Abstract
Aim: Morphogenetic switching between the replicating and nonreplicating states of Mycobacterium tuberculosis is regulated by oxygen, nitric oxide, and carbon monoxide levels. The mechanisms by which M. tuberculosis senses these diatomic gases remain poorly understood. In this study, we have examined whether virulence factor SenX3 plays any role in oxygen sensing. Results: In this study, we demonstrate that the virulence factor SenX3 is a heme protein that acts as a three-way sensor with three levels of activity. The oxidation of SenX3 heme by oxygen leads to the activation of its kinase activity, whereas the deoxy-ferrous state confers a moderate kinase activity. The binding of nitric oxide and carbon monoxide inhibits kinase activity. Consistent with these biochemical properties, the SenX3 mutant of M. tuberculosis is capable of attaining a nonreplicating persistent state in response to hypoxic stress, but its regrowth on the restoration of ambient oxygen levels is significantly attenuated compared with the wild-type and the complemented mutant strains. Furthermore, the presence of signaling concentrations of nitric oxide and carbon monoxide was able to inhibit the regrowth of M. tuberculosis in response to ambient oxygen levels. Innovation and Conclusions: Evidence presented in this study delineates a plausible mechanism explaining the oxygen-induced reactivation of tuberculosis diseases in humans after many years of latent infection. Furthermore, this study implicates nitric oxide and carbon monoxide in the inhibition of mycobacterial growth from the nonreplicating state. Antioxid. Redox Signal. 22, 603–613.
Introduction
The capability of Mycobacterium tuberculosis (Mtb) to transit from the actively replicating state into a nonreplicating and metabolically quiescent latent state and vice versa is central to the success of Mtb as a pathogen. Oxygen (O2) tension is recognized as a major physiologically relevant environmental factor that regulates mycobacterial replication (2, 23, 32, 37). A gradual exposure to hypoxia inhibits mycobacterial growth and leads to the phenotypic changes associated with latency, whereas active replication is resumed on re-oxygenation of the culture (36). Other studies have also implicated an important role for nitric oxide (NO) and carbon monoxide (CO) in regulation of mycobacterial replication (8, 10, 13, 34). However, the molecular mechanisms that regulate the switching between active replication and a nonreplicating persistent state in response to hypoxia, NO, and CO are poorly understood. Theoretically, the initiation and maintenance of the nonreplicating persistent state will need sensor/s of hypoxia, whereas regrowth will require sensor/s of oxygen. Mtb responds to hypoxia, NO, and CO by inducing Dos regulon (10, 13, 26) consisting of ∼48 genes under the control of DosR (26). The DosR is a response regulator protein that receives signals from heme-based sensor histidine kinases DosS and DosT (10, 19, 24, 29). The kinase activity of DosS and DosT is induced by NO and CO and is inhibited by O2 (10, 29); thus, these sensor kinases possess the required capability to facilitate the transition into the nonreplicating state. However, the role of DosR in Mtb latency and reactivation remains controversial with some reports suggesting an important role (3, 11), while others describe only minor survival defects during hypoxia in DosR mutant strain (22, 23). These observations suggest the presence of alternative sensors and novel metabolic pathways that guide the switching between latency and reactivation.
Innovation.
Previous studies have suggested that sensor kinases DosS and DosT sense hypoxia, nitric oxide (NO), and carbon monoxide (CO) and facilitate the transition of actively replicating bacteria into the nonreplicating persistent state. However, the sensing mechanism and the genetic pathways that govern oxygen controlled regrowth is not known. This study leads to a paradigm shift in our understanding of oxygen-controlled mycobacterial replication and provides evidence which suggests that the virulence factor SenX3 is a heme-based oxygen sensor that senses the presence of oxygen to induce active replication of persistent Mycobacterium tuberculosis (Mtb). Furthermore, inhibition of SenX3 by NO and CO provides a plausible mechanism of arrest of regrowth of Mtb by NO CO.
Two component proteins and serine threonine kinases are often used by prokaryotes in sensing changes in the environment. One of the two component proteins, SenX3 has a PAS domain that commonly exists in proteins involved in sensing O2, redox potential, and light (30). Furthermore, SenX3-RegX3 knockout mutant is attenuated for growth in THP-1 macrophages and in guinea pigs/mice model of tuberculosis (TB), suggesting that SenX3-RegX3 is a virulence factor (15, 17, 18). The mutant also exhibits downregulation of a number of genes involved in DNA replication and protein synthesis (15). The mutant strain also has altered expression of cydB and gltA1, encoding cytochrome D oxidase and citrate synthase, respectively (20). These genes are related to survival of bacteria in response to hypoxia, implying a link between oxygen tension and SenX3. The literature cited earlier suggest that SenX3-RegX3 could be involved in regulation of mycobacterial replication and metabolism in response to O2 levels. However, what is the signal for SenX3 and its sensing mechanism remains unknown. To address these issues, we tested the hypothesis that SenX3 PAS domain binds to a redox active factor. We further tested the hypothesis that O2, NO, and CO are ligands of SenX3 and that their interaction with SenX3 modulates its kinase activity. Finally, we have examined the role of SenX3 in the Mtb latency and reactivation.
Results
SenX3 is a heme protein
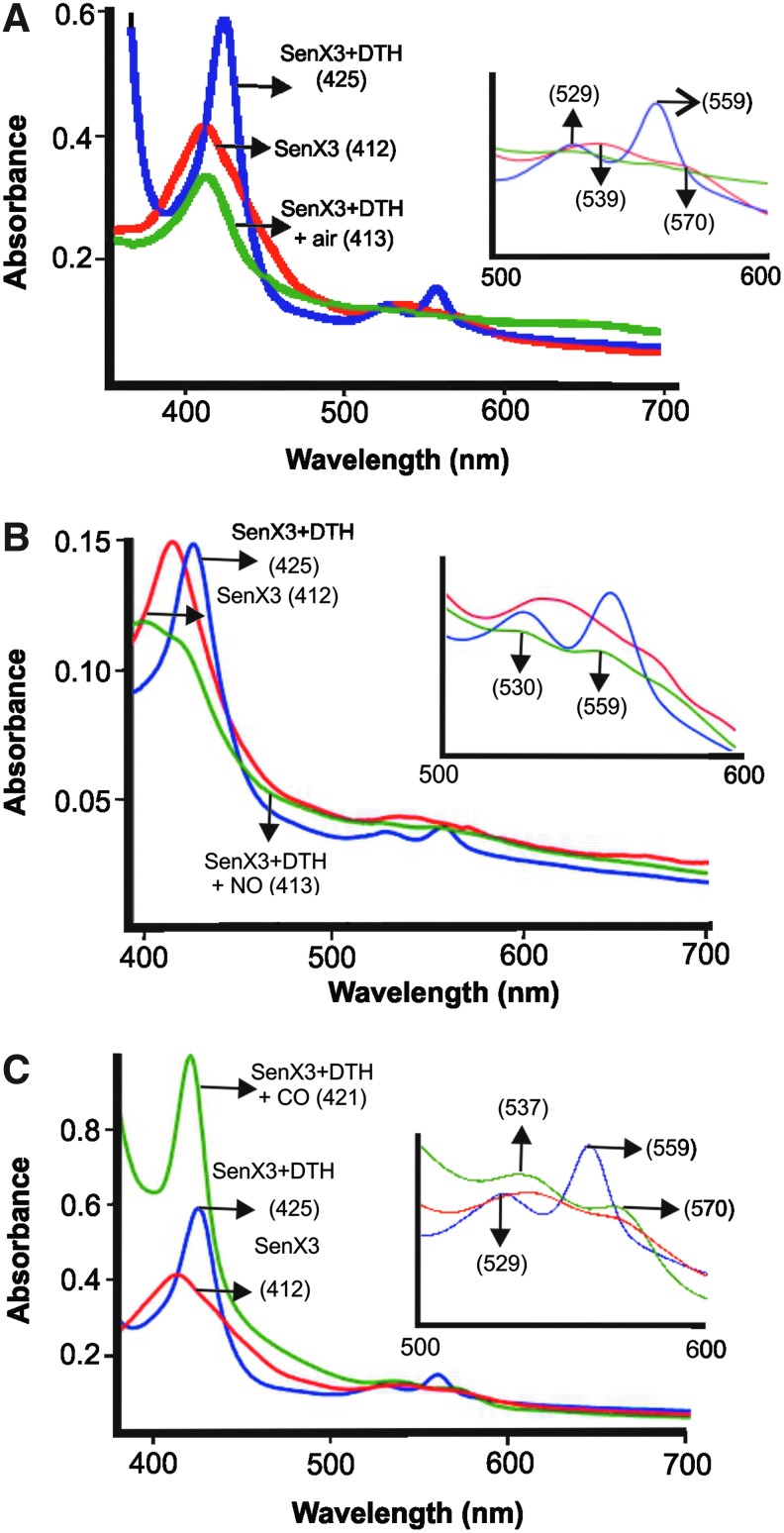
Sequence analysis has revealed that MtbSenX3 possess a PAS domain highly similar to the PAS domain of heme-based hypoxia sensor FixL (17), suggesting that SenX3 could also be a heme-based sensor. To test this hypothesis, we performed a comprehensive biochemical analysis of SenX3. We cloned and purified the full-length SenX3 in the heterologous host Escherichia coli. Purified SenX3 was reddish brown in color (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars), indicating the presence of a co-factor such as heme or an Fe-S cluster bound to the purified protein. Purified SenX3 could be oxidized by ortho-dianisidine in the presence of hydrogen peroxide in polyacrylamide gels (5) (Supplementary Fig. S1B), confirming that SenX3 binds heme. To further characterize the type of heme associated with SenX3, a pyridine hemochromogen assay (1) was performed. The pyridine hemochromogen assay revealed that SenX3 binds to type b heme molecules with a stoichiometry of 0.7:1 (data not shown). Type B heme is often associated with heme-based sensor proteins. Furthermore, UV-visible absorption spectroscopy using purified SenX3 revealed a sharp Soret band at 412 nm, an α band at 570 nm, and a β band at 539 nm (Fig. 1A). These features indicate the presence of a hexa-coordinated, high spin heme bound to SenX3.
FIG. 1.
SenX3 binds heme and is responsive to O2, NO, and CO. (A) SenX3 reacts reversibly with O2. UV-visible absorption spectra of native SenX3 (3 μM in 50 mM sodium phosphate, 200 mM NaCl, and pH 8.0) indicate that a hexa-coordinated heme bound to the protein. Treatment of native SenX3 with a 100-fold molar excess of DTH followed by re-exposure to air leads to reversible spectral changes, as observed with classical hypoxia/O2 sensors. (B, C) NO and CO are ligands of SenX3. DTH-treated SenX3 was exposed to a 50-fold molar excess of NO donor ProliNONOate in an anaerobic glove box, and absorption spectra were recorded (B). DTH-treated SenX3 was exposed to a 100-fold molar excess of CO donor CORM-2, and the absorption spectra were recorded (C). Insets display enlarged images of the 500–600 nm regions. Numbers in parentheses indicate the absorption maxima in nanometers. CO, carbon monoxide; CORM-2, carbon monoxide-releasing molecule-2; DTH, sodium dithionite; NO, nitric oxide; O2, oxygen. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SenX3 interacts with O2, NO, and CO
After establishing that SenX3 is a heme protein, we used UV-visible absorption spectroscopy to analyze whether O2, NO, and CO are ligands of SenX3. Since these diatomic gases primarily interact with deoxy-ferrous form, we treated the aerobically purified SenX3 protein with a 100-fold molar excess of single-electron donor sodium dithionite (DTH). Treatment of SenX3 with DTH in an anaerobic environment caused a red shift in the Soret band from 412 to 425 nm, while β and α produced minor and major bands at 529 and 559 nm, respectively (Fig. 1A and Supplementary Table S1). These spectral changes are consistent with the spectra of the deoxy form of the E. coli direct oxygen sensor (4). When DTH-treated SenX3 was exposed to air for 2 min, the absorption spectrum shifted back to that of the aerobically purified SenX3. This interaction with O2 and DTH was reversible, as observed with classical hypoxia/O2 sensors. To further examine whether SenX3 interacts with NO, we utilized NO donor ProliNONOate that release 2 moles of NO for each mole. To study the effect of NO on SenX3 absorption spectra, DTH-treated SenX3 was exposed to a 50-fold molar excess of NO donor ProliNONOate inside an anaerobic glove box. This exposure resulted in a shift of the Soret band to 413 nm and produced β and α bands at 530 and 559 nm, respectively (Fig. 1B and Supplementary Table S1). These spectral changes are characteristic of the nitrosyl heme complex (21, 25), suggesting that NO forms a nitrosyl heme complex with SenX3. We also exposed SenX3 to an equal amount of spent ProliNONOate. This exposure did not result in any spectral change, ruling out nonspecific effect of the solvent used for ProliNONOate (Supplementary Fig. S2A). Furthermore, we also utilized a 10-fold molar excess of NO generated by ProliNONOate (5-fold molar excess), and similar spectral changes were recorded (Supplementary Fig. S2B). To study the effect of CO on the absorption spectra of SenX3, we utilized carbon monoxide-releasing molecule-2 (CORM-2), which releases 0.9 moles of CO for every mole. Exposure of DTH-treated SenX3 with a 100-fold molar excess of CORM-2 resulted in a shift of the Soret band to 421 nm and β and α bands at 537 and 570 nm, respectively (Fig. 1C and Supplementary Table S1), suggesting the formation of a carbonyl-heme complex (33). We also exposed the DTH-treated SenX3 to equal amounts of DMSO to rule out any effect of the solvent on the SenX3 absorption spectra (Supplementary Fig. S3A). Furthermore, exposure of DTH-treated SenX3 to a 100-fold molar access of CO dissolved in phosphate-buffered saline (PBS) also resulted in similar spectral changes (Supplementary Fig. S3B). Similar spectral changes were observed on exposure of DTH-treated SenX3 with a 10-fold molar excess of CORM-2 (Supplementary Fig. S3C). These data suggest that the SenX3 heme could interact with O2, NO, and CO, and, thus, could be exploited by Mtb to regulate latency and reactivation in response to these diatomic gases.
SenX3 heme is oxidized by O2
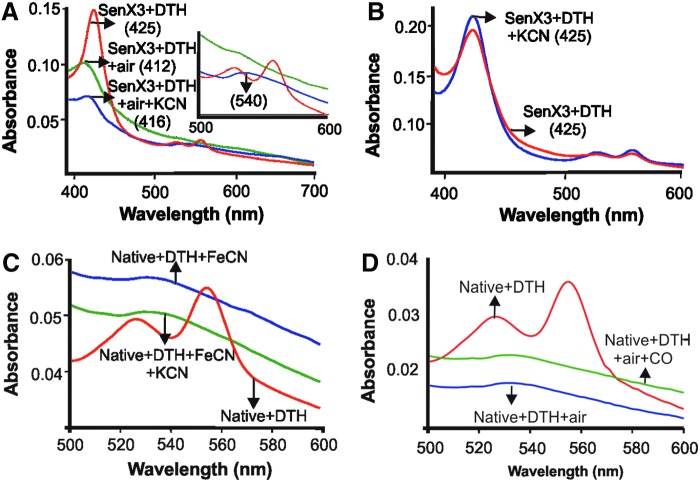
Oxygen-responsive heme proteins can either ligate with O2 to form a stable oxy-ferrous complex (as occurs with DosT) or become oxidized to met or ferric heme (as occurs with DosS). However, ligation with O2 is typically preferred in heme proteins, as conversion to the met form necessitates reducing proteins/factors to create a reversible regulatory circuit. To establish whether the O2 ligates with or oxidizes SenX3, air-exposed SenX3 was treated with potassium cyanide (KCN). CN− only reacts with the met form of heme, forming a met-cyanide complex with a characteristic band at 540 nm (10). The KCN exposure resulted in the shift of the Soret band to 416 nm, with the β (570 nm) and α (539 nm) bands converging into a single band at 540 nm, indicating that exposure to air for 2 min leads to the oxidation of the SenX3 heme group (Fig. 2A). In addition, longer air exposure (up to 2 h) did not result in any detectable spectral changes (Supplementary Fig. S4A, B), suggesting that 2 min of air exposure is sufficient for the oxidation of SenX3 heme. Consistent with these observations, heme bound to the reduced SenX3 did not react with KCN (Fig. 2B). However, on oxidation with Fe(CN)63−, the reduced SenX3 reacted with KCN (Fig. 2C). In agreement with these observations, treating air-exposed SenX3 with Fe(CN)63− did not result in any spectral change (Supplementary Fig. S5), suggesting that air exposure leads to the complete oxidation of the SenX3 heme. Since CO does not react with met-heme, it was used as a tool to reconfirm the redox state of the air-exposed SenX3. Exposure to a 100-fold molar excess of CORM-2 did not alter the absorption spectra of the air-exposed SenX3, confirming that O2 leads to the oxidation of the SenX3 heme (Fig. 2D). Taken together, these data suggest that SenX3 heme is redox sensitive and is oxidized by atmospheric O2.
FIG. 2.
SenX3 is oxidized by O2. (A) SenX3 protein was exposed to air for 2 min after DTH treatment. The air-exposed SenX3 was then treated with a 100-fold molar excess of KCN, which produced a peak at 540 nm, indicating the presence of a met-CN− complex. Insets display enlarged images of the 500–600 nm regions. (B) DTH-treated SenX3 does not react with KCN. Exposure of DTH-treated SenX3 to a 100-fold molar excess of KCN does not result in any spectral changes, but it only reacts with KCN after treatment with a 500-fold molar excess of the chemical oxidant Fe(CN)63− (C). (D) Air-exposed SenX3 does not react with CO. Air-exposed SenX3 was treated with a 100-fold molar excess of CO donor CORM-2. It is very well known that CO does not react with met-heme proteins. In agreement with this, we did not observe any spectral changes in the air-exposed SenX3 on CO treatment. Numbers in parentheses denote absorption maximum in nanometers. KCN, potassium cyanide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SenX3 is activated by O2-mediated oxidation
After identifying that O2 rapidly oxidizes SenX3, we further examined whether the oxidation of SenX3 by O2 affects the autophosphorylation of the sensor kinase SenX3. Aerobically purified SenX3 was divided into two aliquots in an anaerobic glove box. One aliquot was treated with DTH, while the other was left untreated. Both of these aliquots were then examined for autophosphorylation at his 167 through the incorporation of radiolabeled phosphate from [γ-32P] ATP. We observed that aerobically purified met-SenX3 possesses greater autokinase activity (visualized by a band with a high intensity arising due to higher incorporation of radiolabeled phosphate) than the deoxy-ferrous form (Fig. 3A), implying that the met form of SenX3 is the “active or on state.” These data also imply that the deoxy-ferrous form of SenX3 is an “inactive or off state.” To further analyze whether O2-mediated oxidation leads to enhanced SenX3 kinase activity, deoxy-ferrous SenX3 was anaerobically divided into two aliquots. One aliquot was exposed to air for 2 min, and the other was left untreated in an anaerobic glove box. The air exposure resulted in enhanced phosphorylation compared with the deoxy-ferrous SenX3 (Fig. 3B). To further validate that the redox state of SenX3 heme iron regulates its autokinase activity, we utilized the chemical oxidant Fe(CN)63− instead of O2 to oxidize the SenX3 heme iron, and determined the effect of chemical oxidation on autokinase activity. Oxidation with Fe(CN)63− enhanced the autokinase activity of SenX3, similar to O2 (Fig. 3C). In summary, these data demonstrate that SenX3 is activated by the presence of O2 through the oxidation of the redox-sensitive heme.
FIG. 3.
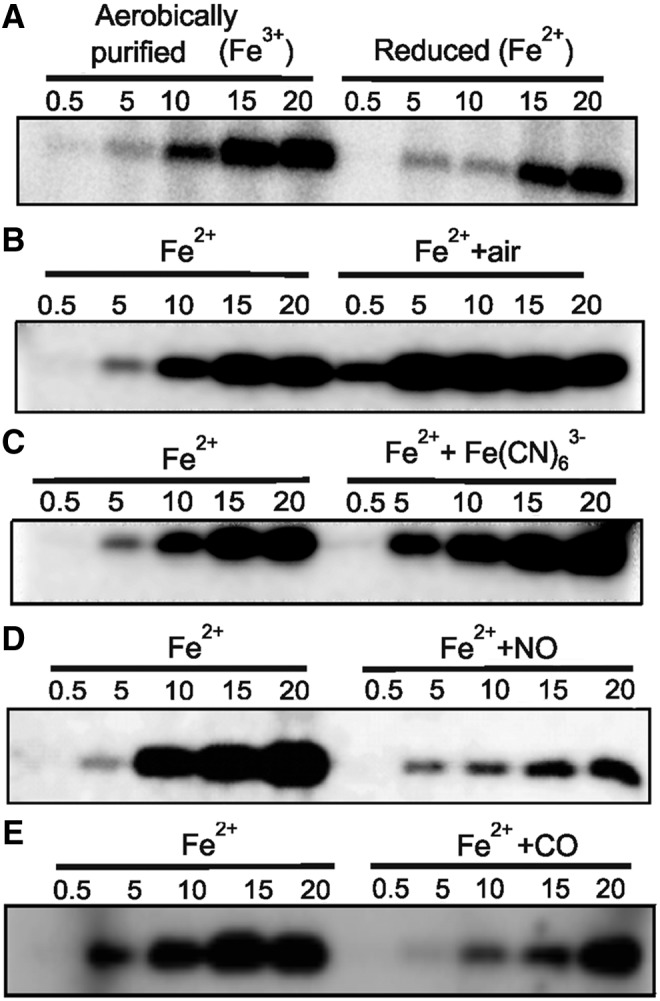
Oxygen-catalyzed oxidation of SenX3 heme activates its autokinase activity. (A) Purified native SenX3 was exposed to DTH (Fe2+ form) and analyzed for autokinase activity. The treatment of 5 μM native protein with the single electron donor DTH (100-fold molar excess) generates deoxy-ferrous heme ligated to SenX3. This change in the redox state of heme resulted in a significant inhibition of kinase activity. (B) The autokinase activity of DTH-reduced SenX3 (5 μM in 50 mM sodium phosphate, 200 mM NaCl, and pH 8.0) was compared with that of the protein exposed to air for 2 min. The activity of air-exposed SenX3 was found to be significantly enhanced compared with ferrous SenX3. (C) A 3 μM solution of DTH-reduced SenX3 in 50 mM sodium phosphate, 200 mM NaCl, pH 8.0 was exposed to a 100-fold molar excess of Fe(CN)63− to generate met-SenX3 in the absence of O2. The chemical oxidation by Fe(CN)63− in the absence of O2 enhanced the autokinase activity of SenX3, similar to the air-oxidized protein. Numbers on each lane indicate the incubation time at 37°C in minutes. (D–E) A 6 μM solution of SenX3 in 50 mM sodium phosphate, 200 mM NaCl, pH 8.0 was exposed to a 100-fold molar excess of DTH to create the deoxy-ferrous form (Fe2+), followed by treatment with a 50-fold molar excess of NO donor ProliNONOate (D) and a 100-fold molar access of CO donor CORM-2 (E). Both NO and CO inhibited the autophosphorylation of SenX3. The numbers on each lane indicate the incubation time at 37°C in minutes.
SenX3 is inhibited by NO and CO
In addition to hypoxia, NO and CO also play an important role in the induction of the Dos/Dormancy regulon of Mtb. Physiologically relevant low concentrations of NO have been shown to be capable of inducing the nonreplicating persistent state in Mtb (34), and the absence of NO increases the susceptibility of mice to TB (12). Since SenX3 heme is capable of forming nitrosyl-heme and carbonyl-heme complexes with NO and CO, we next determined whether the formation of these complexes modulates the kinase activity of SenX3. Native SenX3 was reduced with a 100-fold molar excess of DTH and then either treated or not treated with a 100-fold molar excess of NO (generated by NO donor ProliNONOate) or CO (released by CO donor CORM-2). This was followed by the analysis of the autokinase activity of each sample. Exposure to NO (Fig. 3D) and CO (Fig. 3E) inhibited the kinase activity of SenX3. We also observed that the spent ProliNONOate does not inhibit the autokinase activity of the SenX3 (Supplementary Fig. S6A). We also confirmed the inhibition of the autokinase activity of the SenX3 using the CO released from the PBS saturated with CO instead of CO donor CORM-2 (Supplementary Fig. S6B). We also further established that DMSO without CORM-2 does not inhibit the autokinase activity of SenX3 (Supplementary Fig. S6C). In summary, NO and CO inhibit the kinase activity of the sensor kinase SenX3.
NO and CO lock SenX3 in an inactive state
Heme proteins have higher affinities for NO and CO along with considerably lower dissociation rates. Therefore, we tested whether the nitrosyl-heme and carbonyl-heme complexes of SenX3 are stable in the presence of ambient O2 levels. Nitrosyl-heme and carbonyl-heme complexes of Senx3 were formed inside the glove box and then exposed to air. These complexes were found to be stable, locking the SenX3 in the “off” state, even in the presence of the kinase-activating O2 (Fig. 4A, B). To test this possibility, the autokinase activity of nitrosylated (generated by exposure of DTH-treated SenX3 to NO donor ProliNONOate) and carbonylated SenX3 (generated by exposure of DTH-treated SenX3 to CO donor CORM-2) was measured after air exposure. We observed that SenX3 with a heme-NO complex (Fig. 4C) or a heme-CO complex (Fig. 4D) remained in the “off state” for kinase activity, even in the presence of air. These data suggest a plausible mechanism in which the reactivation of Mtb growth by O2 could be inhibited by the presence of NO and CO. However, the inhibition of SenX3 kinase activity by NO and CO is dependent on the reduction of met SenX3 by unidentified factor/s, as the kinase activity of air-exposed SenX3 was not inhibited on exposure to NO or CO (Fig. 4E, F, respectively). In summary, the presence of NO and CO could lock SenX3 in an inactive state that can persist even in the presence of air.
FIG. 4.
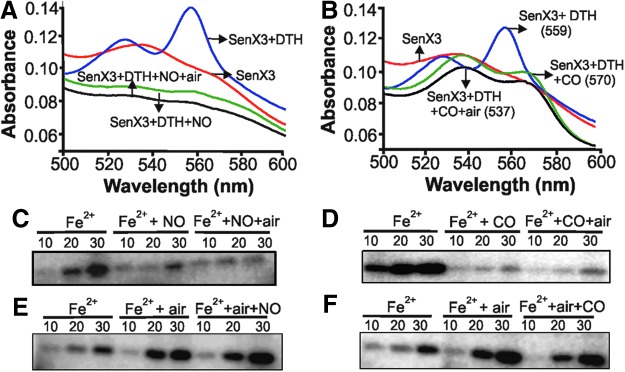
The carbonyl-heme and nitrosyl-heme complexes of SenX3 are stable in the presence of O2, locking SenX3 in the inactive conformation. A 3 μM solution of SenX3 in 50 mM sodium phosphate, 200 mM NaCl, pH 8.0 was treated with a 100-fold molar excess of DTH followed by treatment with a 50-fold molar excess of NO donor ProliNONOate (A) and a 100-fold molar access of CO donor CORM-2 (B), and the absorption spectra were recorded. CO and NO form stable nitrosyl and carbonyl-heme complexes, respectively, thus locking the protein in an inactive conformation. Exposure of DTH-treated SenX3 to NO (using ProliNONOate) (C) and CO (using CORM-2) (D) locks SenX3 in the inhibited state, which remains unchanged on exposure to air. (E, F) Inhibition of SenX3 kinase activity by NO (donated by ProliNONOate) and CO (released by CORM-2) depends on reductant. A 3 μM solution of SenX3 protein in 50 mM sodium phosphate, 200 mM NaCl, pH 8.0 was treated with a 100-fold molar excess of DTH followed by exposure to air for 2 min. The air-exposed protein was then treated with a 100-fold molar excess of NO (donated by ProliNONOate) (E) or CO (released by CORM-2) (F), and the kinase activities were measured. NO and CO did not inhibit the kinase activity of air-exposed SenX3. Numbers on each lane indicate the time of incubation at 37°C in minutes. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
SenX3 is required for the reactivation of growth in response to the restoration of aerobic conditions
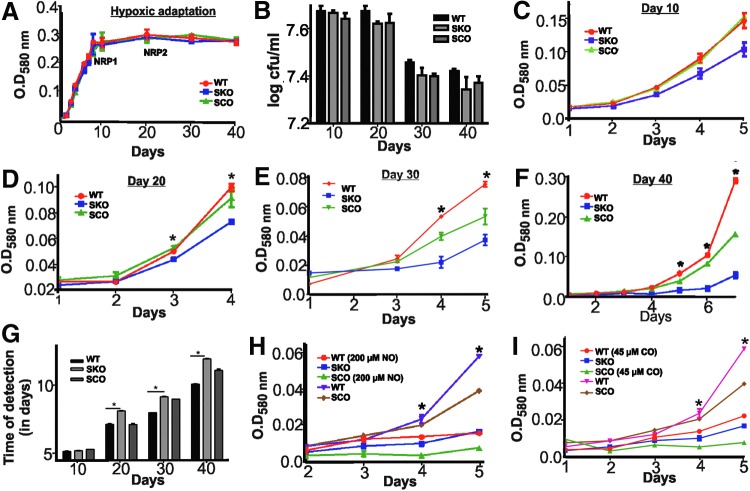
Since the kinase activity of SenX3 is enhanced by O2 and inhibited by NO and CO, SenX3 could activate the replication of Mtb in response to oxygen. To test this hypothesis, we used the “Wayne model” of nonreplicating persistence. The Wayne model enables the bacteria to gradually consume O2 and enter a nonreplicative state with the physiological characteristics of latent bacteria. In the same model, re-oxygenation leads to the resumption of growth, indicating reactivation (36). To analyze the role of SenX3 in hypoxia-induced dormancy and oxygen-activated reactivation, we analyzed the growth of the wild-type Mtb strain, the senX3 mutant strain, and the complemented senX3 mutant strain in the Wayne model of dormancy and reactivation. The SenX3 mutant strain and its complement were kind gifts from Dr. Buxton (Mycobacterial Research, National Institute for Medical Research, London), and their construction has already been described in detail (17). The senX3 mutant strain was found to be able to enter and maintain the NRP1 and NRP2 stages of hypoxic adaptation, similar to the wild-type and the complemented mutant strains (Fig. 5A, B); however, the regrowth of the senX3 mutant was significantly attenuated on the re-oxygenation of the culture at days 20, 30, and 40 (Fig. 5D–F) compared with the wild-type strain. Episomal overexpression of SenX3 in the mutant strain resulted in a significant decrease in the attenuation of regrowth in response to oxygen, further confirming the role of SenX3 in the oxygen-controlled regrowth of Mtb. A minor but statistically insignificant difference in the reactivation of the senX3 mutant strain was observed on the re-oxygenation of cultures at day 10 (Fig. 5C). These results were verified using the BACTEC MGIT 960 mycobacterial growth detection system (Fig. 5G). Interestingly, although the SenX3 mutant is attenuated, it is capable of resuming delayed reactivation. The delayed resumption of growth of the senX3 mutant on re-oxygenation suggests the presence of alternate SenX3-independent pathways for Mtb reactivation. In summary, SenX3 plays an important role in the O2-induced resumption of growth of Mtb.
FIG. 5.
SenX3 is required for the regrowth of Mtb in response to the restoration of ambient oxygen levels. (A) Wild-type, senX3 mutant, and senX3 complementing strains were found to have similar growth profiles in the Wayne model. (B) No significant differences in colony-forming units between wild-type, mutant, and complementing strains were observed during hypoxic adaptation after days 10, 20, 30, and 40. After 10 days (C), 20 days (D), 30 days (E), and 40 days (F) of hypoxic adaptation, the wild-type, SenX3 mutant, and SenX3 complementing strains were exposed to O2 and allowed to resume growth. (G) The reactivation of the wild-type, ΔsenX3 mutant, and complemented strains was assessed based on the time required for the development of a detectable growth signal by the BACTEC MGIT 960 system. A consistent and statistically significant delay in regrowth after the restoration of O2 was observed in all cultures of the ΔsenX3 mutant (at 20, 30, 40, and 130 days) compared with the wild type. However, episomal expression of SenX3 in the mutant strain led to a decrease in that attenuation. WT, SKO, and SCO indicate the wild-type, senX3 knockout Mtb, and senX3 complementing Mtb, respectively. (H, I) The results of the kinase assay were further validated in the Wayne model by exposing the hypoxic culture at day 40 to 200 μM of NO (generated by DETA NONOate) (H) and 50 μM of CO (released by CORM-2) (I) in the presence of ambient oxygen. Exposure of the wild-type and complemented strains to NO and CO leads to an arrest in regrowth in response to ambient oxygen similar to that observed in the SenX3 mutant strain. *p<0.05. Statistical significance was determined using Student's t-test. These figures (A–I) are representative of at least three independent experiments performed in triplicate. Mtb, Mycobacterium tuberculosis; SCO, Senx3 complementing strain of Mtb; SKO, SenX3 knockout strain of Mtb. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NO and CO inhibit the resumption of mycobacterial growth in the presence of O2
Since the biochemical properties of SenX3 suggest that NO or CO binding can lock SenX3 in a kinase inactive form, we hypothesized that NO and CO could arrest the resumption of mycobacterial growth in response to the presence of oxygen. To test this hypothesis, we exposed the hypoxic cultures (at day 40) of the wild-type Mtb strain and the complemented mutant strain to ambient oxygen levels in the presence or absence of micro-molar concentrations of CO (released by the CO donor CORM-2) and NO released by the NO donor (DETA NONOate). Interestingly, the presence of signaling concentrations of CO and NO arrested the regrowth of the wild-type and complemented strains in the presence of ambient oxygen (Fig. 5H, I). The growth patterns of the wild-type and complemented strains in the presence of NO and CO were similar to those of the senX3 mutant strain, suggesting that SenX3 is a key regulator of mycobacterial replication in response to O2, NO, and CO (Fig. 5H, I). To further rule out nonspecific effects of solvent used for dissolving DETA NONOate, we also utilized spent DETA NONOate. We observed that the spent DETA NONOate did not inhibit the regrowth of Mtb, suggesting that the observed effects arise specifically from NO (Supplementary Fig. S7A). We also observed that CO dissolved in PBS could inhibit the regrowth of the Mtb in response to ambient oxygen (Supplementary Fig. S7B), while the DMSO without the CO donor CORM-2 did not inhibit the regrowth (Supplementary Fig. S7C), suggesting that the inhibition specifically results from the presence of CO.
Discussion
While O2, NO, and CO levels have long been recognized to regulate the latency and reactivation of Mtb, the sensors utilized by Mtb to monitor the levels of these diatomic gases and the pathways that they modulate have remained poorly characterized. In this study, we have demonstrated that the virulence factor SenX3 is an O2 sensor which could play an important role in the O2-induced reactivation of mycobacterial growth. Further, SenX3 was demonstrated to act as a three-way switch with three levels of activity: The most active met form is generated by O2-mediated oxidation of SenX3, the deoxy-ferrous form has moderate activity, and the nitrosylated and carbonylated forms possess the least activity. Evidence in this study delineates a plausible mechanism explaining the reactivation of TB diseases in humans after many years of latent infection. Previously, NO and CO were thought to be involved only in inducing the nonreplicating persistent state, but this study further implicates NO and CO in the inhibition of mycobacterial growth from the nonreplicating state.
Levels of O2 play a critical role in whether bacteria enter a latent state in which the bacteria remain hidden from the hostile immune system or resume active growth and induce active TB disease. Hypoxia is known to induce an NRP state, while normoxic levels support active growth. Thus, it is logical to think that the initiation and maintenance of latency would involve a hypoxia sensor, whereas reactivation would require an O2 sensor. The hypoxia sensor would inhibit the en-mass biosynthesis of DNA, RNA, and protein and activate alternative metabolic pathways that could provide energy and biochemical resources for the sustenance of life. Conversely, O2 sensors would overcome the inhibition of the biosynthesis of DNA, RNA, and proteins and activate metabolic pathways to generate the energy and resources required for replication, transcription, translation, and cell division. Previous studies have demonstrated that the kinase activities of DosS and DosT are inhibited by O2 and that the deoxy-ferrous state is the “on state” for these proteins (10, 29). These observations suggest that DosS and DosT are hypoxia sensors. In line with these observations, growth defects were observed in DosR mutants for survival in hypoxia (3, 11). However, before our study, no true O2 sensor that modulates the initiation of mycobacterial replication had been described. The evidence generated in this study suggests that the autokinase activity of SenX3 is activated by O2 through the oxidation of heme iron, suggesting that SenX3 is a redox-dependent O2 sensor which could regulate the metabolic pathways required for the transition from the nonreplicating state into the actively dividing state. To analyze the role of SenX3 in latency and reactivation, we have utilized the Wayne model of nonreplicating persistence and regrowth. This model is the gold standard for studying hypoxia-induced nonreplicating persistence and subsequent oxygen-induced reactivation. In the Wayne model, the SenX3 mutant was found to be able to attain the NRP1 and NRP2 states in response to hypoxia, but its resumption of growth on the restoration of oxygen in the hypoxic cultures was significantly attenuated. These observations directly implicate the SenX3-RegX3 system in the regulation of the mycobacterial replication and metabolic pathways governing the resumption of Mtb growth. This study is the first step toward a systems-level understanding of Mtb reactivation and will pave the way for the identification of reactivation-specific metabolic pathways. Previous studies have identified that the SenX3-RegX3 system plays an important role in regulating protein synthesis, respiration, and aerobic metabolism (15, 20). Furthermore, the SenX3-RegX3 system is also induced on reaeration of hypoxic cultures (27). In agreement with these observations, our biochemical analysis and in vivo data suggest that SenX3 facilitates the transition from a nonreplicating state into an actively replicating state. However, SenX3 is not the only regulator of the reactivation of growth in response to the restoration of aerobic conditions, as the senX3 mutant can also be reactivated after a significant delay. This study is the first to establish the molecular basis of oxygen-induced reactivation of mycobacterial growth, and it holds promise to explain the reactivation of TB inside the human host. However, more research work is required to establish the role of SenX3-RegX3 in oxygen-induced reactivation inside the host. This task is especially challenging given that animal models of TB do not mimic natural latency and reactivation. The role of SenX3 in reactivation is supported by the observation that the growth and persistence of SenX3-RegX3 mutants are attenuated in the host (15, 17). Note that a number of factors apart from oxygen levels may modulate the latency and reactivation inside the host, making it difficult to examine the specific role of SenX3-RegX3 in oxygen-modulated latency and reactivation inside the host.
The presence of NO and CO could also induce the nonreplicating persistent state of Mtb, and, in principle, could inhibit the reactivation of growth (8, 13, 28, 34). In agreement with this hypothesis, SenX3 autokinase activity is strongly inhibited by NO and CO, thereby suggesting that the presence of NO and CO could inhibit the reactivation of Mtb growth. In this study, we have utilized 50 μM CO and 200 μM of NO released from the established donors of CO (CORM-2) and NO (DETA NONOate). Similar concentrations of NO (13, 35) and CO (8, 10, 28) were earlier used to establish that low concentrations of these diatomic gases specifically induce the Dos dormancy regulon and do not lead to initiation of the nonspecific stress pathway. Interestingly, in this study, we have observed that NO-heme and CO-heme complexes of SenX3 are highly stable and are able to inhibit the autokinase activity of SenX3, even in the presence of O2. This study has further demonstrated that the resumption of growth in response to ambient levels of oxygen is arrested by NO and CO in the wild-type strain and the SenX3 mutant strain with an episomal copy of the SenX3. These studies are in agreement with the observation that NO can even inhibit mycobacterial growth in the presence of ambient O2 levels (34). These properties of SenX3 are different from those of the hypoxia sensors DosS and DosT (8). NO and CO can lock DosS and DosT in the active confirmation, even in the presence of ambient O2 levels, thereby activating pathways that facilitate the transition from the active growth state into the nonreplicating persistent state. These studies establish a new paradigm of dormancy and reactivation. In this paradigm, hypoxia, NO, and CO induce DosS and DosT and inhibit SenX3 to facilitate the transition into the nonreplicating persistent state. Restoration of aerobic conditions inhibits DosS and DosR and induces SenX3 to facilitate the transition into the actively replicating state (Fig. 6).
FIG. 6.
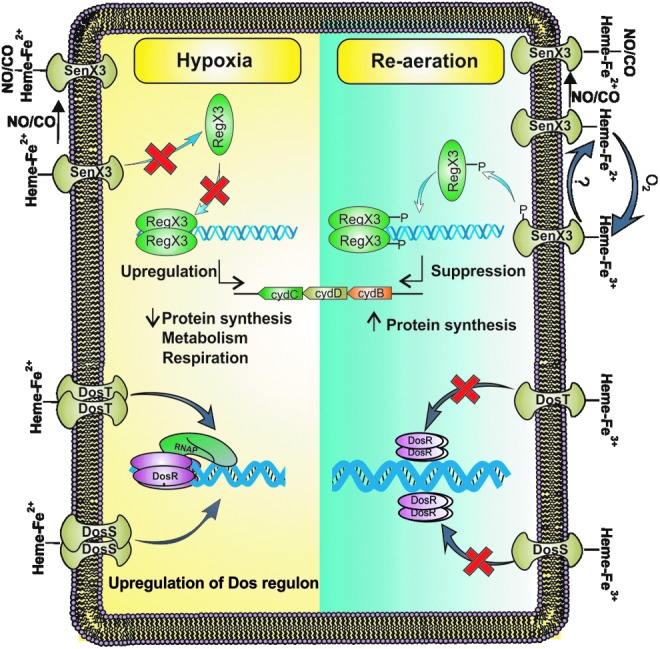
Model depicting the role of SenX3 in the reactivation of Mtb in response to O2. The current paradigm of oxygen sensing and mycobacterial response suggests that hypoxia is sensed by the heme-based sensors DosS and DosT. These sensors relay the signal to DosR, which induces the DosR regulon on phosphorylation. The DosR regulon plays a critical role in the hypoxia-induced transition into nonreplicating persistence. This model further suggests that apart from inducing DosS and DosT, hypoxia also inhibits senX3 (as demonstrated in this study). NO and CO also induce DosS and DosT while inhibiting SenX3 kinase activity. While ambient levels of oxygen induce SenX3 kinase activity, they simultaneously inhibit the kinase activity of DosS and DosT. SenX3 relays this signal to RegX3, which regulates the replication and respiration of Mtb. This model further suggests that the presence of NO and CO could inhibit the kinase activity of SenX3, thus inhibiting replication and activating the DosR regulon through the activation of DosS and DosT. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In the model presented earlier (Fig. 6), SenX3-mediated reactivation can also be blocked by the abundance of still unknown factors that could reduce the SenX3 heme. It is well established that the intracellular environment of Mtb becomes reducing on the inhibition of respiration or growth by fatty acids or culture under hypoxic conditions (16, 34). Therefore, we hypothesize that under these conditions, the accumulated reductants such as NADH, ubiquinol, cytochrome C, FADH, Acetyl CoASH, and mycothiol could reduce SenX3 and then maintain it in the reduced form with a low level of activity, thus inhibiting SenX3-mediated reactivation. This model also predicts the control of reactivation via the reduction of SenX3 by unidentified proteins, thus capturing the level of complexity employed by mycobacteria to control latency and reactivation.
Some previous studies have also linked the SenX3-RegX3 system with inorganic phosphate (Pi) sensing (6, 7, 18, 31). Phosphate limiting conditions are known to induce nonreplicating persistence in Mtb. The ability of Mtb to survive under phosphate limiting conditions has been attributed to the SenX3-RegX3 system (18). Furthermore, RegX3 was demonstrated to bind directly with the promoter of the phosphate transporter pstS and secreted alkaline phosphatase phoA (6). Although these studies suggest that the SenX3-RegX3 system may be involved in the regulation of intracellular Pi levels, no conclusive evidence has been presented demonstrating that SenX3 autokinase activity is modulated by Pi, and more research work is required in this area. It is tempting to speculate that intracellular Pi is linked to Mtb metabolism and may influence the reactivation of Mtb via SenX3-RegX3.
In conclusion, we have demonstrated that SenX3 is a heme protein and that exposure to O2 leads to the oxidation of the ferrous heme into ferric heme, suggesting that SenX3 is a redox sensor (as depicted in the model shown in Fig. 6). We have further shown that O2-mediated oxidation leads to the activation of the kinase activity of SenX3, while binding with NO and CO leads to the inhibition of the kinase activity of SenX3. We have also demonstrated that the SenX3 mutant of Mtb is capable of entering latency and surviving during hypoxia, but that its reactivation of replication in response to the restoration of ambient O2 is significantly attenuated. These findings are important in that they establish a mechanism for the regulation of Mtb reactivation in response to O2.
Materials and Methods
Cloning, expression, and purification of SenX3
SenX3 was PCR amplified from the genomic DNA of Mtb H37Rv and cloned into the pET-15B expression vector to obtain His-tagged full-length SenX3. This vector was transformed into Rosetta (DE3) pLysS (Novagen) competent cells for protein expression. Rosetta (DE3) pLysS cells containing the expression vector were grown in 2 L of Luria Bertani (LB) medium containing 75 μg/ml of ampicillin to an OD600 of 0.8–1.0 at 37°C, followed by the addition of 20 μM hemin and a 1 h incubation period at room temperature. SenX3 expression was induced by the addition of 1 mM IPTG at 37°C for 4 h. The cells were pelleted at 5000 g for 8 min, then resuspended in lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 10 mM imidazole, 1 mg/ml lysozyme, and pH 8.0), and incubated on ice for 30 min. The cells were lysed using sonication (30-s on and 30-s off pulses), and then centrifuged at 15,000 g for 15 min to remove the membrane fraction. The Ni-NTA resin (Qiagen) was equilibrated with wash buffer (50 mM sodium phosphate, 300 mM NaCl, 20 mM imidazole, and pH 8.0), and the supernatant containing soluble protein was loaded onto the column. After loading, the column was washed with wash buffer (10 bed volumes), and the recombinant protein was eluted into elution buffer (50 mM sodium phosphate, 300 mM NaCl, 150 mM imidazole, and pH 8.0) followed by running a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. The purified protein was dialyzed in buffer (50 mM sodium phosphate, 200 mM NaCl) to eliminate imidazole.
Spectroscopic analysis of SenX3
Purified SenX3 protein (3 μM) was deoxygenated for 15 min by constant purging with argon gas, transferred into an anaerobic glove box (mBRAUN), and divided into aliquots. The aliquots were treated with a 100-fold molar excess of sodium dithionite (DTH; Sigma-Aldrich), KCN, Fe(CN)63−, ProliNONOate (Cayman Chemical) (dissolved in 0.01 N NaOH), and tricarbonylchlororuthenium(II) dimer (CORM-2; Sigma-Aldrich) (dissolved in DMSO) when required. The protein was then analyzed for absorption in the wavelength range from 200 to 700 nm. We have also used CO dissolved in PBS for experiments involving analysis of effect of CO on SenX3 (see Supplementary Note I section). As a control of ProliNONOate, we also utilized spent ProliNONOate as an appropriate control (see Supplementary Note II section).
In vitro phosphorylation assay
The recombinant His-tagged SenX3 (6 μg) was assayed for autokinase activity in a reaction mixture containing 0.5 μCi {γ32-P} ATP (BRIT), 10 mM MgCl2, and 25 mM Tris-HCl (pH 7.6) in a final volume of 20 μl. The reaction was incubated at 37°C for 0–20 min. The reaction was stopped by the addition of 10 μl 3×Laemmli buffer, boiled at 100°C for 10 min, and loaded onto a 15% SDS-PAGE gel. The gel was analyzed using a PhosphorImager (FLA-9000; Fujifilm). All of the assays were performed inside an anaerobic glove box.
Wayne model
The growth of Mtb H37Rv, the SenX3 mutant, and the complemented mutant strains under hypoxic conditions and their regrowth on the restoration of oxygen were analyzed using the Wayne model as previously described (36). The nonreplicating persistence in response to hypoxic adaptation and the regrowth on the restoration of ambient oxygen were monitored after 10, 20, 30, and 40 days of dormancy. To monitor the reactivation, 10-, 20-, 30-, and 40-day-old hypoxic cultures of wild-type and ΔSenX3 strains were exposed to fresh medium and ambient oxygen levels and allowed to resume growth. Growth was measured every day by measuring the absorbance at 580 nm. The growth was also monitored using BACTEC MGIT 960. The effect of NO and CO on the regrowth was analyzed using NO donor DETA NONOate or CO-releasing molecules CORM-2. Appropriate solvent controls were used as and when required.
Heme staining of polyacrylamide gels
The heme staining was performed using previously described methods (5). Briefly, the purified protein was electrophoresed using SDS-PAGE. O-dianisidine (0.2 g; Sigma-Aldrich) was dissolved in 20 ml of acetic acid, and 160 ml of distilled water was added to this solution. After electrophoresis, the gel was washed in 200 ml of 12.5% TCA for 30 min and then washed with water for 30 min. Then, 20 ml of 0.5 M sodium citrate buffer (pH 4.4) and 0.4 ml of 30% H2O2 were added to the o-dianisidine solution, and this solution was poured on the gel, which was placed on a shaker until the bands developed.
Statistical analysis
The results are expressed as the mean±SE and were derived from at least three independent biological experiments performed in triplicate. The Student's t-test was used to analyze the statistical significance.
Supplementary Material
Abbreviations Used
- CO
carbon monoxide
- CORM-2
carbon monoxide-releasing molecule-2
- DTH
sodium dithionite
- K3 Fe(CN)6
potassium ferricyanide (III)
- KCN
potassium cyanide
- Mtb
Mycobacterium tuberculosis
- NO
nitric oxide
- O2
oxygen
- PBS
phosphate-buffered saline
- Pi
inorganic phosphate
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SCO
Senx3 complementing strain of Mtb
- SKO
SenX3 knockout strain of Mtb
- TB
tuberculosis
Acknowledgments
The Mtb SenX3 KO strain and its respective complementing strain used in this study were kind gifts from Dr. Roger S. Buxton, National Institute of Medical Research, London. The authors wish to acknowledge help from Dr. Sanjay Gupta with UV-Visible absorption spectroscopy. N.S. is grateful to the CSIR for SRF. This work was supported by a grant from the Department of Science and Technology (SR/SO/BB-0037/2013). A.K. is supported by DBT (BT/PR15097 and BT/PR15086/GBD/27/307/2011) and CSIR (OLP-70, BSC0119F, HCP0001, BSC0211E, and BSC0210G) research grants. These funders had no role in the study design, data collection and analysis, decision to publish, or preparation of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Berry EA. and Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem 161: 1–15, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Bhat SA, Singh N, Trivedi A, Kansal P, Gupta P, and Kumar A. The mechanism of redox sensing in Mycobacterium tuberculosis. Free Radic Biol Med 53: 1625–1641, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Boon C. and Dick T. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J Bacteriol 184: 6760–6767, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado-Nixon VM, Gonzalez G, and Gilles-Gonzalez MA. Dos, a heme-binding PAS protein from Escherichia coli, is a direct oxygen sensor. Biochemistry 39: 2685–2691, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Francis RT., Jr. and Becker RR. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem 136: 509–514, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Glover RT, Kriakov J, Garforth SJ, Baughn AD, and Jacobs WR., Jr.The two-component regulatory system senX3-regX3 regulates phosphate-dependent gene expression in Mycobacterium smegmatis. J Bacteriol 189: 5495–5503, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James JN, Hasan ZU, Ioerger TR, Brown AC, Personne Y, Carroll P, Ikeh M, Tilston-Lunel NL, Palavecino C, Sacchettini JC, and Parish T. Deletion of SenX3-RegX3, a key two-component regulatory system of Mycobacterium smegmatis, results in growth defects under phosphate-limiting conditions. Microbiology 158: 2724–2731, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, Kramnik I, Agarwal A, and Steyn AJ. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem 283: 18032–18039, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.This reference has been deleted.
- 10.Kumar A, Toledo JC, Patel RP, Lancaster JR, Jr., and Steyn AJ. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc Natl Acad Sci U S A 104: 11568–11573, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, and Voskuil MI. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol 192: 1662–1670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, and Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 94: 5243–5248, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Jr., and Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol 5: 637–648, 2003 [DOI] [PubMed] [Google Scholar]
- 14.This reference has been deleted.
- 15.Parish T, Smith DA, Roberts G, Betts J, and Stoker NG. The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149: 1423–1435, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Rao SP, Alonso S, Rand L, Dick T, and Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 105: 11945–11950, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickman L, Saldanha JW, Hunt DM, Hoar DN, Colston MJ, Millar JB, and Buxton RS. A two-component signal transduction system with a PAS domain-containing sensor is required for virulence of Mycobacterium tuberculosis in mice. Biochem Biophys Res Commun 314: 259–267, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rifat D, Bishai WR, and Karakousis PC. Phosphate depletion: a novel trigger for Mycobacterium tuberculosis persistence. J Infect Dis 200: 1126–1135, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Roberts DM, Liao RP, Wisedchaisri G, Hol WG, and Sherman DR. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J Biol Chem 279: 23082–23087, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts G, Vadrevu IS, Madiraju MV, and Parish T. Control of CydB and GltA1 expression by the SenX3 RegX3 two component regulatory system of Mycobacterium tuberculosis. PLoS One 6: e21090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romberg RW. and Kassner RJ. Nitric oxide and carbon monoxide equilibria of horse myoglobin and (N-methylimidazole)protoheme. Evidence for steric interaction with the distal residues. Biochemistry 18: 5387–5392, 1979 [DOI] [PubMed] [Google Scholar]
- 22.Rustad TR, Harrell MI, Liao R, and Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One 3: e1502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rustad TR, Sherrid AM, Minch KJ, and Sherman DR. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol 11: 1151–1159, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Sardiwal S, Kendall SL, Movahedzadeh F, Rison SC, Stoker NG, and Djordjevic S. A GAF domain in the hypoxia/NO-inducible Mycobacterium tuberculosis DosS protein binds haem. J Mol Biol 353: 929–936, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Sharma VS, Traylor TG, Gardiner R, and Mizukami H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry 26: 3837–3843, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, and Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc Natl Acad Sci U S A 98: 7534–7539, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherrid AM, Rustad TR, Cangelosi GA, and Sherman DR. Characterization of a Clp protease gene regulator and the reaeration response in Mycobacterium tuberculosis. PLoS One 5: e11622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiloh MU, Manzanillo P, and Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3: 323–330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa EH, Tuckerman JR, Gonzalez G, and Gilles-Gonzalez MA. DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis. Protein Sci 16: 1708–1719, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor BL. and Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 63: 479–506, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tischler AD, Leistikow RL, Kirksey MA, Voskuil MI, and McKinney JD. Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect Immun 81: 317–328, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi A, Singh N, Bhat SA, Gupta P, and Kumar A. Redox biology of tuberculosis pathogenesis. Adv Microb Physiol 60: 263–324, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Tsubota S, Ishikawa T, Tamura M, and Yamazaki I. The reaction of carbon monoxide with myoglobin in solution, in an amorphous state, and in crystals. J Biochem 94: 257–265, 1983 [DOI] [PubMed] [Google Scholar]
- 34.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, and Schoolnik GK. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198: 705–713, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voskuil MI, Visconti KC, and Schoolnik GK. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis 84: 218–227, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Wayne LG. and Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64: 2062–2069, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wayne LG. and Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol 55: 139–163, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.