Abstract
Clathrin-mediated endocytosis (CME) is vital for the internalization of most cell-surface proteins. In CME, plasma membrane-binding clathrin adaptors recruit and polymerize clathrin to form clathrin-coated ‘pits’ into which cargo is sorted. AP2 is the most abundant adaptor, and is pivotal to CME. By determining a new structure of AP2 that includes the clathrin-binding β2-hinge and developing an AP2-dependent budding assay, we reveal the existence of an autoinhibitory mechanism that prevents clathrin recruitment by cytosolic AP2. A large-scale conformational change driven by the plasma membrane phosphoinositide PtdIns(4,5)P2 and cargo relieves this autoinhibition, so triggering clathrin recruitment and hence clathrin-coated bud formation. This molecular switching mechanism constitutes an unsuspected layer of regulation that couples AP2’s membrane recruitment to its key functions of cargo and clathrin binding.
Clathrin adaptors provide an essential physical bridge connecting clathrin, which itself lacks membrane binding activity (1), to the membrane and to embedded transmembrane protein cargo. A central player in CME is the AP2 (Assembly Polypeptide 2) complex, (Figs 1A, S1), which both coordinates CCP formation and binds the many cargo proteins that contain ‘acidic dileucine’ and Yxxφ endocytic motifs (φ denotes a bulky hydrophobic residue) through its membrane proximal core (2, 3). Cargo binding is activated by a large-scale conformational change from the ‘locked’ or ‘inactive’ cytosolic form to an ‘open’ or ‘active’ form driven by localization to membranes containing the plasma membrane phosphoinositide PtdIns(4,5)P2 (4, 5). The C-terminal ‘appendages’ of the α and β2 subunits bind other clathrin adaptors as well as CCV (clathrin-coated vesicle) assembly and disassembly accessory factors (3, 6-8). The flexible ‘hinge’ separating the β2-appendage from the β2-trunk binds the N-terminal beta-propeller of the clathrin heavy chain using a canonical clathrin box motif (LLNLD; Fig 1A,B (9)). The β2 appendage domain also binds clathrin, albeit weakly, but both interactions are necessary for robust clathrin binding (10).
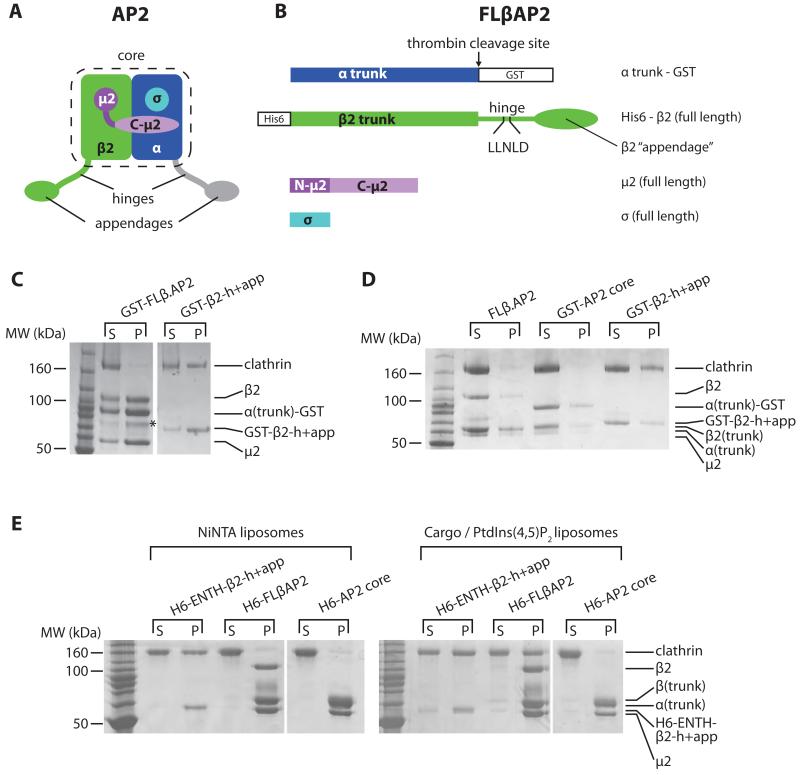
Fig. 1. AP2 can bind clathrin only when it is attached to a PtdIns(4,5)P2- and cargo-containing membrane.
(A). AP2 schematic, colour-coded by subunit: α, blue; β2, green; N-μ2, dark magenta; C- μ2 pale magenta; σ, cyan. The unstructured α and β2 ‘hinge’ and ‘appendage’ subdomains, together with the ‘core’ of the complex, are indicated. Parts shown in grey are not present in FLβ.AP2. Schematics of constructs are shown in Fig S1.
(B). FLβ.AP2 showing the positions of the clathrin box and purification tags.
(C). Coomassie stained SDS-PAGE of glutathione-sepharose pulldowns using GST-FLβ.AP2 or GST-β2-h+app. Supernatant (‘s’) and pellet (‘p’). The band marked with an asterisk results from proteolysis of β2.
(D). Coomassie stained SDS-PAGE of clathrin cage assembly assays using 2.5 μM clathrin and 1.5 μM adaptors (as indicated), overnight at 21°C, centrifuged to separate clathrin cages (‘p’) from supernatants (‘s’).
(E). Coomassie stained SDS-PAGE of liposome pulldown assays. Liposomes were sequentially incubated with adaptors and clathrin, then centrifuged to separate unbound material (‘s’) from the liposome pellet (‘p’).
A version of AP2 comprising full-length β2, μ2 and σ2 subunits, and the α-trunk domain, (FLβ.AP2) (Fig 1B)(11) was expressed in E.coli, avoiding contamination with other CCV components inherent to purification from brain tissue (12, 13). Despite most FLβ.AP2 possessing an intact β2 subunit (Fig 1C-E), it bound clathrin very poorly in pulldowns when immobilized on either glutathione sepharose beads (Fig 1C) or via its N-terminal His6 tag (similarly positioned to the β2 PtdIns(4,5)P2 binding site Fig1B (4, 5).) to liposomes containing the nickel-attached lipid NiNTA-DGS (Fig 1E): in both cases the FLβ.AP2 will be in its locked cytosolic conformation (4). FLβ.AP2 also failed to stimulate clathrin cage assembly efficiently at physiological pH (Fig 1D). In contrast, the isolated β2 hinge-appendage (‘GST-β2-h+app’, Fig S1) bound clathrin efficiently (Fig 1C) and stimulated cage assembly (Fig 1D). We next compared clathrin recruitment to synthetic DOPC/DOPE liposomes supplemented either with NiNTA-DGS, or with a mixture of PtdIns(4,5)P2 and a lipid-linked YxxΦ endocytic motif (5, 14). β2-h+app fused to His6-tagged epsin ENTH domain (His6-ENTH-β2-h+app), which can bind NiNTA-DGS or PtdIns(4,5)P2, recruited clathrin efficiently to both types of liposomes. In contrast, FLβ.AP2 recruited clathrin only when bound to PtdIns(4,5)P2- and YxxΦ-containing liposomes (Fig 1E). These results indicate that no additional proteins are required to prevent clathrin binding to AP2 in solution and are consistent with immunoprecipitation data (15). We conclude that the clathrin-binding activity of AP2 is autoinhibited in the cytosol to restrict inappropriate clathrin recruitment and that only upon encountering its physiological membrane ligands (PtdIns(4,5)P2 and cargo) can AP2 recruit clathrin efficiently, and that previous reports that AP2 purified from brain could bind and polymerise clathrin(12) was likely due to other contaminating clathrin adaptors such as AP180(13).
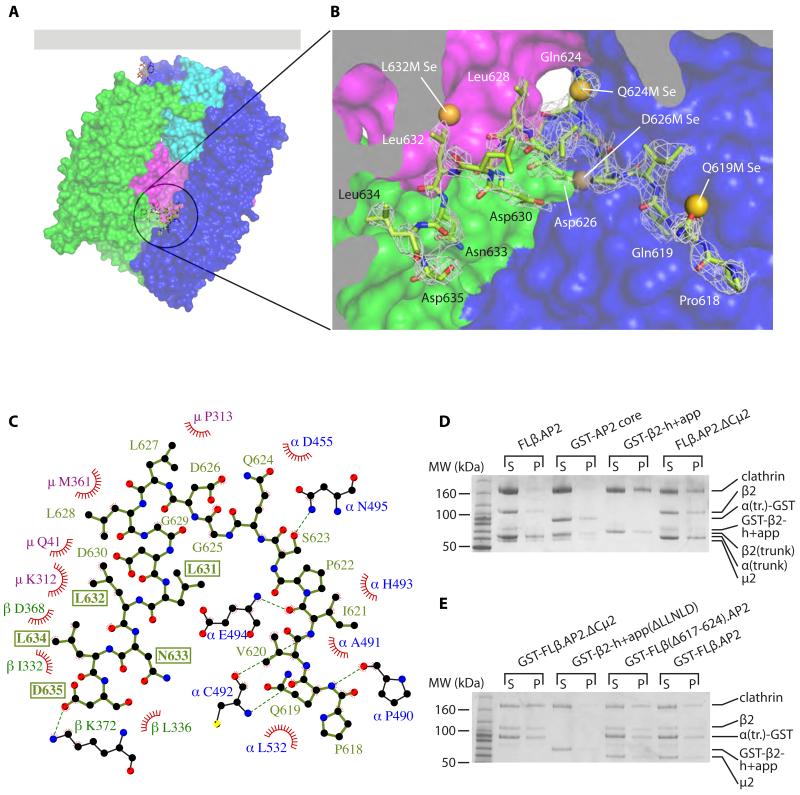
We were unable to crystallize FLβ.AP2, so we determined the structure of a form of AP2 (βhingeHis6.AP2) whose β2 (residues 1-650) includes the clathrin box-containing hinge but not the β2-appendage. The structure closely resembles that of the ‘locked’ conformation of the AP2 core but additional protein difference electron density was visible in the centre of the AP2 core Fig.S2. Crystallographic analysis of AP2s truncated at Leu636 and Gln619 of β2 suggested that this electron density corresponded to the region of β2 between these points (11). A series of mutant AP2s with single methionine substitutions throughout the hinge region was created, the mutants crystallized as selenomethionyl derivatives and their structures solved (Fig. S3). This allowed us to assign unambiguously this density to residues 618-634 of β2 and refine the structure (Fig. 2A,B, Fig. S2 and Movies S1 and S2). There are two regions of contact: a β-sheet interaction between β2 residues 618-624 and α trunk residues 490-493, and packing between the clathrin box itself and the short helix immediately preceding it and residues of C-μ2 and the β2 trunks (Fig 2C and S4). In this position the LLNLD clathrin box is inaccessible, explaining FLβ.AP2’s lack of clathrin binding (Fig 1), since deletion of only the LLNLD clathrin motif in GST-β2-h+app abolishes clathrin recruitment (Fig S5). β2 618-636 is well-conserved across a wide range of species (Fig S6), and between β2 and β1 (the equivalent subunit of the AP1 adaptor). This suggests that in AP1, clathrin binding will be similarly regulated by membrane attachment, albeit stimulated by binding to Arf1:GTP (16, 17). Interfering with the interactions that trap the clathrin box in the AP2 core should release the hinge, allowing increased clathrin recruitment. Indeed, deletion of C-μ2 (termed FLβ.AP2.ΔCμ2), disrupting key interactions with β2 625-635 (that includes the clathrin motif; Fig. S4), had a profound effect, resulting in efficient clathrin binding and cage assembly in solution (Fig. 2D and S7). Deletion of the hinge residues 617-624, removing the largely backbone mediated interaction with 490-493 of the α-subunit (Fig. S4), resulted in a modest but significant increase in AP2-mediated clathrin polymerization in solution at physiological pH (25% ± 3.1% clathrin polymerized (mean ± s.e.m., 3 experiments) vs 9.9% ± 3.8%; difference tested by Student’s t-test, p = 0.037; Fig 2E).
Fig. 2. The AP2 β2 subunit LLNLD clathrin binding motif is buried in the centre of the core.
(A, B). Overall (A) and closeup (B) views of the structure of βhingeHis6.AP2. The residues of the hinge resolved in the structure are shown in green as a stick representation. The AP2 core is depicted in a surface representation, coloured as in Fig. 1. The residues of the buried hinge are indicated in (B), with electron density shown as mesh (2mFo-DFc map, contoured at 0.34 e Å−3). Also shown are the positions of the selenium sites found in the bowl for each of the methionine mutants indicated, showing good agreement with the positions of the corresponding wild-type residues that were mutated. Individual LLG maps are shown in Fig S3.
(C). Ligplot+ (25) diagram showing interactions between buried hinge residues (in pale green) with residues of α (blue), μ2 (magenta) and β2 (dark green). Red fans indicate hydrophobic interactions; dashed green lines indicate hydrogen bonds. The residues of the clathrin-binding motif are boxed. See also Fig. S4.
(D). Clathrin cage assembly assays. (D) is identical to Fig. 1D (2.5 μM clathrin, 1.5 μM adaptors) with the addition of the FLβ.AP2.ΔCμ2 lane.
(E). Assays performed as in (D) but at 28°C and with 2 μM clathrin and 4 μM adaptors as shown.
The structure suggests a mechanism by which clathrin binding is triggered by AP2’s membrane recruitment in the cell. Aligning the ‘open’ and ‘locked’ conformations on residues 480 to 510 of the α subunit, (a TLS group used in refinement(5) that juxtaposes the buried hinge fragment) reveals that the membrane and cargo-bound ‘open’ conformation is incompatible with the autoinhibitory sequestration of the hinge (Figs. 3, S4 and S8): the β2 trunk now blocks the point of entry of the hinge into the bowl, and the relocation of C-μ2 removes one face of the pocket in which the helical β2 hinge segment rests (Figs 2C and S4). Therefore, transition of AP2 from the locked to the open conformation, triggered by association with the plasma membrane, stimulates clathrin binding by releasing the clathrin-box containing β2-hinge from the centre of the core (5).
Fig. 3. The ‘open’, activated form of AP2 is not compatible with the β2 hinge binding back into the core.
Release of the clathrin-binding motif stimulated by conformational change. Top panels: ‘locked’AP2 core, in solution or transiently bound to the plasma membrane via PtdIns(4,5)P2 (left) and ‘open’ AP2 stably attached to the membrane via multiple PtdIns(4,5)P2s and cargo. Lower panels: views of the hinge binding site in each conformational state; in the ‘open’ state (right), the hinge residues from the ‘locked’ state βhingeH6.AP2 structure are superposed onto the ‘open’ structure and shown in grey.
To address whether cargo binding is absolutely required to stimulate clathrin recruitment we prepared synthetic DOPC/DOPE liposomes supplemented either with PtdIns(4,5)P2 alone, or with a mixture of PtdIns(4,5)P2 and a lipid-linked Yxxφ motif, and mixed these with FLβ.AP2 and clathrin both at plausible cellular concentrations of 0.4 μM (18)) (Fig 4A). As expected, FLβ.AP2 binds more tightly to the cargo-containing liposomes (Fig 4A)(5, 14); but, in addition, the ratio of clathrin to AP2 present in the cargo-containing liposome pellets is significantly greater (26.5% ± 5.3%) than in the PtdIns(4,5)P2-only liposomes (5.2% ± 1.9%; means ± s.e.m., 4 experiments; difference tested by Student’s t-test, p=0.0094), suggesting that PtdIns(4,5)P2-and cargo-bound FLβ.AP2 is able to recruit clathrin more efficiently than FLβ.AP2 bound only via PtdIns(4,5)P2, consistent with PtdIns(4,5)P2 driving the conformational change in AP2 that is then stabilized by cargo binding (5, 19).
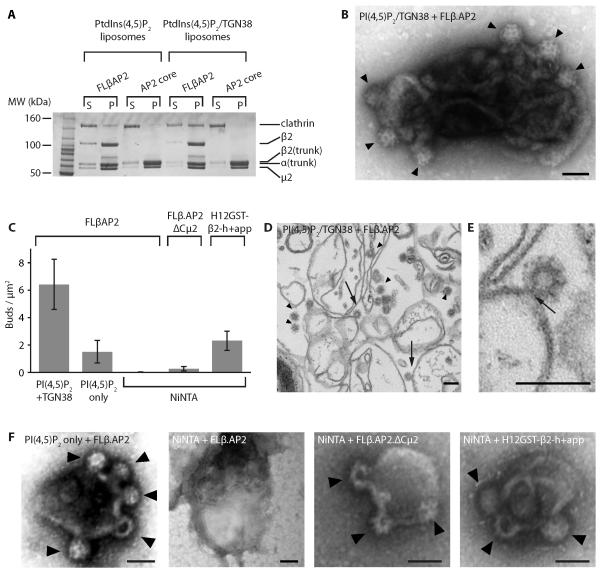
Fig. 4. AP2 and clathrin are sufficient to generate clathrin-coated buds on membranes.
(A). Clathrin recruitment to liposomes. Synthetic liposomes supplemented with PtdIns(4,5)P2 , or with PtdIns(4,5)P2 and TGN38 peptide (as indicated), were incubated with adaptor and clathrin as shown (both at 0.4μM); supernatants (‘s’) and pellets (‘p’) were then separated and analyzed by gel electrophoresis.
(B). Liposomes supplemented with PtdIns(4,5)P2 and lipid-linked TGN38 internalization incubated sequentially with FLβAP2 and clathrin, and analyzed by negative stain EM, showing clear clathrin coated membrane buds (arrowheads).
(C). Number of buds formed per μm2 of membrane, estimated for various combinations of lipid and adaptor. Note that no buds were found on PtdIns(4,5)P2 liposomes ± TGN38 incubated sequentially with the AP2 core and clathrin, or with clathrin only (Fig. S9).
(D, E). Liposomes supplemented with PtdIns(4,5)P2 and lipid-linked TGN38 internalization incubated sequentially with FLβAP2 and clathrin, analyzed by ultra-thin sectioning. Arrowheads indicate examples of clathrin-coated structures; arrows indicate coated buds where the connection to the liposome is clearly visible. (E) shows an enlarged image of a bud showing the “neck”.
(F). Examples of liposomes supplemented with the lipids indicated (PtdIns(4,5)P2 or NiNTA-DGS) incubated sequentially with adaptors (as indicated) and clathrin, examined by negative stain EM. Note that some buds produced by FLβAP2 on PtdIns(4,5)P2-only liposomes seemed less invaginated than those shown here; this can be seen more clearly in ultrathin sections (Fig S10).
Finally, we sought to determine whether AP2 alone is sufficient to initiate and drive clathrin-coated bud formation on appropriate membranes. When FLβ.AP2-loaded PtdIns(4,5)P2- and lipid-linked Yxxφ cargo-supplemented liposomes were incubated with clathrin and the results examined by negative stain EM (Fig 4B), we observed numerous clathrin-coated buds, of ~80nm diameter, which by ultra-thin sectioning were revealed to encapsulate invaginated membrane/vesicles of 30-40nm diameter similar to CCVs isolated from brain (Fig 4D,E). Fewer buds were found on PtdIns(4,5)P2 liposomes incubated with FLβ.AP2 and clathrin (Fig 4C,F). We were unable to find buds on NiNTA liposomes similarly treated with FLβ.AP2 (Fig 4C,F), but some clathrin-coated buds were found on NiNTA liposomes incubated with clathrin and C-μ2-deleted FLβ.AP2 (FLβ.AP2.ΔCμ2), whilst His12-tagged GST-β2-h+app produced ample buds on NiNTA liposomes (Fig 4C,F). Thus, once activated by binding to its physiological ligands, AP2 is sufficient to drive clathrin-coated bud formation (at least in vitro); no other clathrin adaptors, including those currently described as driving membrane curvature, are required.
AP2 is the most abundant endocytic clathrin adaptor (20), and the first to be recruited to sites of CCP formation (2, 8); and AP2 knockdown results in a ~12-fold reduction in CCP formation in HeLaM cell (21). Together with our findings that PtdIns(4,5)P2-activated and cargo-stabilized AP2 is sufficient to drive bud formation on liposomes, these data suggest that in vivo the dominant mechanism for endocytic CCP initiation will be the recruitment of AP2 to PtdIns(4,5)P2-enriched sites in its locked form with its clathrin-binding autoinhibited, followed by transition to the open form if sufficient PtdIns(4,5)P2 is present (5, 19). AP2’s conformational change will expel the β2 hinge, allowing clathrin triskelia to bind. The presence of cargo will stabilize AP2’s open form and increase its dwell time on the membrane (5, 14, 19, 22), thus increasing the chances of it binding clathrin and forming a sufficiently stable nucleating structure. Once a small nucleus of AP2 and clathrin has formed, further AP2 and clathrin, and other clathrin adaptors that bind the α appendage, can then be recruited in random order to produce a CCP, which can ultimately be severed from the membrane (3, 23, 24).
Supplementary Material
Acknowledgments
We would like to thank the I02, I03 and I04-1 beamline staff at the Diamond Lightsource, Airlie McCoy and Phil Evans for crystallographic advice, Chris Oubridge for advice and assistance with SeMet mapping of the hinge residues, Nick Bright, Christos Savva and Sharon Miller for advice and assistance with EM, Corinne Smith for reagents and H. Böning and H. Ungewickell for expert technical assistance. D.J.O. and B.T.K. are supported by Wellcome Trust Principal Research Fellowship (090909/Z/09/Z). S.H. is supported by a grant of the German Science Foundation (SFB 635, TP A3). S.C.G. is supported by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (098406/Z/12/Z). CIMR is supported by a Wellcome Trust Strategic Award (079895). Coordinates have been deposited in the Protein Data Bank with PDB ID XXX.
References and Notes
- 1.Dannhauser PN, Ungewickell EJ. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat. Cell Biol. 2012;14:634–639. doi: 10.1038/ncb2478. [DOI] [PubMed] [Google Scholar]
- 2.Cocucci E, Aguet F, Boulant S, Kirchhausen T. The first five seconds in the life of a clathrin-coated pit. Cell. 2012;150:495–507. doi: 10.1016/j.cell.2012.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traub LM. Regarding the amazing choreography of clathrin coats. PLoS Biol. 2011;9:e1001037. doi: 10.1371/journal.pbio.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 5.Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Höning S, Evans PR, Owen DJ. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010;141:1220–1229. doi: 10.1016/j.cell.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 7.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MJ, Perrais D, Merrifield CJ. A High Precision Survey of the Molecular Dynamics of Mammalian Clathrin-Mediated Endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Haar E, Harrison SC, Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc. Natnl. Acad. Sci. U.S.A. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Materials and methods are available as supplementary material on Science Online.
- 12.Zaremba S, Keen JH. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J. Cell Biol. 1983;97:1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindner R, Ungewickell E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J. Biol. Chem. 1992;267:16567–16573. [PubMed] [Google Scholar]
- 14.Höning S, Ricotta D, Krauss M, Späte K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Hirst J, Borner GH, Edgar J, Hein MY, Mann M, Buchholz F, Antrobus R, Robinson MS. Interaction between AP-5 and the hereditary spastic paraplegia proteins SPG11 and SPG15. Mol. Biol. Cell. 2013;24:2558–2569. doi: 10.1091/mbc.E13-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X, Farias GG, Canagarajah BJ, Bonifacino JS, Hurley JH. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell. 2013;152:755–767. doi: 10.1016/j.cell.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. The quantitative proteome of a human cell line. Mol. Syst Biol. 2011;7:549. doi: 10.1038/msb.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borner GH, Antrobus R, Hirst J, Bhumbra GS, Kozik P, Jackson LP, Sahlender DA, Robinson MS. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J. Cell Biol. 2012;197:141–160. doi: 10.1083/jcb.201111049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu AP, Aguet F, Danuser G, Schmid SL. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J.Cell Biol. 2010;191:1381–1393. doi: 10.1083/jcb.201008117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodsky FM. Diversity of clathrin function: new tricks for an old protein. Annu. Rev. Cell Dev. Biol. 2012;28:309–336. doi: 10.1146/annurev-cellbio-101011-155716. [DOI] [PubMed] [Google Scholar]
- 24.Aguet F, Antonescu CN, Mettlen M, Schmid SL, Danuser G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell. 2013;26:279–291. doi: 10.1016/j.devcel.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. Journal of chemical information and modeling. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.