was not statistically significant Even with a conservative correction for multiple comparisons, the association of allele 558T with susceptibility to MTB remained highly statistically significant (P = .0026 for all types of Mtb vs. controls; P < .001 for TBM vs. controls). SNPs 303A and 539T had lower frequencies in the group with pulmonary TB (0.003 and 0.003) than in controls (0.013 and 0.012), but the comparison was not statistically significant The lack of significance may have been due to decreased power caused by small sample numbers. The frequency of SNP 164A was not different between case subjects and controls.
Table 1. Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) single-nucleotide polymorphism (SNP) allele and genotype frequencies in the control and tuberculosis (TB) groups.
| Allele |
Genotype |
Allelic comparison |
Genotypic comparison |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP, group | 0 | 1 | 00 | 01 | 11 | OR (95% CI) | P | x2 (2 df) | P |
| G164A | |||||||||
| Control | 621 (0.794) | 161 (0.224) | 247 (0.632) | 127 (0.325) | 17 (0.043) | 1 | |||
| All TB | 562 (0.787) | 152 (0.213 | 220 (0.618) | 121 (0.340) | 15 (0.042) | 1.04 (0.81–1.48) | .74 | 0.19 | .91 |
| Pulmonary TB | 281 (0.776) | 81 (0.224) | 110 (0.608) | 62 (0.343) | 9 (0.050) | 1.11 (0.82–1.50) | .49 | 0.34 | .85 |
| TBM | 281 (0.798) | 71 (0.202) | 110 (0.629) | 59 (0.337) | 6 (0.034) | 0.98 (0.72–1.33) | .87 | 0.31 | .86 |
|
| |||||||||
| G303A | |||||||||
| Control | 766 (0.987) | 10 (0.013) | 378 (0.974) | 10 (0.026) | 0 | 1 | |||
| All TB | 692 (0.991) | 6 (0.009) | 342 (0.983) | 6 (0.017) | 0 | 0.67 (0.24–1.84) | .43 | .46a | |
| Pulmonary TB | 351 (0.997) | 1 (0.003) | 175 (0.994) | 1 (0.006) | 0 | 0.22 (0.03–1.71) | .11 | .10a | |
| TBM | 341 (0.986) | 5 (0.014) | 167 (0.971) | 5 (0.029) | 0 | 1.13 (0.38–3.31) | .83 | .78a | |
|
| |||||||||
| C539T | |||||||||
| Control | 769 (0.988) | 9 (0.012) | 380 (0.977) | 9 (0.023) | 0 | 1 | |||
| All TB | 704 (0.992) | 6 (0.008) | 348 (0.983) | 6 (0.017) | 0 | 0.73 (0.26–2.06) | .55 | .61a | |
| Pulmonary TB | 361 (0.997) | 1 (0.003) | 180 (0.994) | 1 (0.006) | 0 | 0.24 (0.03–1.88) | .14 | .18a | |
| TBM | 343 (0.986) | 5 (0.008) | 168 (0.971) | 5 (0.029) | 0 | 1.25 (0.41–3.74) | .70 | .77a | |
|
| |||||||||
| C558T | |||||||||
| Control | 753 (0.965) | 27 (0.035) | 366 (0.938) | 21 (0.054) | 3 (0.008) | 1 | |||
| All TB | 659 (0.926) | 53 (0.074) | 308 (0.868) | 41 (0.115) | 6 (0.017) | 2.25 (1.40–3.62) | <.001 | 10.82 | .004 |
| Pulmonary TB | 344 (0.950) | 18 (0.05) | 162 (0.895) | 19 (0.105) | 0 | 1.55 (0.85–2.82) | .22 | 6.26 | .044 |
| TBM | 315 (0.900) | 35 (0.100) | 146 (0.839) | 22 (0.126) | 6 (0.034) | 3.02 (1.79–5.09) | <.001 | 15.04 | .001 |
NOTE. For calculation of odds ratios (ORs), each group was compared with the control group. All of the TIRAP SNPs were in Hardy-Weinberg equilibrium. The frequencies of SNPs G164A, C303A, C539T, and C558T in the National Center for Biotechnology Information database were 0.026, 0.047, 0.089, and 0.167, respectively. 0, common allele; 1, allele with minor frequency; CI, confidence interval; TBM, tuberculous meningitis.
Fisher’s exact test was used because of small sample numbers.
We examined SNP558 further by comparing genotype frequencies to determine whether there was an increased association in homozygous (558TT) individuals. We used a dominant model of analysis and compared the genotype frequencies of 558CC and CT (combined) versus 558TT. The frequency of genotype 558TT was 0.034 in subjects with TBM, compared with 0.008 in controls (OR, 4.61 [95% CI, 1.14–18.64]; P = .019); this suggested a stronger association with susceptibility in homozygotes. We next constructed haplotypes to analyze whether there were additive associations among the different alleles. The haplotype containing the 558T allele (the GGCT haplotype, representing SNPs 164-303-539-558) was higher in subjects with TBM (0.095) than in controls (0.031) and was statistically significant (OR, 3.33; P < .001) (table 2).
Table 2. Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) haplotype frequencies in the control and tuberculosis (TB) groups.
| Haplotype (G164A_G303A_C539T_C558T) |
||||
|---|---|---|---|---|
| Group | GGCC 0000 | GGCT 0001 | GATC 0110 | AGCC 1000 |
| Control | 577.8 (0.753) | 24.0 (0.031) | 7.2 (0.009) | 158.2 (0.206) |
|
| ||||
| All TB | 481.5 (0.700) | 51 (0.074) | 5.5 (0.008) | 149.5 (0.217) |
| All TB vs. control | ||||
| OR (95% CI) | 1 | 2.55 (1.55–4.20) | 0.92 (0.30–2.80) | 1.13 (0.88–1.46) |
| P | <.001 | .88 | .33 | |
|
| ||||
| Pulmonary TB | 250 (0.714) | 19 (0.054) | 1 (0.003) | 80 (0.229) |
| Pulmonary TB vs. control | ||||
| OR (95% CI) | 1 | 1.83 (0.98–3.40) | 0.32 (0.04–2.61) | 1.17 (0.86–1.59) |
| P | .05 | .26 | .32 | |
|
| ||||
| TBM | 231.5 (0.686) | 32 (0.095) | 4.5 (0.013) | 69.5 (0.206) |
| TBM vs. control | ||||
| OR (95% CI) | 1 | 3.33 (1.92–5.77) | 1.56 (0.48–5.12) | 1.10 (0.80–1.51) |
| P | <.001 | .46 | .57 | |
NOTE Data are estimated haplotype no. (frequency), unless otherwise indicated. Haplotypes represent alleles composed of 4 TIRAP single-nucleotide polymorphisms: G164A_G303A_C539T_C558T. Odds ratios (ORs) were calculated in reference to the 0000 haplotype. “All TB” includes both pulmonary and meningeal TB (TBM). 0, common allele; 1, allele with minor frequency; CI, confidence interval.
The analysis suggested that the association of SNP 558T with susceptibility to TB was predominantly observed with meningeal, as opposed to pulmonary, disease. We examined this possibility by directly comparing the polymorphism frequencies in subjects with meningeal and pulmonary TB. SNP C558T was associated with susceptibility to TBM with a significantly higher allele frequency in subjects with TBM (0.10) than in subjects with pulmonary TB (0.05) (OR, 2.12; P = .01). Genotype 558TT also had a higher frequency in subjects with TBM (0.034) than in subjects with pulmonary TB (0.00) (P = .013). We further analyzed the TBM group and compared subjects with or without pulmonary involvement in the setting of meningitis. There was no difference in the frequency of the 558T allele between these 2 groups (558T allele frequency, 0.118 with pulmonary disease vs. 0.098 without pulmonary disease: OR, 1.23; P >.05). These results suggest that the primary association of SNP558T is with meningitis and that the additional presence of pulmonary involvement does not further influence this association. Together, the results of the allelic, genotypic, and haplotypic analyses suggest that SNP 558T is associated with an increased risk of TB and that this effect is predominantly observed with meningeal, as opposed to pulmonary, TB. Furthermore, the association is not substantially altered by other alleles in the haplotype analysis and is strongest for individuals who are homozygotes for 558TT.
Association of SNP558TT with decreased interleukin (IL)–6 production ex vivo
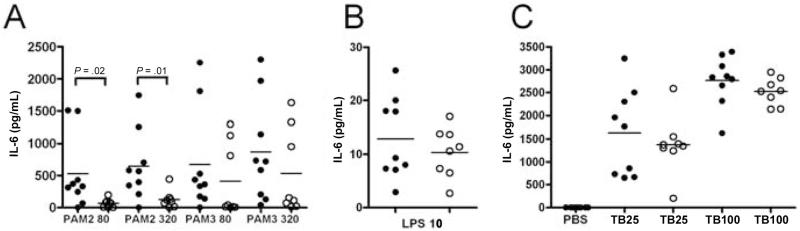
Because of the association of SNP558TT with susceptibility to TBM, we examined the capacity of individuals with this genotype to mediate cytokine production using an ex vivo assay. We stimulated TIRAP-dependent pathways in whole blood from individuals with different TIRAP genotypes and then measured cytokine production by ELISA. Stimulation with different lipopeptides resulted in high levels of IL-6 production and minimal levels of other cytokines, such as tumor necrosis factor-α and IL–1β (figure 1 and data not shown). Samples from individuals with the 558TT genotype had significantly decreased IL-6 production, compared with those with the wild-type 558CC genotype, when stimulated with a diacylated lipopeptide (PAM2), a ligand for the TLR2/TLR6 pathway (median [interquartile range] for 80 ng/mL PAM2: 329.9 [155.3–965.3] pg/mL for CC vs. 72.7 [6.7–91.2] pg/mL for TT; P = .02, Mann-Whitney U test; for 320 ng/mL PAM2: 567.4 [276.6–979.5] pg/mL for CC vs. 102.2 [41.3–142.9] pg/ mL for TT; P = .01) (figure 1A). There was a similar, although statistically nonsignificant trend toward a difference between the CC and TT genotypes for stimulation with a triacylated lipopeptide (PAM3), a ligand for TLR2/TLR1. In comparison, there was no significant difference in LPS-induced IL-6 production (figure 1B). Similarly, whole-cell lysates of TB H37Rv did not stimulate different levels of IL-6 (figure 1C). Together, these results suggest that TIRAP SNP 558TT mediates cytokine production less efficiently than SNP 558CC in response to TLR2 stimulation.
Figure 1. Ex vivo whole-blood cytokine response and Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) single-nucleotide polymorphism (SNP) C558T.
Whole blood was harvested from individuals with TIRAP genotypes 558CC (black circles) or 558TT (white circles). Cells were stimulated with 80 or 320 ng/mL lipopeptides PAM2Cys-SKKK (PAM2) or PAM3Cys-SKKK (PAM3) (A), 10 ng/mL lipopolysaccharide (LPS) (B), PBS, or 25 or 100 μg/mL whole-cell H37Rv Mycobacterium tuberculosis (TB) lysates (C). Cells were stimulated for 18 h, and supernatants were assayed for cytokine production by ELISA. Data are combined from 2 separate experiments, each of which were performed in triplicate. Individual data points are plotted, with the median indicated by a line. Significant P values are noted and were calculated using the Mann-Whitney U test.
DISCUSSION
We report here the firs association of a TIRAP polymorphism (C558T) with susceptibility to any infectious disease. Although these results suggest an association of TIRAP with susceptibility to Mtb, we cannot exclude that SNP C558T is in linkage disequilibrium with a polymorphisms in a nearby causative gene other than TIRAP. However, the biological plausibility of TIRAP’s association with TB is compelling, and no nearby genes are more likely candidates. TIRAP is a critical adaptor protein in the TLR signaling pathway, and it mediates signals from TLR1, -2, -4, and -6. These 4 TLRs recognize a broad spectrum of pathogens, including fungi, viruses, parasites, gram-negative and -positive bacteria, and mycobacteria. Because TIRAP plays a central role in the innate immune recognition of a wide variety of pathogens, we speculate that TIRAP polymorphisms will be associated with susceptibility to a wide spectrum of human infections. With an allele frequency of 0.035-0.10, TIRAP SNP 558T may influence susceptibility to TB in a substantial portion of the population. However, the population-attributable fraction of meningitis that is associated with SNP 558T is low and suggests that further genes will be identified that influence susceptibility to TBM.
A potential limitation of our study is the misclassification of controls, given that some subjects who provided cord-blood samples would become case subjects if exposed to Mtb after birth. However, because only 10% of individuals with latent TB develop active disease, the misclassification rate would be small and would lower, rather than artefactually inflate the magnitude of the association. In Vietnam, ~65% of adults have evidence of previous exposure to Mtb [37]. Thus, the potential misclassification rate would be even lower (~6.5%) if the control group included healthy adults with no history of TB—a common inclusion criterion for control groups in TB genetics studies [38]. Furthermore, the risk of misclassification is substantially lower for TBM than that for pulmonary TB, because only a small fraction of individuals with active TB develop meningitis [39, 40]. The other potential confounding effect of using cord-blood controls is the influence of early childhood mortality on SNP frequencies. However, the influence of childhood mortality on the associations observed in our study would be small, given that Vietnam’s death rate among children <5 years old is 23 deaths/1000 live births (2.3%) [41]. In addition, this potential confounding influence would likely lower the magnitude of our observed effect rather than create a false association. The results of previous genetic studies in infectious diseases have supported the utility of using cord-blood controls and included evidence that polymorphism frequencies are comparable between cord-blood samples and adult control subjects [42-44]. To directly address the possible influence of control misclassification we reanalyzed our data and used subjects with pulmonary TB as a reference group for the TBM group. This analysis also supported a statistically significant association of SNP C558T with TBM and suggested that there was no obvious artefactual association attributable to the use of cord-blood controls.
To better understand the role of TIRAP in the pathogenesis of TB, we examined the association of SNP C558T with cellular immune responses. Compared with genotype 558CC, genotype 558TT individuals had decreased IL-6 levels in whole blood stimulated with bacterial lipopeptide (a TLR2 ligand) but not LPS (a TLR4 ligand). These data suggest that SNP C558T mediates signaling differently for TLR2-induced activation than for TLR4 stimulation. This difference may be attributable to selective utilization of adaptor proteins by TLR2 and TLR4. TLR2 uses MyD88 and TIRAP, whereas TLR4 uses MyD88, TIRAP, TRIF, and TRAM [45]. Because Mtb is known to contain lipopeptides that activate through TLR2 and TIRAP-dependent pathways, the impaired response to PAM2 and PAM3 suggests that genotype 558TT individuals may also have an altered response to TB. Interestingly, stimulation of cells with whole-cell lysates of Mtb was not associated with different cytokine responses in genotype 558CC versus 558TT individuals, which suggests that other Mtb molecules can stimulate cells by TIRAP-independent pathways or that some molecules stimulate in a TIRAP-dependent manner that is not influence by the C558T polymorphism. A number of Mtb molecules are known to stimulate the innate immune response through TLRs, including cell-wall components such as LAM and lipoproteins such as the 19-kDa protein [19, 46]. Mtb stimulates a variety of cellular responses, including cytokine production, dendritic-cell activation, and apoptosis. Future studies will address whether these cellular phenotypes are affected by TIRAP SNP C558T. Together, these results suggest that SNP C558T is associated with a deficient initial cellular inflammatory response to some, but not all, TLR stimuli. Thus, it is plausible that TIRAP SNP C558T could influence monocyte, macrophage, and T cell inflammatory responses that subsequently affect the immunopathogenesis of TBM.
The molecular mechanism underlying the association of TIRAP SNP C558T with susceptibility to TB is not known. SNP C558T is a synonymous polymorphism that does not encode an amino acid change. SNP 558 may be in linkage disequilibrium (LD) with a regulatory region polymorphism in a non-coding region that controls expression levels of TIRAP. It is also plausible that another coding region SNP is in LD with 558. In future studies, we will investigate whether an additional SNP is in LD with C558T and examine further the molecular mechanism of the variation in TIRAP signaling associated with different genotypes. The only previously described association of a TLR-pathway polymorphism with susceptibility to pulmonary TB is with TLR2 SNP R753Q [47]. Because TLR2 and TIRAP interact during signaling, it will be of interest to examine possible interactions between these 2 genes in future genetic studies.
The pathogenesis of TBM is not well understood at a molecular or cellular level [2]. In addition, the factors that affect host susceptibility to TBM are poorly understood. We were particularly interested that TIRAP SNP C558T was more strongly associated with TBM than with localized, uncomplicated pulmonary TB. Most previous host genetic studies of tuberculosis have examined the association of polymorphisms with susceptibility to pulmonary disease. Few studies have compared susceptibility to pulmonary and TBM, and none had the sample size of the present investigation. Rossouw et al. [48] reported an association of a polymorphism in interferon-γ with susceptibility to TB in South Africa (OR, 1.64 [95% CI, 1.16–2.30]). The case group included 241 subjects with pulmonary TB and 72 subjects with TBM, and there were no difference in allele frequencies between these groups. A second study of a mannose-binding protein polymorphism found an association with both pulmonary and TBM [49]. Our data are the firs report of an association of a polymorphism in any gene with predominant susceptibility to meningeal, as opposed to pulmonary, TB. An alternative explanation of our data is that this TIRAP SNP is associated with disease severity rather than with clinical subtype, given that the pulmonary group was deliberately chosen to have localized disease that was less severe. We cannot exclude this alternative explanation, which would also be of great interest—previous studies have not uncovered SNPs that are specifically associated with the severity of TB disease. By studying and comparing different clinical forms of TB, we hope to gain insight into the immunopathogenesis of tuberculosis in humans.
TIRAP could affect several steps in the pathogenesis of TBM. At a macroscopic level, the development of TBM likely begins when the meninges or brain parenchyma are seeded with tubercle bacilli during a period of bacteremia during primary infection [2]. These foci of bacilli can rupture into the subarachnoid space and cause clinical symptoms of meningitis, as well as neuropathological symptoms. TIRAP could plausibly affect pathogenesis at several steps in this process. Because of its central role in mediating innate immune inflammatory responses, TIRAP SNPs could alter the initial host containment of bacilli in monocytes or macrophages and the early bacteremia that results in seeding of the meninges. Alternatively, containment of the bacilli in granulomas in the Rich foci could also be controlled by TIRAP. Finally, TIRAP SNPs could regulate inflammatory responses, such as cytokine production, that lead to neuropathological sequelae.
Acknowledgments
We thank the directors and staff at Pham Ngoc Thach Hospital for Tuberculosis and the Hospital of Tropical Diseases, for the clinical and microbiological work associated with the study; the Vietnamese individuals who took part in the study; Sarah Li, for expert genotyping assistance; and the National Institutes of Health–sponsored TB Vaccine Testing and Research Materials Program at Colorado State University.
Financial support: National Institute of Health (grants to T.R.H., L.P.Z., and A.A.); Dana Foundation (grant to T.R.H.); Wellcome Trust of Great Britain (grant to J.J.F.).
Footnotes
Presented in part: “Determinants of Host Resistance, Susceptibility or Immunopathology to Pathogens: Integrating Knowledge from Experimental Models to Human Disease” Keystone Meeting, Keystone, CO, 6–11 January 2006 (abstract 133).2
Potential conflicts of interest: none reported.
References
- 1.Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001;345:189–200. doi: 10.1056/NEJM200107193450307. [DOI] [PubMed] [Google Scholar]
- 2.Thwaites G, Chau TT, Mai NT, Drobniewski F, McAdam K, Farrar J. Tuberculous meningitis. J Neurol Neurosurg Psychiatry. 2000;68:289–99. doi: 10.1136/jnnp.68.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2:967–77. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 4.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy R, Ruwende C, Corrah T, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–4. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson R, Llewelyn M, Toossi Z, et al. Influence of vitamin D receptor and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 7.Comstock GW. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117:621–4. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 8.Malik S, Schurr E. Genetic susceptibility to tuberculosis. Clin Chem Lab Med. 2002;40:863–8. doi: 10.1515/CCLM.2002.153. [DOI] [PubMed] [Google Scholar]
- 9.Newport MJ, Nejentsev S. Genetics of susceptibility to tuberculosis in humans. Monaldi Arch Chest Dis. 2004;61:102–11. doi: 10.4081/monaldi.2004.707. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell JM, Goswami T, Evans CA, et al. SLC11A1 (formerlyNRAMP1) and disease resistance. Cell Microbiol. 2001;3:773–84. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellamy R. Genetic susceptibility to tuberculosis. Clin Chest Med. 2005;26:233–46. doi: 10.1016/j.ccm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–9. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 13.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–9. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 14.Hawn TR, Verbon A, Lettinga KD, et al. A common dominant TLR5 stop codon polymorphism abolishes flagelli signaling and is associated with susceptibility to legionnaires’ disease. J Exp Med. 2003;198:1563–72. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawn TR, Verbon A, Janer M, Zhao LP, Beutler B, Aderem A. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires’ disease. Proc Natl Acad Sci USA. 2005;102:2487–9. doi: 10.1073/pnas.0409831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 19.Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 2002;4:937–44. doi: 10.1016/s1286-4579(02)01611-8. [DOI] [PubMed] [Google Scholar]
- 20.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced pro-inflammatory signaling in macrophages. Proc Nat Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 22.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 23.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170:3451–4. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 24.Krutzik SR, Ochoa MT, Sieling PA, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–32. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 25.Stenger S, Modlin RL. Control of Mycobacterium tuberculosis through mammalian Toll-like receptors. Curr Opin Immunol. 2002;14:452–7. doi: 10.1016/s0952-7915(02)00355-2. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 27.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificit for Toll-like receptors. Nature. 2002;420:329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 28.McGettrick AF, O’Neill LA. The expanding family of MyD88-like adaptors in Toll-like receptor signal transduction. Mol Immunol. 2004;41:577–82. doi: 10.1016/j.molimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 30.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 31.Thwaites GE, Simmons CP, Than Ha Quyen N, et al. Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J Infect Dis. 2003;188:1105–15. doi: 10.1086/378642. [DOI] [PubMed] [Google Scholar]
- 32.Gordon D, Abajian C, Green P. CONSED: a graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 33.Storm N, Darnhofer-Patel B, van den Boom D, Rodi CP. MALDI-TOF mass spectrometry-based SNP genotyping. Methods Mol Biol. 2003;212:241–62. doi: 10.1385/1-59259-327-5:241. [DOI] [PubMed] [Google Scholar]
- 34.Hawn TR, Wu H, Grossman JM, Hahn BH, Tsao BP, Aderem A. A stop codon polymorphism of Toll-like receptor 5 is associated with resistance to systemic lupus erythematosus. Proc Natl Acad Sci USA. 2005;102:10593–7. doi: 10.1073/pnas.0501165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawn TR, Ozinsky A, Williams LM, et al. Hyper-IgE syndrome is not associated with defects in several candidate toll-like receptor pathway genes. Hum Immunol. 2005;66:842–7. doi: 10.1016/j.humimm.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Mander AP. Haplotype analysis in population-based association studies. Stata J. 2001;1:58–75. [Google Scholar]
- 37.Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007–14. doi: 10.4049/jimmunol.176.3.2007. [DOI] [PubMed] [Google Scholar]
- 38.Bellamy R, Ruwende C, Corrah T, McAdam K, Whittle H, Hill A. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–4. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 39.de March-Ayuela P. Trend in tuberculous meningitis in Barcelona in children aged 0-4 years: correlation with the annual risk of tuberculous infection. Tuber Lung Dis. 1994;75:423–8. doi: 10.1016/0962-8479(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 40.Styblo K, Meijer J, Sutherland I. Tuberculosis Surveillance Research Unit report no. 1: the transmission of tubercle bacilli; its trend in a human population. Bull Int Union Tuberc. 1969;42:5–104. [PubMed] [Google Scholar]
- 41.United Nations Children’s Fund (UNICEF) The state of the world’s children 2006. UNICEF; New York: 2005. [Google Scholar]
- 42.Ackerman H, Usen S, Jallow M, Sisay-Joof F, Pinder M, Kwiatkowski DP. A comparison of case-control and family-based association methods: the example of sickle-cell and malaria. Ann Hum Genet. 2005;69:559–65. doi: 10.1111/j.1529-8817.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- 43.Koch O, Awomoyi A, Usen S, et al. IFNGR1 gene promoter polymorphisms and susceptibility to cerebral malaria. J Infect Dis. 2002;185:1684–7. doi: 10.1086/340516. [DOI] [PubMed] [Google Scholar]
- 44.Roy S, Knox K, Segal S, et al. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 2002;359:1569–73. doi: 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- 45.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 46.Quesniaux V, Fremond C, Jacobs M, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004;6:946–59. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Ogus AC, Yoldas B, Ozdemir T, et al. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219–23. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 48.Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene. Lancet. 2003;361:1871–2. doi: 10.1016/S0140-6736(03)13491-5. [DOI] [PubMed] [Google Scholar]
- 49.Hoal-Van Helden EG, Epstein J, Victor TC, et al. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr Res. 1999;45:459–64. doi: 10.1203/00006450-199904010-00002. [DOI] [PubMed] [Google Scholar]