Abstract
In Caenorhabditis elegans, several manipulations that affect nutrition slow development, reduce fecundity, and increase life span. These are viewed as dietary restriction (DR) and include culture in semidefined, nutrient-rich liquid medium that is axenic (i.e., there is no microbial food source). Here we describe convenient ways to exert DR by culture on agar plates containing axenic medium. We used these to explore whether effects of axenic culture really reflect DR. Our results imply that major nutrient components of axenic medium, and overall caloric content, are not limiting for life span. However, adding growth-arrested Escherichia coli as an additional food source rescued the effects of axenic culture. We then sought to identify the component of E. coli that is critical for normal C. elegans nutrition using add-back experiments. Our results suggest that C. elegans has a nutritional requirement for live, metabolically active microbes or, possibly, an unidentified, heat-labile, nonsoluble component present in live microbes.
Keywords: Dietary restriction, C. elegans, E. coli, Aging, Axenic culture
DIETARY restriction (DR), the controlled reduction of food intake without inducing starvation, increases life span in a number of animal species including rodents and dogs (1–7). Studies of the mechanisms involved may provide insight into the biological mechanisms of longevity and aging. The nematode Caenorhabditis elegans is widely used as a model organism for studying aging, and its life span is also extended by DR. A variety of different methods have been used to subject C. elegans to DR. These include dilution of the Escherichia coli bacterial food source (8), use of agar plates with limited amounts of the E. coli food source (9,10) culture on agar plates containing nematode growth medium (NGM) but no E. coli food source (11,12), use of Eat mutants, where food intake is reduced due to defects in the function of the pharynx (13), and axenic culture (in the absence of E. coli) in semidefined, liquid culture medium (14). Whether the effects on aging of all of these treatments involve the same pathways and biochemical mechanisms remains uncertain (15).
To study the mechanisms by which DR extends life span in C. elegans, we initially surveyed the effects on life span of several DR protocols: bacterial dilution in liquid culture (8), the use of Eat mutants (13), bacterial dilution on agar plates (9,10), and axenic liquid culture (14). In our hands, culture in axenic medium (AXM) was by far the best method in terms of reproducibility of results and magnitude of effects (Keaney M, Walker G, Gems D, unpublished results, 2007). However, a problem with axenic culture is that it is unclear whether it really represents a form of DR or involves another mechanism altogether.
AXM contains 3% soy peptone, 3% yeast extract, and 0.05% hemoglobin, which is added after autoclaving of the basal medium. The increase in life span of C. elegans in axenic culture has long been considered to reflect DR because, like DR, axenic culture results in delayed development, reduced body size, and greatly reduced fecundity (14,16,17). Moreover, as in most other forms of DR (e.g., bacterial dilution, use of Eat mutants, and culture on NGM without E. coli), life extension in axenic culture does not require the FOXO transcription factor DAF-16 (11–13,16,18). Furthermore, when long-lived insulin/insulin-like growth factor 1 signaling mutants are subjected to putative DR via axenic culture, use of Eat mutants, or bacterial dilution, there are additive interactions between the effects of these two manipulations on adult life span (11–13,16,19). Yet it remains unclear why culture in AXM should lengthen life span in the way that it does, because this medium is rich in protein, carbohydrate, vitamins, and minerals. It has been suggested that in axenic culture there is a failure of endocytic uptake of nutrients from the intestinal lumen (20,21), but this remains unconfirmed.
The use of axenic culture to study DR also has several practical shortcomings. First, it is conducted in liquid culture, whereas most studies of C. elegans are performed using agar plates; thus, the use of liquid culture is inconvenient and introduces an extra variable (liquid vs solid phase culture). Second, growth in axenic culture results in developmental asynchrony and reduced body size, which can complicate studies of, e.g., global transcript profiles. We therefore developed new protocols for culture with AXM to facilitate C. elegans DR studies. These protocols use agar plates containing AXM, and small quantities of radiation- or antibiotic-arrested E. coli to raise nematode population cohorts that are both developmentally synchronized and full sized. While we were conducting our investigations, two other groups reported formally similar methods of performing DR on C. elegans, by axenic culture on agar plates, although the plates contained NGM rather than standard axenic culture medium (11,12). Potentially, life extension by axenic culture involves the same mechanism whether the culture method uses AXM in liquid or plate culture, or NGM in plate culture; however, further work will be required to confirm this.
Using axenic culture on plates, we explored the mechanism by which life span is extended by axenic culture. Addition of growth-arrested, but live E. coli restored the normal short life span of C. elegans, as it does for nematodes cultured on NGM (11,12). We next attempted to identify the factors present in live but not autoclaved E. coli that are critical for effects on life history. Our studies suggest that there exists a peculiarity in C. elegans nutrition: a requirement for metabolic activity in its microbial food source. An alternative possibility that we could not exclude is that there is a heat-labile, nondiffusible component of E. coli that C. elegans requires.
METHODS
Strains
The wild-type C. elegans strain used in this study was the N2 male stock (22) (from which we took the hermaphrodites), obtained from the Caenorhabditis Genetics Center (CGC). The bacterial strains used as a food source for C. elegans were E. coli K12 (wild type) and the slow growing, uracil auxotroph OP50, which is derived from K12 (23). Use of E. coli OP50 is standard in C. elegans research (24). We used E. coli K12 to more readily obtain the large quantities of bacteria needed for growing bulk C. elegans cultures. Bacteria were cultured overnight on an orbital shaker in Luria Broth (LB) medium at 37°C.
Axenic Culture Using Agar Plates
We developed and optimized protocols for C. elegans DR studies using agar plates containing AXM. Semi-defined AXM is composed of 3% soy peptone (Sigma-Aldrich, St. Louis, MO), 3% yeast extract (YE) (Becton-Dickinson, Franklin Lake, NJ), and 0.05% hemoglobin (bovine; Serva, Heidelberg, Germany) (diluted from 100X stock in 0.1 N KOH, autoclaved for 10 minutes), which is added after autoclaving the basal medium (21,25).
In one approach, worms were supplemented with live E. coli K12 cells that were prevented from further proliferation by β-irradiation (see following section). The severity of the DR effect can be varied by adding different amounts of irradiated E. coli cells to the plates. In a second approach, worms were maintained until day 1 of adulthood on AXM plates with live E. coli OP50 treated with an antibiotic (see section Antibiotic Treatment of E. coli) to prevent growth of the bacteria on the plates.
Irradiation of E. coli
β-irradiation of E. coli was performed using the 15 MeV, 2% duty factor high-power linear electron accelerator (26) at Ghent University. This accelerator can deliver electron beams with a well-defined energy between 3 and 15 MeV, and a maximum average beam power of approximately 5 kW at 10 MeV beam energy. For these irradiations, an electron beam with an energy of 10 MeV and a mean current of approximately 75 μA was produced. This beam traversed a water-cooled vacuum window and 80 cm of air, so that the lateral dose distribution was flattened by scattering. In the exit window, the electrons lose, on average, about 2 MeV. As such, the mean energy of the electrons hitting the samples was about 8 MeV. An irradiation up to 10 kGy took about 150 seconds. Absolute dose calibration was performed using a dosimeter (Far West Technology, Goleta, CA), calibrated against ferrous sulphate (Fricke) dosimeters.
Antibiotic Treatment of E. coli
Carbenicillin was also used to prevent bacterial growth. This antibiotic interferes with the synthesis of the bacterial cell wall and, consequently, E. coli treated with it will stop growing after a few more cell divisions. Carbenicillin treatment was usually performed 2 days before the nematode culture plates were required. Freshly grown E. coli OP50 cells were washed and diluted to a concentration of 3 × 109 cells/mL in M9 buffer supplemented with 0.5 mM carbenicillin. The bacteria were then incubated in an orbital shaker at 37°C for 3 hours. Next, the cells were pelleted, resuspended in M9 buffer to 1.5 × 1010 cells/mL, and spread onto AXM plates containing 1 mM carbenicillin (added after autoclaving). Approximately 50 μL of carbenicillin-treated bacteria were spread onto each small (6.0-cm diameter) Petri dish. The bacteria treated in this way were unable to proliferate, even if streaked onto LB plates without antibiotic. Assessment of viability using the LIVE/DEAD BacLight Viability Kit (Molecular Probes, now part of Invitrogen, Carlsbad, CA) (see section Testing of Viability of Growth-Arrested E. coli), shows that bacteria prepared in this way are alive, despite being unable to divide.
Life History Analysis of C. elegans
Synchronous nematode populations were obtained as described previously (25). For lifespan analysis on plates, worms were maintained in small Petri dishes at a density of 6–8 animals per plate. Life-span analysis in liquid culture was performed in 5-mL Sarstedt tubes (75 × 12 mm), containing 3–5 worms in 300 μL of AXM. In each case, 5-fluoro-2′-deoxyuridine was added to 50 μM to prevent progeny production, and worms were usually maintained in the same vessels throughout life. Life-span measurements were initiated at the fourth larval stage (L4), which was treated as 0 days old. Survival was monitored at regular time points. Worms were scored as dead if they did not show any movement after prodding with a platinum wire or tapping of the tube.
For assessment of fecundity (on agar), nematodes were transferred to individual plates in early adulthood and transferred to fresh plates every 2 days, and progeny counted.
For measurement of worm volumes, several hundred animals were washed off agar plates with S buffer, and killed by addition of 75-fold diluted commercial bleach. Body length and width were measured with a Rapid View Particle Analysis System (Beckman Coulter, Fullerton, CA), and body volume was calculated treating C. elegans as cylindrical in shape. All life history experiments were performed at 20°C or 22.5°C.
Preparation of Various Culture Supplements
Bacterial filtrates for add-back tests were prepared as follows: Bacteria were sonicated on ice using a sonicator (MSE Soniprep 150; SANYO Gallencamp, Loughborough, U.K.) by means of six 10-second pulses at 80% power separated by 20-second rests. Bacterial suspensions were sonicated in 1-mL volumes at a concentration 3 × 1010 cells/mL. Filtrates were then passed through a 0.2-μm filter before addition to AXM at the concentrations shown.
Plant extracts for DR rescue tests were obtained using a standard household juicer (Moulinex Juice Extractor, Type Y36). The extracts were filtered through a 0.22-μm syringe filter and added to the axenic medium at the ratios shown.
Testing of Viability of Growth-Arrested E. coli
The LIVE/DEAD BacLight Bacterial Viability Kit was used to assess the viability of irradiated or carbenicillin-treated E. coli. This kit uses a mixture of SYTO 9 (green fluorescence) and propidium iodide (red fluorescence) to distinguish live and dead cells. These dyes are both nucleic acid specific, but differ in their spectral properties and the ability to enter live cells. SYTO 9 stains all cells in a population irrespective of whether they are alive or dead, resulting in green fluorescence. Propidium iodide, in contrast, only enters dead cells, masking SYTO 9 fluorescence and causing dead cells to fluoresce red. Bacteria were stained for microscopic observation according to the manufacturer’s instructions. Bacteria were washed with 0.85% NaCl (wt/vol) and diluted to 108 cells/mL. Equal amounts of SYTO 9 and propidium iodide were mixed, and 3 μL of this mixture was added to each milliliter of bacterial suspension. The samples were incubated in the dark at room temperature for 15 minutes, after which microscopic slides were prepared. These slides were viewed using a standard fluorescein longpass filter set, allowing one to simultaneously observe live (green) and dead (red) E. coli cells.
The measurement of oxygen consumption by bacterial cells was essentially performed as described previously for C. elegans (27). A six-channel Strathkelvin respirometer (Glasgow, Scotland) with Clark-type electrodes was used. This device comprises a regulated water bath (37°C) with six respirometer cells equipped with a magnetic stirrer. For electrode calibration, a stream of air was bubbled through distilled water at 4°C for 5 minutes. One milliliter of this oxygen-saturated water was transferred to the cells and stirred for 5 minutes to allow the temperature in the cells to rise to 37°C and excess oxygen to escape. Next, the electrodes were inserted and the oxygen concentration was set at 214 μmol/L (=100%). Finally, the water was replaced by 4 × 108 E. coli cells in LB medium, and oxygen consumption was monitored at 37°C for 10–30 minutes, depending on the rate of oxygen consumption. The oxygen concentration was plotted as a function of time, and the slope of the linear part of the graph was used to derive the oxygen consumption rate.
The rate of metabolic heat dissipation of live cells or tissues is most accurately measured by microcalorimetry, which was performed as described previously for C. elegans using a Thermal Activity Monitor (Thermometric, Järfälla, Sweden) (27). The bacteria (4 × 108 cells in 1 mL of LB medium) were transferred to a glass ampoule, which was then sealed. An ampoule containing assay medium (without bacteria) was included as a control. Next, the ampoules were transferred to the measuring units. These were inserted in the ultrathermostatted water bath (37°C) where heat flows are monitored. The device was first allowed to equilibrate. Stable signals were usually obtained after 1–2 hours of equilibration.
Statistical Analysis
Survival fractions were calculated using the Kaplan–Meier method. Life spans were compared using the log-rank test in the application SPSS 14.0.2 (SPSS Inc., Chicago, IL). A general linear mixed model incorporated in the application SAS 9.1 (SAS Institute Inc., Cary, NC) was used for the statistical analysis of worm volumes. This model takes into account the possible effects of replicate experiments on a dependent variable, thereby allowing one to model fixed effects (e.g., the effect of E. coli concentration on worm volume), as well as the error structure, by means of random effects. This model was chosen because not all E. coli concentrations could be included in all experiments, possibly causing misinterpretation of results. Because the error structure of a count is Poisson distributed, rather than normally distributed, a generalized linear mixed model was used for statistical analysis of worm fecundity, and carried out using SAS 9.1. Pairwise comparison of the means was performed with the post hoc Tukey test. Linear regression was used for the statistical analysis of the metabolic activity data of E. coli, and carried out using SPSS 14.0.2.
RESULTS
Culture on AXM Plates Extends C. elegans Life Span
To combine the robust DR effect of axenic culture with the convenience of agar plate culture, we developed protocols for performing DR using agar plates containing AXM. This approach is similar to the recently developed approach of culture on NGM agar plates without E. coli (11,12). In initial tests, C. elegans raised on AXM agar alone showed highly asynchronous development, and many did not reach adulthood (data not shown). The nematodes were therefore initially provided with live E. coli. To prevent bacterial proliferation on the nutrient-rich AXM agar, we initially exposed E. coli to an electron beam (10 kGy). Although unable to divide, bacteria irradiated in this way mostly remain alive (see section Rescue of Axenic Culture Effects is Correlated with the Presence of Metabolic Activity in E. coli).
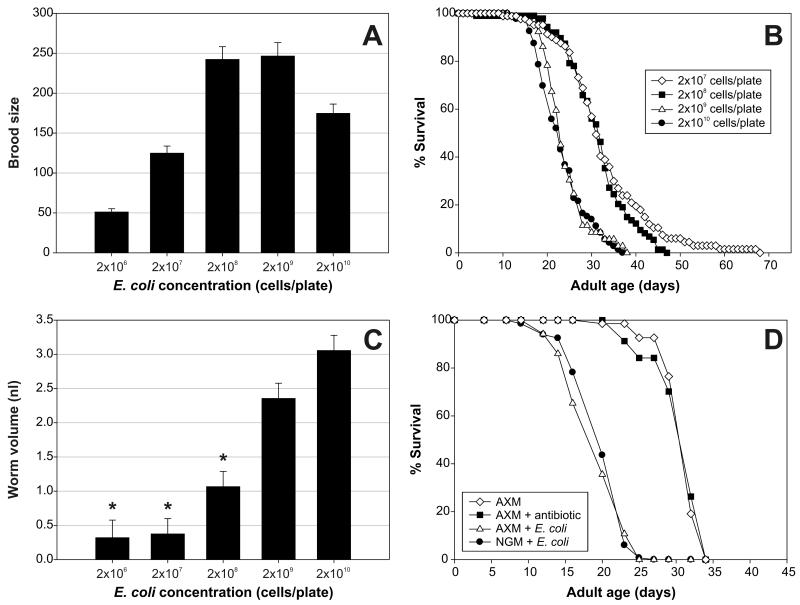
C. elegans raised in liquid axenic culture show reduced fecundity and increased life span (17). We first explored whether similar effects occurred on AXM agar, and whether we could selectively rescue the poor nematode growth on AXM agar by addition of small quantities of E. coli. To this end, we tested the effects on fecundity and life span by adding 2 × 106, 2 × 107, 2 × 108, 2 × 109, and 2 × 1010 E. coli K12 cells to AXM agar-cultured nematodes, from the L1 stage onwards. Addition of E. coli produced a concentration-dependent increase in brood size from 51 up to ~245 progeny on 2 × 108 to 2 × 109 cells/plate (Figure 1A). By contrast, lower levels of E. coli (2 × 106 to 2 × 108 cells/plate) had little effect on life span, whereas higher concentrations (2 × 109 and 2 × 1010 cells/plate) suppressed the DR effect on life span (Figure 1B). Thus, one can dissociate the effects of axenic culture on fecundity and aging. This is reminiscent of the effects of insulin/insulin-like growth factor 1 signaling (IIS), where partial loss can result in normal development and fecundity but increased life span (28,29). We also examined the effect of these culture conditions on body size. A maximal worm volume was reached on a food concentration of 2 × 1010 cells/plate, and a gradual decline in body size is observed with decreasing food concentration (Figure 1C).
Figure 1.
Effects of dietary restriction (DR) on axenic medium (AXM) plates, and rescue by addition of Escherichia coli. A, Fecundity of wild-type Caenorhabditis elegans at 20°C on AXM plates with addition of different numbers of radiation-arrested E. coli K12 (10 kGy). Data shown are the average of results from eight partially overlapping replicate trials. There was a small replicate effect on fecundity (variance between replicates = 0.028 ± 0.02). For each concentration, 50–90 adults were scored. Decreasing the concentration of bacteria on the plates below 2 × 108 to 2 × 109 cells caused a substantial decline in progeny production (p < .0001 for 2 × 107 vs 2 × 108 cells, and for 2 × 106 vs 2 × 108 cells). A higher concentration of E. coli similarly caused a reduction of fecundity (p < .0001 for 2 × 1010 vs 2 × 109 cells). B, Survival of N2 at 20°C on AXM plates with different concentrations of radiation-arrested E. coli K12 (10 kGy). There is no difference in life span between worms grown on 2 × 1010 and 2 × 109 cells (mean adult life span on 2 × 1010 cells = 23.4 ± 0.6 days, N = 77 (dead)/22 (censored); mean adult life span on 2 × 109 cells = 24.3 ± 0.8 days, N = 48/44; p = .39). When the E. coli concentration on the plates was diluted to 2 × 108 or 2 × 107 cells, life span was extended substantially, but to the same extent (mean adult life span on 2 × 108 cells = 31.7 ± 0.8 days, N = 81/19; mean adult life span on 2 × 107 cells = 32.9 ± 1.2 days, N = 70/30; p < .0001 for both comparisons). Three other replicate experiments showed largely similar results (data not shown). Censored worms were mainly a consequence of crawling off the plates in search of food on low concentrations of E. coli, and of crawling in the agar at higher E. coli concentrations. Note that worms, at a density of 6–8 per plate, were maintained on the same plates throughout life; thus at low bacterial cell densities, it is possible that E. coli density declined over time. C, Maximal worm volume at 20°C on AXM plates with different concentrations of radiation-arrested E. coli K12 (10 kGy). Average of four partially overlapping replicate trials. There was no effect of replicate on body size (variance between replicates = 0). Body size is maximal on the highest food concentration and declines substantially with decreasing E. coli concentration on the plates (p < .0001). *Indicates significant differences as compared to 2 × 1010 cells (p < .0001 for comparison with 2 × 106 and 2 × 107 cells/plate and p = .0004 for comparison with 2 × 108 cells/plate). Note that the volumes on the two lowest bacterial concentrations are probably underestimated due to the combination of substantial asynchrony on plates with such low food concentration on the one hand, and the bulk measurement of the length and width of the nematodes on the other hand. Length and width were affected in a similar fashion (data not shown). D, Survival of N2 at 22.5°C on nematode growth medium (NGM) and AXM plates with or without a rescuing concentration of carbenicillin-arrested E. coli OP50. Worms maintained on AXM with carbenicillin but without E. coli (mean adult life span = 31.7 ± 0.4 days, N = 68/82) were longer lived than animals grown on NGM or AXM with E. coli (mean adult life span on NGM with OP50 = 20.3 ± 0.3 days, N = 133/25; mean adult life span on AXM with OP50 = 19.5 ± 0.3 days, N = 121/29; p < .0001 for comparison between AXM with and without E. coli). The axenic condition without bacteria and without carbenicillin was included as a test for possible toxic effects of the antibiotic. The results show that carbenicillin does not affect the long life span of axenic cultured worms (mean adult life span on AXM = 31.7 ± 0.3 days, N = 75/68; p = .93). Similar results were obtained in a second trial (data not shown).
Because few laboratories have access to a particle accelerator, we developed a second protocol. Animals were raised until the first day of adulthood on AXM agar plates plus E. coli treated with an antibiotic (carbenicillin). The resulting populations showed developmental synchrony and a body size similar to that of populations raised under standard C. elegans growth conditions (NGM agar and live E. coli; data not shown). After day 1 of adulthood, nematodes were transferred to axenic plates containing carbenicillin, which prevents any further proliferation of E. coli. C. elegans cultured in this way showed an increase in adult life span typical of axenic culture (Figure 1D).
Maintenance on AXM agar in the presence of E. coli throughout adulthood fully rescued the increase in nematode life span. We directly compared life spans of C. elegans on AXM agar with those on NGM agar (with antibiotic present in each case), and they were not significantly different (Figure 1D). Thus, the property of AXM plates that increases life span is fully rescued by addition of E. coli. The increased longevity of nematodes on NGM agar without E. coli is also rescued by addition of live E. coli (11,12). Comparison of populations on axenic plates with or without carbenicillin showed that this antibiotic does not affect C. elegans life span at the concentration used (Figure 1D).
That addition of E. coli could suppress the slowed development and decreased fecundity seen in axenic culture implies that the E. coli cells provide a nutritional requirement that is missing from axenic plates. Later in adulthood, however, there appears to be some detrimental component of viable E. coli that contributes to a shortening of life span. That life span was extended by axenic culture after day 1 of adulthood confirms an earlier observation (30) that the critical period for the effects of axenic culture on life span is during adulthood and not during development.
Concentration of Nutrients in AXM is not a Determinant of the DR Effect
Our results suggest that AXM is deficient in critical dietary components that are present in E. coli. To investigate what these might be, we first compared the nutrient content of AXM with that of a suspension of E. coli OP50 (in water) at a concentration approximating that which is sufficient to rescue the effects of axenic culture (3.8 × 107 cells/mL). This analysis was performed by a commercial food analysis service (Leatherhead Food International, Wells, U.K.). We first compared the caloric content of these two culture media. Whereas the energy content of 100 mL of 1X AXM was ~20 kCal, that of the same volume of E. coli suspension was below the limit of detection for the methods used (<3 kCal). Thus, AXM is not able to support normal C. elegans growth despite having a caloric content that is at least 6-fold higher than a concentration of E. coli that is sufficient to rescue fully the effects of axenic culture. This high nutrient concentration implies that the effects of AXM on life span are not due to an insufficiency of caloric content. Also notable in the nutritional breakdown of AXM is that it is rich in protein, less so in carbohydrates, and the level of fat was below the limit of detection (Table 1).
Table 1.
Nutrient Analysis of Axenic Medium and E. coli (3.8 × 107 cells/mL in Water).
| Food Component | Detection Limit | Axenic Medium | E. coli |
|---|---|---|---|
| Protein | <0.1 g/100 mL | 4.6 g/100 mL | Below detection limit |
| Carbohydrate | <0.1 g/100 mL | 0.5 g/100 mL | Below detection limit |
| Fat | <0.2 g/100 mL | Below detection limit | Below detection limit |
| Vitamins | |||
| B1 | <0.05 mg/100 mL | 0.68 mg/100 mL | Below detection limit |
| B2 | <0.004 mg/100 mL | 0.12 mg/100 mL | Below detection limit |
| B5 | <0.001 mg/100 mL | 1.02 mg/100 mL | Below detection limit |
| B6 | <0.003 mg/100 mL | 0.003 mg/100 mL | Below detection limit |
| B8 | n.a. | 2.76 μg/100 mL | 2.58 μg/100 mL |
| Niacin B3 | <0.01 mg/100 mL | 3.96 mg/100 mL | Below detection limit |
| Minerals | |||
| Chloride | <0.1 g/100 mL | 0.1 g/100 mL | Below detection limit |
| Calcium | <1 mg/100 mL | 8 mg/100 mL | Below detection limit |
| Iron | n.a. | 0.2 mg/100 mL | 0.1 mg/100 mL |
| Magnesium | <1 mg/100 mL | 4 mg/100 mL | Below detection limit |
| Phosphorus | n.a. | 46 mg/100 mL | 6 mg/100 mL |
| Potassium | n.a. | 182 mg/100 mL | 2 mg/100 mL |
| Sodium | n.a. | 61 mg/100 mL | 12 mg/100 mL |
| Zinc | <0.1 mg/100 mL | 0.3 mg/100 mL | Below detection limit |
| Others | |||
| Folic acid | <0.123 μg/100 mL | 3.93 μg/100 mL | Below detection limit |
Notes: The measurements were above detection limit in both culture media. In cases of “n.a.” (not applicable), the detection limit was not provided by the food analysis company. E. coli, Escherichia coli.
To test the possibility that slow growth and aging on AXM may reflect limiting, low concentrations of specific constituents, we tested the effect on life span of varying its concentration from 5% to 200% of the standard recipe. In rodents, maximal increases in life span result from reducing food intake to ~60% of ad libitum levels (31). In Drosophila, food dilution to 60% of standard sugar yeast fly medium maximized life span (32). Our expectation, therefore, was that a stepwise reduction of the medium concentration would first increase life span as DR became more severe, and then at very low concentrations decrease it, as animals tipped over into starvation. We expected that increasing the medium concentration would lessen DR and reduce life span.
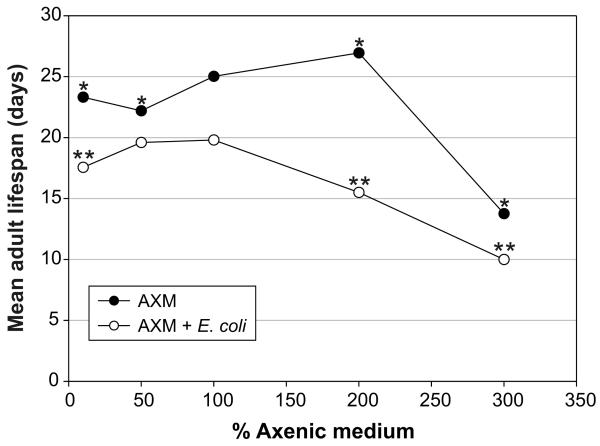
Against expectation, the concentration of AXM had little effect on life span (Figure 2). Reducing the medium concentration from 100% to 50% or 10% did not increase life span, but instead marginally decreased it. Increasing the AXM concentration from 100% to 200% slightly increased life span, whereas 300% caused a large drop in life span. Similar results were obtained using axenic liquid culture: There were no significant differences in life span in 50%, 80%, 100%, and 200% AXM; however, mean life span was reduced by 57% in 0.5% liquid AXM (p < .0001) (Keaney M, Gems D, unpublished results, 2004). Thus, the life-extending effects of DR appear not to reflect limited availability of calories or of major nutrient classes (e.g., protein, carbohydrate) in this medium. Instead, it appears that something else critical for C. elegans nutrition is absent.
Figure 2.
Mean adult life span of wild-type (N2) Caenorhabditis elegans hermaphrodites at 22.5°C on different concentrations of axenic medium (AXM) in agar plates. Standard AXM (100%) was diluted to 50% and 10%, and concentrated to 200% and 300%. *Indicates statistical significant changes as compared to 100% AXM (mean adult life span on 100% AXM = 25.0 ± 0.3 days, N = 156 (dead)/44 (censored); on 10% AXM = 23.3 ± 0.7 days, N = 35/165; on 50% AXM = 22.2 ± 0.3 days, N = 187/35; on 200% AXM = 26.9 ± 0.5 days, N = 90/70; on 300% AXM = 13.8 ± 1.1 days, N = 29/121) (p < .0001 for all comparisons). Between 10% and 200% AXM life span was statistically significantly, but only marginally affected by the medium concentration. Carbenicillin-treated Escherichia coli OP50 was added to 7.5 × 108 cells/plate to a separate series of AXM concentrations to control for toxic effects of the medium. **Indicates statistically significant changes as compared to 100% AXM with E. coli (mean adult life span on 100% AXM + E. coli = 19.8 ± 0.2 days, N = 204/16; on 10% AXM + E. coli = 17.6 ± 0.2 days, N = 128/26; on 50% AXM + E. coli = 19.6 ± 0.2 days, N = 133/10; on 200% AXM + E. coli = 15.5 ± 0.3 days, N = 172/25; on 300% AXM + E. coli = 10.0 ± 0.3 days, N = 62/38) (p < .0001 for all comparisons).
As a control for toxic effects of this range of AXM concentrations unlinked to the DR effect, we also examined life span over this range in the presence of rescuing E. coli. At the highest concentration of AXM (300%), there was a decrease in life span (Figure 2). This implies that the shortening of life span of worms on 300% AXM alone may not be due to suppression of the DR effect by increased nutrient intake, but rather, it may be caused by mildly toxic effects, possibly high osmolarity, of the AXM at this high concentration.
E. coli Factor that Rescues Effects of Axenic Culture is Heat Labile and Nondiffusible
E. coli appears to contain a nutritional factor not present in AXM. To identify this, we tested the capacity of derivatives of E. coli to rescue the effects of culture in liquid AXM. In the following experiments, we initially tested the capacity to rescue the effects of axenic culture on fecundity and then, in some cases, life span too.
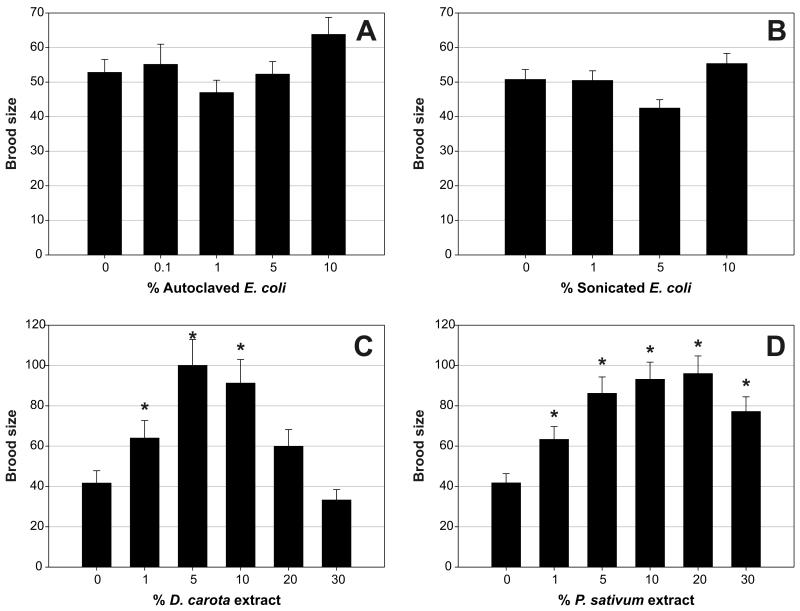
We first tested whether the critical factor in E. coli was thermolabile, by adding autoclaved E. coli to nematodes in liquid axenic culture. Autoclaved E. coli failed to rescue the reduced fecundity (Figure 3A) or increased life span (data not shown). This finding is consistent with previous tests on C. elegans in axenic liquid culture, as well as on AXM plates, where addition of autoclaved E. coli only partially rescued the effects on development (17). Thus, the critical E. coli constituent is heat labile.
Figure 3.
Effect of additional nutrients on fecundity of Caenorhabditis elegans in axenic culture. A, Fecundity of selfed wild-type C. elegans (N2) hermaphrodites at 22.5°C in liquid axenic medium (AXM) supplemented with different concentrations of autoclaved Escherichia coli OP50 (average of three replicate trials). For each concentration, a total of approximately 30 adult broods were scored. One percent of autoclaved bacteria correspond to 108 cells/mL. Although there was a significant effect of E. coli concentration on fecundity (p = .0072), this is unlikely to be biologically meaningful, because the only brood sizes that were significantly different from each other were those on 1% and 10% autoclaved bacteria (p = .0026). B, Fecundity of N2 hermaphrodites at 22.5°C in liquid AXM supplemented with different concentrations of filtrates from sonicated E. coli OP50. Average of two trials. For each concentration, approximately 20 broods were scored. One percent of filtrate corresponds to 108 bacterial cells/mL. As for the above experiment with autoclaved bacteria, the statistically significant effect of E. coli concentration on fecundity (p = .012) is unlikely to be biologically meaningful, because the only brood sizes that were significantly different from each other were those on 5% and 10% sonicated bacteria (p = .0066). C, Fecundity of N2 hermaphrodites at 22.5°C in liquid AXM supplemented with different concentrations of Daucus carota extract. Average of two replicate trials. For each concentration, approximately 20 broods were scored. *Indicates statistically significant changes as compared to unsupplemented liquid AXM. Brood size in liquid AXM increased with addition of 1% or 5% D. carota extract (p = .0098 for comparison between AXM alone or with 1% extract and p < .0001 for comparison between AXM alone or with 5% D. carota extract). The difference in fecundity on 5% and 10% D. carota extract is not significant (p = .9003). Higher concentrations of extract caused fecundity to decline again, with brood size being indistinguishable between worms in AXM and animals in AXM with 20% and 30% D. carota extract (p = .0526 for comparison between 0% and 20% extract and p = .6344 for comparison between 0% and 30% extract). D, Fecundity of N2 hermaphrodites at 22.5°C in liquid AXM supplemented with different concentrations of Pisum sativum extract. Average of two replicate trials. For each concentration, approximately 20 adults were scored. Brood size of axenically cultured worms gradually increased with increasing concentration of P. sativum extract (p < .0001 for comparison between axenically cultured worms and worms in different concentrations of P. sativum extract). Maximum brood size is reached with a concentration of 5%–20% extract. Addition of a higher concentration causes fecundity to slightly decrease again (p = .0213 for comparison between worms in 20% and 30% P. sativum extract).
One possibility is that autoclaved E. coli does not rescue DR effects because C. elegans ingests less of it. To test this, we compared the rate of pumping of the pharynx of nematodes on axenic plates with either live or autoclaved bacteria, but there was no difference (autoclaved bacteria, mean ± standard deviation: 231 ± 25 pumps/min, live bacteria: 221 ± 28 pumps/min; p = .159; N = 31). Thus, autoclaving of the E. coli food source does not obviously affect feeding behavior. However, this test says nothing about the efficiency of uptake of ingested food by intestinal cells.
Next, we tested the possibility that a soluble, heat-labile factor present in E. coli is the critical missing component. We prepared a sterile extract of nonautoclaved E. coli (a filtrate from an E. coli sonicate, prepared using a 0.2-μm filter). Effects of liquid axenic culture on fecundity were not rescued at all by such a filtrate at the concentrations tested (Figure 3B). Thus, the heat-labile constituent that is present in E. coli and promotes fertility, but is missing in AXM, appears not to be soluble.
Weak Rescuing Effects of Extracts of Fresh Plant and Animal Tissues
Next we conducted further tests searching for possible organic components (“nematode vitamins”) missing from AXM, reasoning that such compounds, if they existed, might be present in complex organic mixtures derived from fresh plant and animal tissue. We found that plant extracts from two sources tested, Daucus carota (carrot) and Pisum sativum (pea), partially rescued the effects of axenic culture on fecundity (Figure 3, C and D) but not longevity (data not shown). Addition of skimmed milk has also been shown to improve growth and fecundity of C. elegans in defined medium (33). We therefore tested its capacity to rescue the effect of axenic culture on life span and fecundity, but no rescue was observed (Matthijssens F, Vanfleteren JR, unpublished results, 2003). In summary, these tests failed to find clear evidence for a diffusible requirement for nematode nutrition that AXM lacks. However, more promising results were obtained using extracts of fresh animal tissue. Liver extract has been used previously to improve growth of nematodes in axenic culture (reviewed in 25). In our tests, 20% horse liver extract could largely rescue effects on development but, as with plant extracts, fecundity was only slightly improved. However, liver extract did partially suppress the extended life span of worms in liquid axenic culture (data not shown), an observation that warrants further study.
Another possibility is that AXM possesses some property that is detrimental to C. elegans, and that live E. coli alter the medium and neutralize this detrimental property. We tested this possibility by incubating liquid AXM overnight with live E. coli OP50, then filter sterilizing the medium, and seeing whether it was better able to support C. elegans development. Preincubation with E. coli (37°C), resulting in an increase in bacterial cell number from 4 × 1010 to 6 × 1011 cells/mL, did not reduce worm development time relative to growth in AXM alone (data not shown). This finding suggests that E. coli does not affect C. elegans growth by neutralizing deleterious properties of AXM.
Rescue of Axenic Culture Effects is Correlated with the Presence of Metabolic Activity in E. coli
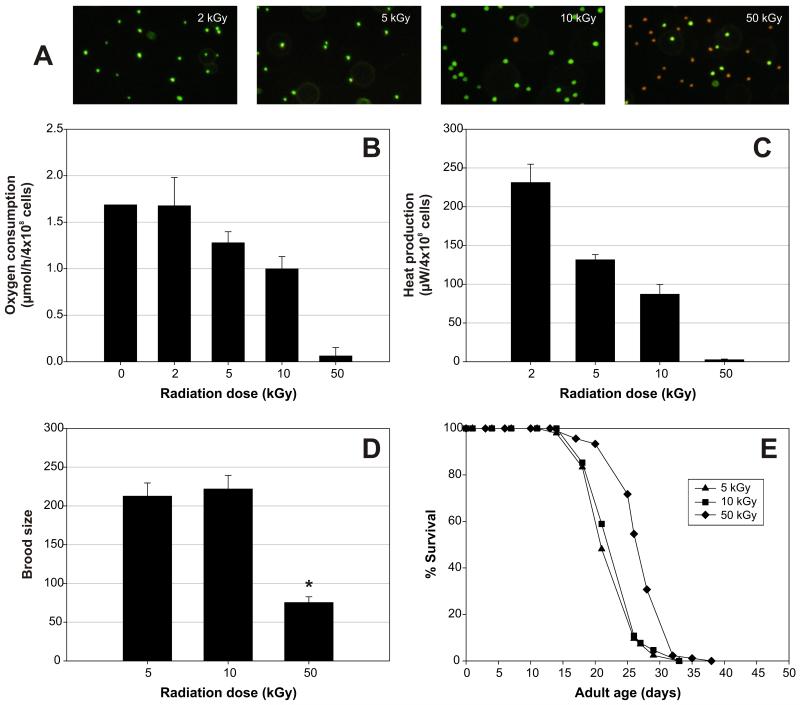
Next, we explored the possibility that C. elegans might require live microbes in their diet for normal digestive function. To this end, we measured the effect of irradiation of E. coli over a range of energy doses on the proportion of cells remaining alive and metabolically active, and their capacity to rescue the effects of AXM on life span. E. coli K12 was irradiated with β-radiation at 2, 5, 10, or 50 kGy. We measured the proportion of cells remaining alive by means of the vital dyes SYTO 9 and propidium iodide (see Methods). The proportion of live cells decreased with increasing dose of β-radiation until at 50 kGy very few cells remained alive (Figure 4A). When bacteria were irradiated with 2 kGy of electrons, there was no effect on oxygen consumption, but given a dose of ≥5 kGy, oxygen consumption showed a dose-dependent decline (Figure 4B). Heat production (an alternative measure of metabolic activity) showed a similar response to irradiation (Figure 4C). Thus, irradiated bacteria are alive and metabolically active after irradiation with doses <50 kGy.
Figure 4.
Capacity of Escherichia coli to rescue effects of axenic culture correlates with presence of microbial metabolic activity. A, Viability staining of E. coli (K12) treated with a range of radiation doses (see Methods for details). Live bacteria stain green, whereas dead bacteria stain red. A magnification of 12.5 × 40 was used. Note that most cells are killed by 50 kGy. B, Assessment of oxygen consumption of E. coli treated with a range of radiation doses. Measurements were performed at 37°C in Luria Broth (LB) medium. E. coli cells (4 × 108) were monitored in 1 mL of medium. Average of three replicate trials. Oxygen consumption decreases with increasing radiation dose (p < .0001). C, Assessment of heat output of E. coli treated with a range of radiation doses. Measurements were performed at 37°C in LB medium. E. coli cells (4 × 108) were monitored in 1 mL of medium. Average of three replicate trials. Heat production declines with increasing radiation dose (p = .001). D, Fecundity of wild-type Caenorhabditis elegans at 20°C on axenic medium (AXM) plates with 2 × 109 E. coli cells treated with different radiation doses. Average of eight partially overlapping replicate trials. There was a small replicate effect on brood size (variance between replicates = 0.045 ± 0.03). For each radiation dose, a total number of 40–100 adults was scored. *Indicates statistically significant changes as compared to worms on AXM plates with 5 kGy-treated bacteria. Data on 2 kGy-treated bacteria are not shown because E. coli cultures treated with such a low dose were not always completely growth-arrested, leading to atypical fecundity results. There is no difference in brood size between worms grown on 5 kGy- or 10 kGy-treated bacteria. The difference between worms on 5 kGy- and 50 kGy-treated bacteria is significant (p < .0001). E, Survival of C. elegans at 20°C on AXM plates with E. coli treated with different radiation doses (2 × 109 cells/plate). There was no difference in life span when C. elegans was cultured on bacteria treated with doses of 2, 5, or 10 kGy (experiment with only 2 and 5 kGy is not shown). Average adult life span was extended by ~15% when worms were grown on 50 kGy-treated bacteria (p < .0001 for comparison with worms on 10 kGy-treated bacteria) (mean adult life span on 5 kGy-treated bacteria = 23.2 ± 0.4 days, N = 85 [dead]/45 [censored]; on 10 kGy-treated bacteria = 24.0 ± 0.5 days, N = 65/63; on 50 kGy-treated bacteria = 27.7 ± 0.4 days, N = 88/55).
Addition of irradiated E. coli to 2 × 109 cells/plate rescued the effects of axenic culture at all except the highest dose (50 kGy). Worms grown on AXM plates with 50 kGy-treated bacteria developed to adulthood in 3 instead of 2 days, and were less fecund than worms on E. coli cells treated with lower doses (Figure 4D). Moreover, worms grown on 50 kGy-treated E. coli were markedly longer lived than those grown on E. coli treated with lower doses (e.g., p < .0001 relative to worms on 10 kGy-treated bacteria) (Figure 4E). Thus, as long as some metabolically active E. coli remained, rescue was possible. This finding suggests the surprising conclusion that metabolic activity in its microbial food source is a nutritional requirement for C. elegans.
DISCUSSION
Culture in liquid AXM has long been studied as a form of DR for C. elegans. Here we have described new and convenient protocols for studying this form of DR which use AXM in agar plates. Surprisingly, applying DR using AXM seems to reflect the lack of critical dietary components that are present only in metabolically active E. coli.
There are several possible mechanisms by which culture in AXM might extend nematode life span. One such is the absence of the harmful effects of proliferating E. coli. As C. elegans age, proliferating E. coli constipate the alimentary tract, such that prevention of E. coli proliferation, e.g., by ultraviolet irradiation or use of antibiotics, increases C. elegans life span (34–36). We therefore expect that the absence of bacterial proliferation effects contributes to longevity in axenic culture, yet this cannot fully account for the effects of axenic culture on life history, because culture on ultraviolet-irradiated E. coli does not retard development or reduce fecundity (35), and the magnitude of the life-span increase in axenic culture (60%–80%) is larger than that resulting from bacterial killing (20%–40%) (35,36).
A second interpretation is that AXM is deficient in major food groups (e.g., lipid, carbohydrate, protein) or overall calories. However, our analysis of the nutrient content of AXM argues against this interpretation. Although fat content appears to be very low, this is also true for a rescuing concentration of E. coli, arguing against a deficiency in fat. Furthermore, varying the concentration of AXM from 5% to 200% of the standard recipe had only marginal effects on life span. Together, this strongly suggests that increased life span in axenic culture is not due to insufficiency of the major nutrient classes.
Our results suggest instead an unexpected conclusion—that C. elegans has a dietary requirement for metabolically active microbes, such that their absence in axenic culture leads to severe DR. Consistent with this conclusion, it was very recently shown that growth on E. coli with impaired respiratory metabolism extends C. elegans life span (Saiki R, Lunceford A, Larsen PL, Clarke CF, personal communication, 2007).
How live E. coli might contribute to C. elegans nutrition is unknown. The presence of a complex community of intestinal microbiota is essential to nutrition in many larger soil invertebrates, for example, termites, isopods, and earthworms (37). In particular, these aid in degradation of recalcitrant biological materials such as cellulose and chitin. Thus, axenic culture of, say, a termite would be predicted to result in lowered food uptake. One possibility is that in the wild, C. elegans digestion is assisted by mutualistic microbiota within their gut. However, given the presence of ample nutrients in AXM, it seems unlikely that release of nutrients due to breakdown by E. coli is important here.
A more plausible interpretation is that a nutritional requirement for live microbes reflects the fact that the C. elegans intestine has evolved in the constant presence of intestinal microbes; therefore, its normal function requires their presence. In the wild, this may aid digestion of other foodstuffs (e.g., fungi, protozoa). Föll and colleagues (38) derived the number of live E. coli cells in the intestine of sucrose-washed worms by growing a ground worm sample on full medium agar plates at 37°C and counting the resulting number of colonies. This gave an estimate of ~3900 live E. coli cells in the gut of a young adult worm. A related possibility is that endocytosed bacterial components, retaining some biochemical activity, contribute to nematode digestion within intestinal cells.
A further possibility is that intestinal microbes influence nutrient transport mechanisms. In C. elegans, pH gradients across the apical membrane of intestinal cells play an important role in nutrient uptake, e.g., by oligopeptide transporters. Disruption of this transport system by mutation phenocopies the effects of DR, including an increase in life span (39). Possibly, microbes condition the C. elegans intestinal redox environment in a way that facilitates the function of pH-dependent intestinal transporters.
Although our findings seem to suggest that metabolically active microbes may be a requirement for C. elegans nutrition, other interpretations of our findings are conceivable. It remains possible that highly labile, high-molecular-weight microbial constituents are the critical dietary component. Furthermore, earlier studies have suggested that C. elegans digestion involves endocytic processes that depend on particles of the size of microbial or subcellular particles generated by the action of the pharyngeal grinder (20,21). The uptake of nutrients in the gut is stimulated by the presence of particles in the culture medium (21,40). These particles are missing from axenic culture, possibly resulting in decreased nutrient uptake. For example, in Caenorhabditis briggsae, stimulation of growth and reproduction by added glycogen is associated with the precipitated part of the medium (41). That various filtered plant extracts had only a weak rescue effect could reflect the need for a food supplement that is composed of particles. That liver extract could partially rescue development and life span in axenic culture could be due to its particulate appearance. If E. coli provides particulate matter that aids digestion, then our results show that this is heat labile.
We postulated above that the DR effects of axenic culture reflect a requirement for a dietary component present in metabolically active microbes. If this nutrient is supplied, development and fecundity are supported, and life span is shortened. By this view, the life-shortening effects of the nutrient may reflect a by-product of the life processes that it supports (e.g., molecular damage), or a secondary toxicity of the nutrient itself. An alternative view is that metabolically active E. coli supplies two distinct elements to C. elegans: a nutrient that supports development and fecundity, and a toxic component or property that reduces life span. Although the first view is more conventional among DR studies, several observations in this study are consistent with the second. First, the threshold for the concentration of E. coli required for normal brood size is 10-fold lower than that which shortens life span (Figure 1). Second, addition of plant extracts supported development and fecundity, but did not reduce life span (Figure 3).
Our results might suggest that DR in C. elegans using AXM agar is different from DR methods in other animal models. In Drosophila, for example, it was shown that axenic culture has no effect on life span (42). However, certain observations suggest that the underlying mechanisms acting on life span may not be so different between worms and mammals. For example, like DR rodents, germ-free rodents exhibit a reduced body weight and increased life span (43,44). Moreover, when DR was applied to germ-free animals, no additive effects on life span were observed, suggesting that, in germ-free animals and in animals subjected to DR, life span is extended by similar mechanisms. Consistent with such a DR-microbe connection, the reproductive capacity of germ-free mice was enhanced following contamination with intestinal bacteria (45), as is observed in C. elegans after addition of β-irradiated E. coli to the AXM plates (Figure 1).
We studied nematodes on agar plates containing AXM to help understand the longstanding observation that axenic culture increases nematode life span. While we were conducting our studies, it was reported that culture of C. elegans in the absence of E. coli on agar plates containing standard NGM also leads to increased life span (11,12). One possibility is that the mechanism of nematode life extension is the same in all forms of axenic culture, whether in standard AXM in liquid culture or agar plates, or on NGM agar plates. In support of this view, extension of the life span of C. elegans by culture either in axenic liquid culture medium or axenically on NGM plates is not dependent on the DAF-16 transcription factor (11,12,16,19). Preliminary tests show that this is also true for culture on agar plates with AXM. Indeed, using a daf-16::gfp reporter strain, we found that DAF-16 remained in the cytoplasm, independently of the concentration of irradiated bacteria (Lenaerts I, Vanfleteren JR, unpublished results, 2006). Further studies are required to verify whether the same mechanisms are at play in each case. Interestingly, starvation in calorie-free liquid medium (S basal) rendered both daf-16(+) and daf-16(lf) animals more sensitive to oxidative stress, and shortened life span (46). In contrast, starvation for only 1–3 days increased life span by 30%–40%, likely by eliciting a hormetic response (47).
In conclusion, we have developed novel, convenient methodologies for exerting DR using axenic culture on agar plates. We used these methodologies to understand the mechanistic basis of this mode of DR. Although the response to DR in terms of delayed development, reduced fecundity, and increased life span is widespread among animal species, the extent to which evolutionarily conserved mechanisms are involved remains unclear. Our results suggest that nematodes may have a nutritional requirement for metabolically active microbes, implying a mechanism of DR the evolutionary conservation of which is currently far from clear.
ACKNOWLEDGMENTS
This work was supported by grants from Ghent University (12050101), the F.W.O.-Vl (G.0025.06), the European Community (LSHM-CT-2004-512020) (to I.L.), and the Wellcome Trust (to G.W. and D.G.). Some strains were obtained from the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health National Center for Research Resources.
We thank Catherine Clarke for critical reading of the manuscript and useful comments, Frederik Hendrickx for advice on statistical analysis, and Sylvie Van Eygen for technical assistance.
REFERENCES
- 1.Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- 2.Chippindale AK, Leroi A, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life history evolution. I. Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–193. [Google Scholar]
- 3.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc R Soc Lond B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr. 1987;117:376–382. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- 5.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon length of life and upon ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 6.Weindruch R. Caloric restriction and aging. Sci Am. 1996;274:46–52. doi: 10.1038/scientificamerican0196-46. [DOI] [PubMed] [Google Scholar]
- 7.Kealy RD, Laxler DF, Ballam JM, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. 2002;220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- 8.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 9.Hosono R, Nishimoto S, Kuno S. Alterations of life-span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp Gerontol. 1989;24:251–264. doi: 10.1016/0531-5565(89)90016-8. [DOI] [PubMed] [Google Scholar]
- 10.Greer EL, Dowlatshahi D, Banko MR, et al. An AMP-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaeberlein TL, Smith ED, Tsuchiya M, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee GD, Wilson MA, Zhu M, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanfleteren JR, Braeckman BP. Mechanisms of life span determination in Caenorhabditis elegans. Neurobiol Aging. 1999;20:487–502. doi: 10.1016/s0197-4580(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 15.Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren J. Life extension via dietary restriction is independent of the Ins/Igf-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 17.Houthoofd K, Braeckman BP, Lenaerts I, et al. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp Gerontol. 2002;37:1371–1378. doi: 10.1016/s0531-5565(02)00173-0. [DOI] [PubMed] [Google Scholar]
- 18.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–556. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 19.Vanfleteren JR, De Vreese A. The gerontogenes age-1 and daf-2 determine metabolic rate potential in aging Caenorhabditis elegans. FASEB J. 1995;9:1355–1361. doi: 10.1096/fasebj.9.13.7557026. [DOI] [PubMed] [Google Scholar]
- 20.Vanfleteren JR. Nematode growth-factor. Nature. 1974;248:255–257. doi: 10.1038/248255a0. [DOI] [PubMed] [Google Scholar]
- 21.Vanfleteren JR. Nematodes as nutritional models. In: Zuckerman BM, editor. Nematodes as Biological Models. Vol. 2. Academic Press; New York: 1980. pp. 47–79. [Google Scholar]
- 22.Gems D, Riddle DL. Defining wild-type life span in Caenorhabditis elegans. J Gerontol Biol Sci. 2000;55A:B215–B219. doi: 10.1093/gerona/55.5.b215. [DOI] [PubMed] [Google Scholar]
- 23.Kayser EB, Sedensky MM, Morgan PG, Hoppel CL. Mitochondrial oxidative phosphorylation is defective in the long-lived mutant clk-1. J Biol Chem. 2004;279:54479–54486. doi: 10.1074/jbc.M403066200. [DOI] [PubMed] [Google Scholar]
- 24.Sulston JE, Hodgkin J. Methods. In: Wood WB, et al., editors. The nematode C. elegans. Laboratory Press; New York: 1988. pp. 587–606. [Google Scholar]
- 25.Vanfleteren JR. Axenic culture of free-living, plant-parasitic, and insect-parasitic nematodes. Ann Rev Phytopathol. 1978;16:131–157. [Google Scholar]
- 26.Mondelaers W, Van Laere K, Goedefroot A, Van den Bossche K. The Gent University 15 MeV high-current linear electron accelerator facility. Nucl Instr Meth A. 1996;368:278–282. [Google Scholar]
- 27.Braeckman BP, Houthoofd K, De Vreese A, Vanfleteren J. Assaying metabolic activity in ageing Caenorhabditis elegans. Mech Ageing Dev. 2002;123:105–119. doi: 10.1016/s0047-6374(01)00331-1. [DOI] [PubMed] [Google Scholar]
- 28.Kenyon C, Chang J, Gensch E, Rudener A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 29.Gems D, Sutton AJ, Sundermeyer ML, et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenaerts I, Van Eygen S, Vanfleteren J. Adult-limited dietary restriction slows Gompertzian aging in Caenorhabditis elegans. Ann N Y Acad Sci. 2007;1100:442–448. doi: 10.1196/annals.1395.049. [DOI] [PubMed] [Google Scholar]
- 31.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 32.Clancy DJ, Gems D, Hafen E, Leevers S, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- 33.Szewczyk NJ, Udranszky IA, Kozak E, et al. Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J Exp Biol. 2006;209:4129–4139. doi: 10.1242/jeb.02492. [DOI] [PubMed] [Google Scholar]
- 34.Garsin DA, Villanueva JM, Begun J, et al. Long-lived C. elegans Daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 35.Gems D, Riddle DL. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics. 2000;154:1597–1610. doi: 10.1093/genetics/154.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garigan D, Hsu AL, Fraser AG, Kamath R, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans. A role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konig H. Bacillus species in the intestine of termites and other soil invertebrates. J Appl Microbiol. 2006;101:620–627. doi: 10.1111/j.1365-2672.2006.02914.x. [DOI] [PubMed] [Google Scholar]
- 38.Föll RL, Pleyers A, Lewandovski GJ, Wermter C, Hegemann V, Paul RJ. Anaerobiosis in the nematode Caenorhabditis elegans. Comp Biochem Physiol B. 1999;124:269–280. doi: 10.1016/s0305-0491(99)00130-3. [DOI] [PubMed] [Google Scholar]
- 39.Nehrke K. A reduction in intestinal cell pH(I) due to loss of the Caenorhabditis elegans Na+/H+ exchanger Nhx-2 increases life span. J Biol Chem. 2003;278:44657–44666. doi: 10.1074/jbc.M307351200. [DOI] [PubMed] [Google Scholar]
- 40.Cheng AC, Lu NC, Briggs GM, Stokstad ELR. Effect of particulate materials on population growth of the free-living nematode Caenorhabditis briggsae. Proc Soc Exp Biol Med. 1979;160:203–207. doi: 10.3181/00379727-160-40420. [DOI] [PubMed] [Google Scholar]
- 41.Hansen EL, Perez-Mendez G, Buecher EJ. Glycogen as a supplement in media for axenic cultivation of nematodes. Proc Soc Exp Biol Med. 1971;137:1352–1354. doi: 10.3181/00379727-137-35786. [DOI] [PubMed] [Google Scholar]
- 42.Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Snyder DL, Pollard M, Wostmann BS, Luckert P. Life span, morphology, and pathology of diet-restricted germ-free and conventional Lobund-Wistar rats. J Gerontol. 1990;45:B52–B58. doi: 10.1093/geronj/45.2.b52. [DOI] [PubMed] [Google Scholar]
- 44.Tazume S, Umehara K, Matsuzawa H, Aikawa H, Hashimoto K, Sasaki S. Effects of germfree status and food restriction on longevity and growth of mice. Jikken Dobutsu. 1991;40:517–522. doi: 10.1538/expanim1978.40.4_517. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu K, Muranaka Y, Fujimura R, Ishida H, Tazume S, Shimamura T. Normalization of reproductive function in germfree mice following bacterial contamination. Exp Anim. 1998;47:151–158. doi: 10.1538/expanim.47.151. [DOI] [PubMed] [Google Scholar]
- 46.Henderson ST, Bonafe M, Johnson TE. daf16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- 47.Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]