Abstract
Over 100 years have passed since the first observation of the notched wing phenotype in Drosophila melanogaster, and significant progress has been made to characterize the role of the Notch receptor, its ligands, downstream targets, and crosstalk with other signaling pathways. The canonical Notch pathway with four Notch receptors (Notch1-4) and five ligands (DLL1, 3–4, Jagged 1–2) is an evolutionarily conserved cell signaling pathway that plays critical roles in cell-fate determination, differentiation, development, tissue patterning, cell proliferation, and death. In cancer, these roles have a critical impact on tumor behavior and response to therapy. Since the role of Notch remains tissue and context dependent, alterations within this pathway may lead to tumor suppressive or oncogenic phenotypes. Although no FDA approved therapies currently exist for the Notch pathway, multiple therapeutics (e.g., demcizumab, tarextumab, GSI MK0752, R04929097, and PF63084014) have been developed to target different aspects of this pathway for both hematologic and solid malignancies. Understanding the context-specific effects of the Notch pathway will be important for individualized therapies targeting this pathway.
Background
One hundred years ago, John S. Dexter observed serrations on the wing margin in Drosophila melanogaster (1). Decades later, Artavanis-Tsakonas and Young independently cloned the Notch receptor (2). The Notch signaling pathway is a highly conserved pathway among species (Fig. 1). In mammals, four type 1 transmembrane Notch receptors (Notch 1-4) are synthesized. After cleavage at the S1 site in the Golgi apparatus by a furin-like convertase, (3) it becomes glycosylated by O-fucosyltransferase (4, 5) and Fringe family N-acetylglucosaminidyl transferases (6); the processed heterodimers re-assemble on the cellular membrane (7). The extracellular subunits, of Notch1 and 2, both have 36 epidermal growth factor (EGF) repeats; Notch3 and Notch4 have 34 and 29 repeats, respectively, which correlate with affinity for their respective ligands (8). Additionally, the receptor contains a negative regulatory region comprised of three cysteine-rich Lin12/Notch repeats and a C-terminal region (9, 10). The other primary difference between the receptors rests within the transactivation domain (TAD) with either strong (Notch1), weak (Notch 2), or absent (Notch4) TAD (11). The Notch3 TAD is specific to activation of the hes5 promoter (12).
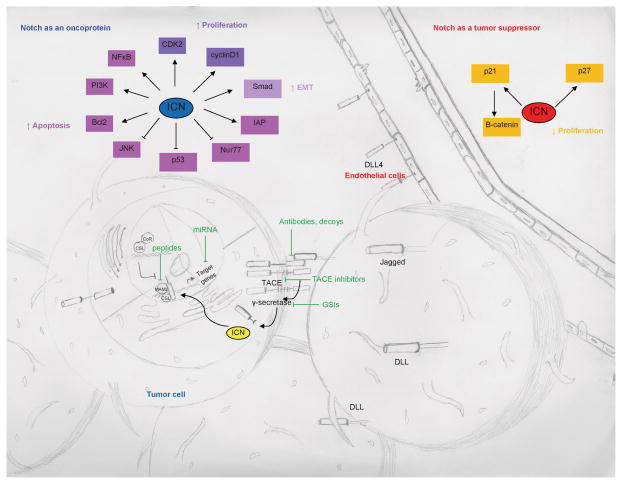
Figure 1.
Notch receptors (Notch1-4) and ligands (DLL1, 3 and 4, Jagged 1-2) are expressed in tumor, normal, and endothelial cells. After ligand binding, the ICN is generated after cleavage events by ADAM/TACE proteases and γ-secretase. The ICN travels into the nucleus, interacts with multiple transcriptional regulators including CSL, displaces CoR, and recruits MAML to activate transcription of target genes. Potential cancer therapeutics that target Notch signaling include antibodies, peptides, miRNAs, TACE inhibitors, and GSIs. Notch can function as a tumor suppressor or is oncogenic and activate/inhibit different downstream targets depending on the malignancy and microenvironment.
Close proximity among cells within the microenvironment is required for ligand-receptor binding and interactions because the ligands remain immobilized as transmembrane proteins. Mammals have four distinct ligands (Jagged1-2, Delta-like [DLL] 1, 3, and 4). Distinct ligand affinities exist for the various receptors, altered by glycosylation, which influences downstream transcriptional activation. Activation of the pathway requires ligand-receptor binding; the ligand undergoes endocytosis within the ligand-emitting cell, which causes a mechanical disruption, changing conformation of the negative regulatory region, and susceptibility of the ectodomain to cleavage by ADAM17 metalloprotease/TNF-α converting enzyme (TACE) at site S2 (13, 14). A subsequent cleavage occurs within the TAD at S3 by presenilin-γ-secretase, liberating the intracellular domain of the Notch receptor (ICN) (15, 16). ICN forms a complex with the inactive DNA-binding factor CSL (CBF1/Suppressor of Hairless/Lag1) and recruits other co-activator proteins from the Mastermind-like family of proteins such as MAML1 (17, 18). The target genes activated by Notch depend on the cell type and ligand-receptor interaction at the cell surface. Frequent target genes include transcriptional repressors of the HES and HEY families, MYC, NF-κB, cyclinD1, p21, CCND1/3, BCL2, pre-Tα (pre-T-cell receptor alpha chain), GATA3, NRARP, Deltex1, and CCR7 (2, 19). Additional non-cognate ligands (e.g. EGFL7) (20) and soluble Jagged ligands have also been described (21).
Notch pathway in cancer
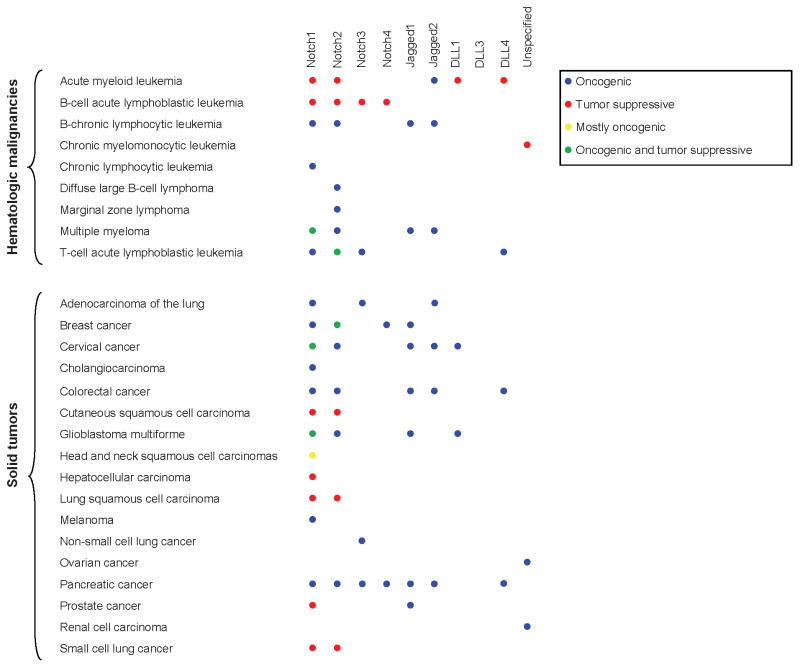
Expression of the four Notch receptors in adult and embryonic tissues varies widely with overlapping expression patterns, but they have unique roles during the generation of hematopoietic stem cells, T-cell and B-cell fate and lineage development, renal progenitor cells, and vascular morphogenesis (2, 22). Dysregulation of the Notch pathway has been implicated in a variety of hematologic and solid malignancies (2). Depending on expression patterns, the Notch pathway can be either oncogenic or tumor suppressive (Fig. 2), involved in either survival or death pathways, proliferation or growth arrest, or differentiation into terminally differentiated cells versus cancer cell “stemness” (23). Abnormal regulation of the Notch pathway may occur by a variety of mechanisms including mutational activation or inactivation, overexpression, post-translational modifications, and epigenetic regulation (2). In general, it seems suppressive in squamous cancers, but activating in hematological malignancies and adenocarcinomas, reflecting its normal functions in those tissues.
Figure 2.
Aberrant Notch signaling occurs in a wide variety of solid and hematologic malignancies, and its role may be oncogenic or tumor suppressive depending on the tissue type and cellular context.
Notch as an oncoprotein
Notch1 is a well-characterized oncoprotein in T-cell acute lymphoblastic leukemia (T-ALL) and lymphomas; activating Notch1 mutations (either in the heterodimerization domain leading to a change in amino acid sequence causing ligand-independent metalloprotease cleavage at site S2 (24) or stop codon or frame shift mutations by deletion of the C-terminal PEST domain) are responsible for approximately 55–60% of T-ALL cases (25). Evidence for Notch as an oncoprotein in melanocytes (26), prostate (27) and breast tissue also exists (28, 29). Constitutively active Notch1 promotes melanoma cell growth, and the oncogenic effect of Notch1 on primary melanoma cells was mediated by beta-catenin (30). The MAPK and PI3K-AKT pathways are both activated in melanoma following Notch1 activation (31). Upregulated Notch signaling has been shown to be oncogenic for multiple hematologic and solid malignancies (2, 19, 32) (Fig. 2).
The mechanisms exploited by Notch for oncogenic effects include inhibition of apoptosis and induction of cellular proliferation. Within solid malignancies, activation of Notch can promote epithelial-to-mesenchymal transition. Anti-apoptotic effects may occur by Notch inhibiting the pro-apoptotic transcription factor, Nur77, upregulation of IAP, Bcl2, and FLIP. Increased proliferation may occur through enhanced CDK2, cyclin D1, and HES1 activity. Notch can suppress p53 expression, promote viability via the PI3K/AKT, ERK and NFκB pathways, and protect against apoptosis via inhibition of JNK activation. Activated Notch can inhibit activity of Smad2-4, leading to decreased TGFβ signaling (Fig. 1) (33).
Notch as a tumor suppressor
Notch has tumor suppressive roles in several malignancies, including skin cancers (Fig. 1). Notch1 knockout mice develop basal cell carcinomas and have increased Wnt and Hedgehog signaling (34). The mechanism of Notch as a tumor suppressor is less well understood, but may be related to inhibition of proliferation and induction of cell cycle arrest through upregulation of p21Cip1 and p27Kip1 and inhibition of β-catenin-mediated Wnt signaling (33).
The mechanisms by which Notch pathway can lead to tumor promoting or suppressive effects in different cell types are not fully understood. These mechanisms may include differential tissue and cell specific target genes, and varying cytokines and growth factors present in distinct microenvironments. For example, p21, a target gene in the keratinocytes in the epidermis may contribute to tumor suppressive effects because it regulates cell cycle progression (35). This may result from CSL only binding the p21 promoter in certain tissue types, or crosstalk between Notch and p62 (36).
Clinical-Translational Advances
Notch antibodies
Antibodies are most frequently targeted to the extracellular negative regulator region of the Notch receptor or the EGF repeats (37). Several Notch specific monoclonal antibodies against Notch1, Notch2, or Notch3 have been developed (38). In pre-clinical models, blocking Notch1 has been shown to decrease T-ALL tumor growth by inhibiting cancer cell growth and by disrupting angiogenesis. However, dual Notch1 and 2 inhibition causes gastrointestinal side effects such as diarrhea (37, 39). A Phase I dose-escalation study of OMP-59R5 (humanized Notch2 and 3 blocking monoclonal antibody) in patients with solid tumors is ongoing (NCT01277146).
Alternative antibodies developed against this pathway target ligands (e.g., anti-DLL4 antibody and soluble DLL4-Fc fusion protein). The DLL4 antibody decreased tumor growth in multiple tumor models and caused defective cell fate differentiation. The role of DLL4 has been shown to be necessary for vascularization, but not vessel maintenance and DLL4 blockade promotes non-productive angiogenesis (40). Multiple early-stage clinical trials are ongoing with demcizumab, an anti-DLL4 monoclonal antibody (41). OMP-21M18 is being tested in combination with gemcitabine with or without Abraxane® (Celgene, Co., Summit, NJ, USA) in pancreatic cancer (NCT01189929), with FOLFIRI in colorectal cancer (NCT01189942), with carboplatin and pemetrexed in lung cancer (NCT01189968), and with paclitaxel in patients with platinum-resistant ovarian cancer (NCT01952249). MedI0639 is another anti-DLL4 antibody being evaluated in patients with advanced solid tumors in phase 1 trials (NCT01577745).
Notch decoys
Soluble decoys of Notch pathway receptors or ligands are highly potent therapeutics. Notch1, DLL1 and Jagged1 decoys (42) remain under current development. A Notch1 decoy reduced downstream signaling and led to decreased tumor angiogenesis with a 58% decrease in microvessel density (43). Monomeric and dimeric forms of DLL1 soluble decoys have been created by fusing the extracellular domain to either a series of myc epitopes or to the Fc portion of human IgG-1, respectively. Non-immobilized DLL1 inhibited downstream Notch functioning (44).
Gamma-secretase inhibitors (GSIs)
A wide variety of GSIs have entered clinical development. Because of the diversity of GSIs and their substrates, the targeting for Notch cleavage is often not highly specific. However, they have cytostatic and cytotoxic properties and act as competitive inhibitors of presenilin activity. RO4929097 is a GSI that pre-clinically showed decreased ICN expression in vitro and antitumor activity in multiple xenografts, with intermittent or daily dosing and prolonged efficacy (45). Tumor growth inhibition values ranged from 66% to 91%. Initial clinical testing suggested a favorable toxicity profile with primarily grade 1 or 2 toxicities of fatigue, thrombocytopenia, rash, chills, and anorexia (46). In a phase I study of patients with refractory metastatic or locally advanced solid tumors, tumor responses included one partial response in a patient with colorectal adenocarcinoma, one mixed response in a patient with sarcoma, and one complete FDG-PET response in a patient with melanoma (46). In a phase II study of 33 evaluable patients with metastatic colorectal cancer, there were no objective radiographic responses and six patients had stable disease. Development of RO4929097 has been discontinued (47). Another GSI, MRK-003, has been shown to induce cell cycle arrest and apoptosis by blocking Notch1 (48), with activity in T-ALL (49) and breast cancer (50). A related compound, MK-0752, which binds to presenilin 1, is being tested in multiple Phase I studies.
Other GSIs include PF-03084014, which has been tested in combination with chemotherapy in colorectal (51), breast (52), pancreatic (53), and triple-negative breast (TNBC) (54) cancer models, and with fludarabine in primary Notch1-mutated CLL (55). Clinical studies with PF-03084014 are ongoing in patients with leukemia, breast or pancreatic cancers. Although preclinical and early clinical activity is encouraging, limitations include non-selectivity and possible toxicities (56).
Inhibition of the Notch signaling pathway by GSIs has been shown to increase sensitivity of tumor cells to both cytotoxic chemotherapy and radiation (57, 58); however, the GSIs are not equally effective in combinations. For example, oxaliplatin activates Notch signaling, so while GSI34 sensitizes colon cancer cells to chemotherapy (59), MRK-003 decreases the apoptotic effect by at least 50% (60). This differential effect of GSIs combined with chemotherapy has also been seen in T-ALL where apoptosis is induced in some cell lines, but not in all (58). In TNBC, the combination of MK-0752 with docetaxel in preclinical and concurrent clinical studies decreased breast cancer stem cells (61). The mechanism for the synergistic activity of MK-0752 and PF-03084014 with docetaxel occurs because docetaxel activates the Notch pathway and suppresses NUMB, an endogenous Notch inhibitor (62).
Blocking peptides
Permeable peptides that interfere with the transcriptional nuclear complex represent attractive therapeutic options. A peptide that forms complexes with Notch1 and CSL, forms a transcriptionally inert nuclear complex, and inhibits the growth of Notch1-transformed T-ALLs (63). Another peptide, SAHM1, binds to the Notch1 and CSL complex and prevents MAML1 from binding (64). The clinical implication and pharmacodynamics of peptides still remain unknown.
There are two basic approaches to targeting the Notch pathway: one is blocking ligands or receptors with monoclonal antibodies and the other blocking downstream signaling with GSIs. Small molecule inhibitors tend to have more off target effects or inhibit multiple pathways, and this is typical with GSIs. Antibodies are more specific and tend to have longer duration of effect. The appropriate selection of therapy will depend on a greater understanding of the molecular pathways involved in a particular cancer. For example, mutations in Notch1 affecting its stability would be logical targets for blocking monoclonal antibodies. For targeting vasculature, DLL4, which is an endothelial specific Notch ligand, can be effectively targeted with a specific antibody.
Rational combination therapies
The Notch pathway interacts with other tumorigenic pathways including the PI3K/AKT, STAT3, and NF-κB pathways. Combinations of GSIs with Hedgehog and Wnt inhibitors suppressed TALL growth in vitro (65). Both the Jagged1 and DLL4 ligands have been previously identified as key players in angiogenesis by separate mechanisms. Jagged1 can positively influence tumor angiogenesis by activating the Notch pathway on tumor endothelial cells (66). Rational combinations include targeting VEGF plus DLL4 since the latter can mediate resistance to anti-VEGF therapy. VEGF induces expression of DLL4 in endothelial and cancer cells in vitro under hypoxic conditions and activation of Notch by immobilized DLL4 led to downregulation of VEGFR2 via promoter methylation. Blocking DLL4 upregulates VEGFR2, and the resultant increase in non-productive angiogenesis may be the result of endothelial cells becoming sensitized to VEGF signaling. This response represents a rational, biologic explanation for why targeting Notch ligands may be complementary to anti-VEGF therapy (67). Notch1 activity has been reported to be related to DNA repair pathways and resistance to DNA cross-linking agents in TNBC; a combination with DNA cross linking agents plus Notch1 inhibitors would be a logical approach (68–70).
In hormonally active malignancies such as ERα-positive breast cancers, combination therapy with GSIs has shown promise in clinical trials (71). Combinations of exemestane and RO4929097 (72) and MK-0752 with tamoxifen or letrozole have led to partial responses and stable disease for some patients (73).
Targeting the Notch pathway via specific siRNA and/or miRNAs also remains a relatively untapped area that could hold future benefit in several malignancies. Notch1 has been identified as a direct target gene of miR-34a. Mir-34a has also been found to be downregulated in glioblastoma multiforme compared with normal brain tissues, and by targeting Notch1, tumor growth was inhibited (74). In pancreatic cancer, miR-34 may be involved in pancreatic cancer stem cell self-renewal, potentially via direct modulation of downstream targets Bcl-2 and Notch (75). Restoring miR-34 may provide future therapeutic benefit in brain and pancreatic malignancies.
Multiple strategies exist for targeting the Notch pathway in malignancies, but the most attractive therapies will target the specific Notch alteration within tumor types while avoiding Notch signaling in normal tissues. Overexpression of Notch receptors and/or ligands does not necessarily imply pathway activation, and pathway activation can lead to tumor suppressive or oncogenic effects. In these cases, simply providing an antibody, peptide, or decoy that targets the Notch receptor may be insufficient to decrease tumor growth or metastasis. Non-specific inhibition of the Notch pathway with GSIs has been toxic. In a phase I study with RO4929097, several responses were noted, but further development has been discontinued.
Patient selection for therapy
As for many targeted therapies, selecting the correct patient is critical. Patients with activating mutations in their tumors seem to be obvious candidates for Notch therapies, but mutations in other genes (FBXW7) can affect Notch degradation. Also, the stroma affects Notch signaling in endothelial cells and does not require tumor mutation. Markers such as ICN, expression of ligands or early dynamic monitoring of response need to be integrated with early clinical studies.
Conclusions
Our understanding of the Notch pathway has progressed substantially over the past century from an observation of the notched wing phenotype in Drospholia melanogaster to the context and tissue-dependent roles of this signaling pathway in hematologic and solid malignancies. No FDA approved Notch targeted therapies currently exist despite the progress in understanding this signaling pathway within different malignancies. GSIs have been the most broadly developed, but due to their lack of specificity, gastrointestinal side effects, and low response rates, further development may be challenging. More selective and potent inhibitors and select combinations with chemotherapy or other biologically targeted drugs should be pursued. Future important directions for this signaling pathway include:
To determine the roles the Notch pathway at different points in tumorigenesis, metastasis, and self-propagation of cancer stem cells
To develop biomarkers for sensitivity of cancers and stroma
To develop rational combination therapies.
Acknowledgments
This work was supported, in part, by NIH grants (P50CA083639, CA109298, P50CA098258, U54CA151668, UH2TR000943, CA016672, U54CA96300, and U54CA96297), the Cancer Prevention Research Institute of Texas (RP110595 and RP120214), an Ovarian Cancer Research Fund Program Project Development Grant, Department of Defense grants (OC120547 and OC093416), the Betty Ann Asche Murray Distinguished Professorship, the RGK Foundation, the Gilder Foundation, the Judi A. Rees Ovarian Cancer Research Fund, the Chapman Foundation, the Meyer and Ida Gordon Foundation (to A.K. Sood), Cancer Research UK (to A.L. Harris), and the Ann Rife Cox Chair in Gynecology (to R.L. Coleman). This research was also supported, in part, by the Blanton-Davis Ovarian Cancer Research Program (to A.K. Sood and R.L. Coleman). R.A. Previs is supported by NIH T32 Training Grant CA101642.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dexter JS. The analysis of a case of continuous variation in Drosophila by a study of its linkage relations. Am Nat. 1914;48:712–58. [Google Scholar]
- 2.Ntziachristos P, Lim JS, Sage J, Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell. 2014;25:318–34. doi: 10.1016/j.ccr.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95:8108–12. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okajima T, Irvine KD. Regulation of notch signaling by o-linked fucose. Cell. 2002;111:893–904. doi: 10.1016/s0092-8674(02)01114-5. [DOI] [PubMed] [Google Scholar]
- 5.Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A. 2003;100:5234–9. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol. 2003;4:786–97. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- 7.Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–91. doi: 10.1016/s0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 8.Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanistsakonas S. Specific Egf repeats of notch mediate interactions with Delta and Serrate - implications for Notch as a multifunctional receptor. Cell. 1991;67:687–99. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- 9.Aster JC, Simms WB, Zavala-Ruiz Z, Patriub V, North CL, Blacklow SC. The folding and structural integrity of the first LIN-12 module of human Notch1 are calcium-dependent. Biochemistry. 1999;38:4736–42. doi: 10.1021/bi982713o. [DOI] [PubMed] [Google Scholar]
- 10.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 11.Kurooka H, Kuroda K, Honjo T. Roles of the ankyrin repeats and C-terminal region of the mouse notch1 intracellular region. Nucleic Acids Res. 1998;26:5448–55. doi: 10.1093/nar/26.23.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong CT, Cheng HT, Chang LW, Ohtsuka T, Kageyama R, Stormo GD, et al. Target selectivity of vertebrate notch proteins. Collaboration between discrete domains and CSL-binding site architecture determines activation probability. J Biol Chem. 2006;281:5106–19. doi: 10.1074/jbc.M506108200. [DOI] [PubMed] [Google Scholar]
- 13.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease. TACE Mol Cell. 2000;5:207–16. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 14.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 15.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 16.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 17.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–83. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JJ, Kovall RA. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell. 2006;124:985–96. doi: 10.1016/j.cell.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80(5):690–701. doi: 10.1016/j.bcp.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Davis GE. Vascular balancing act: EGFL7 and Notch. Blood. 2010;116(26):5791–3. doi: 10.1182/blood-2010-11-314500. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–85. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013;139:95–110. doi: 10.1016/j.pharmthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.South AP, Cho RJ, Aster JC. The double-edged sword of Notch signaling in cancer. Semin Cell Dev Biol. 2012;23:458–64. doi: 10.1016/j.semcdb.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malecki MJ, Sanchez-Irizarry C, Mitchell JL, Histen G, Xu ML, Aster JC, et al. Leukemia-associated mutations within the NOTCH1 heterodimerization domain fall into at least two distinct mechanistic classes. Mol Cell Biol. 2006;26:4642–51. doi: 10.1128/MCB.01655-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 26.Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–70. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–7. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 28.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–86. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 29.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–7. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 30.Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–76. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, et al. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–90. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- 32.Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17:52–9. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–33. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 34.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–21. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 35.Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–36. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–42. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aste-Amezaga M, Zhang N, Lineberger JE, Arnold BA, Toner TJ, Gu M, et al. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One. 2010;5:e9094. doi: 10.1371/journal.pone.0009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li K, Li Y, Wu W, Gordon WR, Chang DW, Lu M, et al. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J Biol Chem. 2008;283:8046–54. doi: 10.1074/jbc.M800170200. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–7. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 40.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 41.Hu W, Lu C, Dong HH, Huang J, Shen DY, Stone RL, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030–9. doi: 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Small D, Kovalenko D, Kacer D, Liaw L, Landriscina M, Di Serio C, et al. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem. 2001;276:32022–30. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- 43.Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 2008;68:4727–35. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113:4313–8. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 45.Luistro L, He W, Smith M, Packman K, Vilenchik M, Carvajal D, et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res. 2009;69:7672–80. doi: 10.1158/0008-5472.CAN-09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–53. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strosberg JR, Yeatman T, Weber J, Coppola D, Schell MJ, Han G, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer. 2012;48:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis HD, Leveridge M, Strack PR, Haldon CD, O’Neil J, Kim H, et al. Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of notch signaling. Chem Biol. 2007;14:209–19. doi: 10.1016/j.chembiol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Tammam J, Ware C, Efferson C, O’Neil J, Rao S, Qu X, et al. Down-regulation of the Notch pathway mediated by a gamma-secretase inhibitor induces anti-tumour effects in mouse models of T-cell leukaemia. Br J Pharmacol. 2009;158:1183–95. doi: 10.1111/j.1476-5381.2009.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandya K, Meeke K, Clementz AG, Rogowski A, Roberts J, Miele L, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer. 2011;105:796–806. doi: 10.1038/bjc.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arcaroli JJ, Powell RW, Varella-Garcia M, McManus M, Tan AC, Quackenbush KS, et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a gamma-secretase inhibitor and irinotecan in colorectal cancer. Mol Oncol. 2012;6:370–81. doi: 10.1016/j.molonc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang CC, Pavlicek A, Zhang Q, Lira ME, Painter CL, Yan Z, et al. Biomarker and pharmacologic evaluation of the gamma-secretase inhibitor PF-03084014 in breast cancer models. Clin Cancer Res. 2012;18:5008–19. doi: 10.1158/1078-0432.CCR-12-1379. [DOI] [PubMed] [Google Scholar]
- 53.Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, et al. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41–51. doi: 10.1016/j.canlet.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang CC, Yan Z, Zong Q, Fang DD, Painter C, Zhang Q, et al. Synergistic effect of the gamma-secretase inhibitor PF-03084014 and docetaxel in breast cancer models. Stem Cells Transl Med. 2013;2:233–42. doi: 10.5966/sctm.2012-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Guerra M, Xargay-Torrent S, Rosich L, Montraveta A, Roldan J, Matas-Cespedes A, et al. The gamma-secretase inhibitor PF-03084014 combined with fludarabine antagonizes migration, invasion and angiogenesis in NOTCH1-mutated CLL cells. Leukemia. 2014 Apr 30; doi: 10.1038/leu.2014.143. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–82. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D, et al. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta. 2010;1806:258–67. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov. 2014;13:357–78. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 59.Meng RD, Shelton CC, Li YM, Qin LX, Notterman D, Paty PB, et al. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69:573–82. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timme CR, Gruidl M, Yeatman TJ. Gamma-secretase inhibition attenuates oxaliplatin-induced apoptosis through increased Mcl-1 and/or Bcl-xL in human colon cancer cells. Apoptosis. 2013;18:1163–74. doi: 10.1007/s10495-013-0883-x. [DOI] [PubMed] [Google Scholar]
- 61.Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–24. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–9. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–64. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–8. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okuhashi Y, Itoh M, Nara N, Tohda S. Effects of combination of notch inhibitor plus hedgehog inhibitor or Wnt inhibitor on growth of leukemia cells. Anticancer Res. 2011;31:893–6. [PubMed] [Google Scholar]
- 66.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 67.Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–7. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brennan K, Clarke RB. Combining Notch inhibition with current therapies for breast cancer treatment. Ther Adv Med Oncol. 2013;5:17–24. doi: 10.1177/1758834012457437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–9. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 70.Hernandez SL, Banerjee D, Garcia A, Kangsamaksin T, Cheng WY, Anastassiou D, et al. Notch and VEGF pathways play distinct but complementary roles in tumor angiogenesis. Vascular cell. 2013;5:17. doi: 10.1186/2045-824X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226–35. doi: 10.1158/0008-5472.CAN-07-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albain KS, Czerlanis C, Zlobin A, Covington KR, Rajan P, Godellas C, et al. Modulation of cancer stem cell biomarkers by the Notch inhibitor MK-0752 added to endocrine therapy for early stage ER+ breast cancer. Cancer Res. 2011;71(24 Suppl):Abstract nr S1–5. [Google Scholar]
- 73.Means-Powell JA, Minton SE, Mayer IA, Abramson VG, Ismail-Khan R, Arteaga CL, et al. A phase Ib dose escalation trial of RO4929097 (a {gamma}-secretase inhibitor) in combination with exemestane in patients with ER + metastatic breast cancer. Cancer Res. 2012;72(24 Suppl):Abstract nr P2-14-04. doi: 10.1016/j.clbc.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li WB, Ma MW, Dong LJ, Wang F, Chen LX, Li XR. MicroRNA-34a targets notch1 and inhibits cell proliferation in glioblastoma multiforme. Cancer Biol Ther. 2011;12:477–83. doi: 10.4161/cbt.12.6.16300. [DOI] [PubMed] [Google Scholar]
- 75.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]