Abstract
The objective of this study was to explore the effects of dendritic cells (DCs) from hepatitis B virus (HBV) transgenic mice-stimulated autologous lymphocytes on in vitro HBV replication. DCs from HBV transgenic mice were induced to maturity by lipopolysaccharide, followed by incubation with hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBcAg) in vitro. Mature DCs and autologous lymphocytes were co-stimulated to form specific sensitized immune effector cells (IEC), which were then co-cultured with the human hepatoma cell line HepG2.2.15. Changes in morphology and activity of hepatocytes were then observed, as well as analysis of changes in liver enzyme, and HBV DNA and inflammatory cytokine levels in the culture supernatant. Intracellular HBV DNA and covalently closed circular DNA (cccDNA) concentration were measured by real-time polymerase chain reaction. Co-stimulation by mature DCs and IEC showed no impact on the morphology and liver enzyme expression level of HepG2.2.15 cells, but the supernatant HBV DNA and intracellular HBV DNA and cccDNA levels decreased significantly compared with those cells co-cultured with immature DCs. Secretion of inflammatory cytokines in the supernatant showed that when HBV DNA was highly expressed, the concentration of IFN-γ and IL-2 decreased, while IL-10 increased. Contrastingly, when HBV DNA had low expression, the concentration of IFN-γ and IL-2 increased and IL-10 decreased. Co-stimulation of HBV-related antigen-induced mature DCs and autologous lymphocytes showed inhibitory effects on ex vivo HBV replication, and cytokines were suggested to mediate this effect.
Introduction
Chronic hepatitis B virus (HBV) infection affects millions of people worldwide, and is responsible for 500,000 deaths every year (1). In China, more than 300,000 patients die of end-stage liver disease related to HBV infection (25). The most effective treatment for HBV-related end-stage liver disease is liver transplantation, but without effective prophylaxis, the risk of HBV re-infection after transplantation can be more than 80% (2,23). Unfortunately, HBV re-infection may lead to graft failure and the need for a second transplantation. A small dose of hepatitis B immunoglobulin (HBIG) combined with nucleoside analogs is a commonly used regime for the prevention of hepatitis B recurrence after liver transplantation, with good results (32). However, there is a high cost of long-term HBIG and nucleoside analog therapy, as well as an increasing shortage of HBIG availability, meaning some patients are unable to maintain this effective regime. In addition, the long-term use of nucleoside analogs may also lead to HBV resistance (14). Application of the HBV vaccine after liver transplantation may potentially lead to the withdrawal of both nucleoside analog and HBIG therapy, although the vaccine is thought to be less effective due to the use of immunosuppressants after transplantation (21,30). In this context, a new, cost-effective prophylactic treatment regime that prevents HBV recurrence after transplantation with complete discontinuation of antiviral agents would represent an important breakthrough in the field.
Dendritic cells (DCs) are the main antigen presenting cells (APC) in the body that can activate naive T-cells, and their powerful antigen presenting ability provides a connection between the innate and adaptive immune system (17). Accordingly, DCs play an important role in various types of viral infection and tumor immunity (24). Liver transplantation is the primary curative treatment for all kinds of end-stage liver diseases, among which 70–80% are HBV-related in China (31). HBV-infected patients waiting for liver transplantation usually receive antiviral treatment to reduce replication of HBV DNA (29), and preoperative HBV DNA concentration is an important predictor for hepatitis B recurrence after transplantation. (16,18) In this regard, increased active immunity and decreased HBV DNA concentration during the wait time for liver transplantation has become an important target for prevention of hepatitis B recurrence after transplantation.(12)
As the most powerful APC in vivo, DCs are able to uptake antigens, then process and present them to T-cells to initiate the corresponding immune response, thereby enhancing specific anti-HBV capacity and improving the patient's active immunity. (22) Here, we studied the effects of dendritic cells on HBV transgenic mice-stimulated autologous lymphocytes on in vitro HBV replication, with an emphasis on covalently closed circular DNA (cccDNA). Our goal was to evaluate changes in specific immune function after HBV infection and its relationship with HBV replication. We believe this work is important with respect to providing a theoretical foundation for the feasibility of applying immunotherapy to improve active immunity of patients waiting for liver transplantation and prevent hepatitis B recurrence after transplantation.
Materials and Methods
Main instruments and reagents
The following instruments and regeants were used: FACS Calibur flow cytometer (Becton Dickinson, Franklin lakes, NJ), microplate reader (Biotek, Vinooski, VT), DCs culture DXF (Promo cell, Heidelberg, Germany), lymphocyte separation medium (Haoxiang Biological Products Technology, Baoji, Sanxi, China), Dulbecco's modified Eagle's medium (DMEM) and RPMI1640 (Gibco, Carlsbad, CA), fetal bovine serum (FBS; General Electric, Fairfield, CT), IFN-γ-APC, IL-4-PE mAb, CD80-APC/CD86-FITC/CD11C-FITC MHC-II-PE/CD83-PE/CD8-PERCP/CD-4FITC mAb (Becton Dickinson), CD3-APC mAb (Biolegend, San Diego, CA), dimethyl sulfoxide (DMSO; Amresco, Solon, OH), hepatitis B core antigen (HBcAg)/hepatitis B surface antigen (HbsAg; Peprotech, Rocky Hill, NJ), enzyme-linked immunosorbent assay (ELISA) kits for IL-10, IL-2, and IFN-γ (Biovalue, Shanghai, China). Other equipment included the fluorescence-based ABI 7500 quantitative real-time polymerase chain reaction (qRT-PCR) detection system (Applied Biosystems, Foster, CA), automatic fluorescence quantitative flow cytometry (Perkin Elmer, Waltham, MA) and RT-6000 automatic microplate reader (Bio-Tek, Vinooski, VT).
Animals and experimental design
Transgenic C57BL/6J mice with the full-length HBV genome (HBV transgenic mice) were bought from Shanghai Research Center for Model Organisms (20). Animals were housed individually in standard animal facilities, maintained on a 12 h light/dark cycle, and provided with commercially available chow and tap water ad libitum before testing. Prior to the start of the experiment, the serum HBsAg, HBeAg, and HBV DNA were positive, intrahepatocellular cccDNA was negative, and liver and kidney function and histopathology were normal in these mice. All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 86–23, revised 1985), and the protocols were approved by Animal Care and Research Committee of Tianjin First Central Hospital, Tianjin, China. All surgery was performed under chloral hydrate anesthesia, and all efforts were made to minimize suffering.
The following experimental groups were studied: Group A—lymphocytes (LC)+immature DCs; group B—HepG2.2.15+immature DC+LCs; group C—LC+HBV-related antigen-stimulated mature DCs; group D—HepG2.2.15+HBV-related antigen-stimulated mature DCs+LC; and group E—HepG2.2.15.
Autologous lymphocyte harvesting
Cell suspensions from the spleen and thymus of C57BL/6J mice were prepared under sterile conditions, and then moved to a centrifuge tube with Percoll lymphocyte separation medium. After centrifugation at 670 g for 20 min, the white middle layer was extracted and centrifuged at 330 g for 8 min, and then the supernatant was discarded. After washing with phosphate-buffered saline (PBS), the lymphocytes were counted and then added to culture medium and transferred to culture plates for co-culture.
Extraction and culture of DCs from HBV transgenic mice and morphological observation and detection of DC surface markers
HBV transgenic mice (6–8 weeks of age; body weight 18–22 g) were euthanized by cervical dislocation. Bone marrow was isolated from the femur and tibia under sterile conditions, washed with PBS, and then centrifuged in a 10 mL tube after which the supernatant was discarded. Red blood cells were lysed using 5 mL of preheated (37°C) red blood cell lysis buffer, and the remaining cells were washed, suspended, and diluted in five volumes of RPMI1640 medium, then centrifuged at 500 g for 5 min. Next, the cells were suspended in RPMI1640 medium containing 100 mL/L FBS, counted, and the cell density adjusted to 1×109/L. The isolated cells were then added to six-well culture plates with 2 mL of culture medium in each well. Bone marrow cells were cultured in an incubator at 37°C, with 50 ml/L CO2 and saturated humidity. Fresh RPMI1640 containing 10 μg/L GM-CSF and 1 μg/L rIL-4 was used for cell culturing. Non-adherent cells were discarded on the second day. A half volume of medium was renewed on the fourth day in culture, and replenished with 10 ng/mL GM-CSF and 1 ng/mL rIL-4. The cultured cells differentiated into immature DCs on the sixth day. Cell morphology and number were observed using an inverted microscope. Flow cytometry was used to detect DC surface markers, including CD3/CD4/CD8, CD80/CD83/CD86 and MHCII/CD11C, according to the manufacturer's protocol.
DC maturation and co-culture with HBsAg and HBcAg
Immature DCs were induced to maturity by lipopolysaccharide (LPS; 100 ng/mL) on the sixth day, followed by incubation with HBsAg (19.6 μg/mL) and HBcAg (10 μg/mL) on the eighth day in culture. On the tenth day, they became mature DCs with HBsAg and HBcAg. Mature DCs were then analyzed for the expression of CD80, CD86, and CD83 by flow cytometry to detect the difference of these cell surface markers between mature and immature DCs.
Preparation and testing of immune effector cells
Autologous lymphocytes were stimulated by mature DCs with HBV-related antigen at a ratio of 1 DC to 10 lymphocytes. After co-culture, some lymphocytes were activated by binding with HBV-related antigen on the DCs, and then differentiated into immune effector cells (IECs), which can recognize specific target cells and kill them. These activated IECs (mainly CD8+) were cultured and activated for more than 10 days of constant division and proliferation. Flow cytometry was used to detect lymphocyte surface markers, cell counts, and proportion of lymphocyte subsets.
HepG2.2.15 and immune effector cell co-culture
The HepG2.2.15 cell line, donated by Wei Lai of the Hepatology Institute of Peking University Affiliated Hospital were cultured in high glucose DMEM medium (containing 10% FBS, 200 mg/L G418, 6 mmol/L glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin), at 37°C in a humidified 5% CO2 atmosphere. IECs were co-cultured with HepG2.2.15 in six-well plates, with a ratio of 10 IECs to 1 hepatoma cell, with the latter seeded at 1×105 cells per well. These cells were co-cultured in an incubator at 37°C, with 50 ml/L CO2 and saturated humidity for 24, 48, and 72 h. At the end of each time point, the cell supernatant and cells were collected to check the concentration of cccDNA and HBV DNA compared with the blank control well. Three different generations of HepG2.2.15 cells were selected to repeat the above experiments, and the cells and supernatant were preserved for further analysis.
Assessment of cytokine and liver enzymes and determination of HBV DNA in supernatants and intracellular cccDNA by qRT-PCR
Secretion of the cytokines IL-10, IFN-γ, and IL-2 into the cell culture supernatant was determined using an ELISA kit (R&D Systems), according to the manufacturer's protocol.
Serum levels of enzymes reflecting liver function, including alanine transaminase (ALT), aspartate aminotransferase (AST), urea, and albumin, were determined using an automatic analyzer (Hitachi, Japan). The supernatant HBV DNA levels were measured using a real-time PCR kit (Shanghai Kehua), according to the manufacturer's instructions, with an ABI 7500 real-time PCR system (Applied Biosystems). Intracellular cccDNA was determined by RT-PCR, as previously described (8).
Statistical analysis
SPSS for Windows v10.0 (SPSS, Inc., Chicago, IL) was used for the statistical analysis. Normally distributed data were shown as the mean±standard deviation from three separate experiments performed in triplicate. Different groups of data were compared by analysis of variance (ANOVA). The results were considered to be statistically significant when a p-value of <0.05 was obtained. GraphPad Prism 5.0 software (GraphPad, La Jolla, CA) was used for graphing data for presentation.
Results
Isolation of DCs from the bone marrow of HBV transgenic mice: morphology and cell surface markers
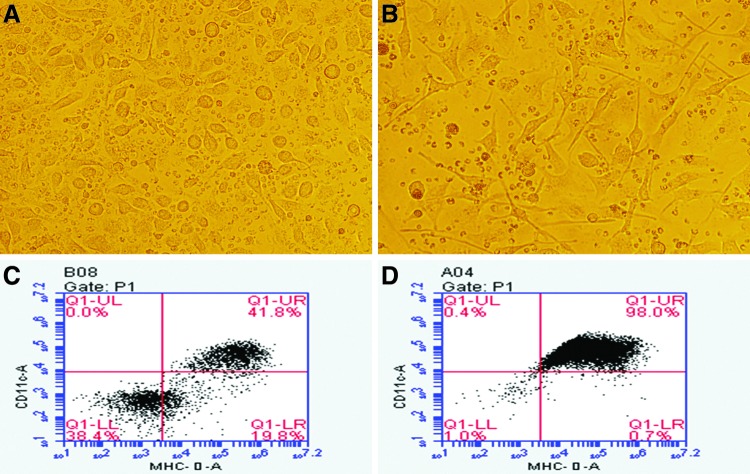
At day 0 in culture, DCs were round mononuclear, adherent, and small in size with no dendrons. Cell shape and volume increased gradually from regular round-shaped cells to strip-shaped cells on day 3 in culture. Later, the cell volume increased significantly with short dendrons or pseudopodia extending from the cell surface on day 6 (Fig. 1A). These cells were grouped as mature or immature according to whether they were stimulated with HBsAg and HBcAg or not. Large amounts of mature cells loaded with theses antigens floated in the medium on day 8 in culture, each with a large volume and two or more obvious pseudopodia (Fig. 1B). At this time, the small round mononuclear cells had gradually disappeared.
FIG. 1.
Morphological comparison between immature and mature dendritic cells (DCs). A representative image of immature DCs on day 6 in culture are shown (A: 400×), appearing round with some short synapses. A representative image of mature DCs after 10 days culture is also shown (B: 400×), appearing to have an increased cell volume with two or more obvious synapses. Flow cytometric graphs showing the proportion of CD11c+ and MHC-II+cells (approximately 40–50%; C) on day 6 in culture, and the proportion of CD11c+and MHC-II+cells (approximately 90–98%; D), on day 10 in culture.
Compared with immature DCs, the surface expression of CD11c/MHC-II/CD83/CD80/CD86 increased significantly in matures DC loaded with antigen on day 6 in culture (Table 1), which suggested that ex vivo HBV-related antigen could stimulate DC maturation. CD11c+MHC-II+cells accounted for 95.50±4.07% of mature DC cells, while they accounted for 45.77±4.53% of immature DC cells (Table 1). Figure 1C and D shows typical changes in cell surface markers between immature and mature DCs.
Table 1.
Change of Phenotype in Different Mature and Immature Dendritic Cells
| Cell surface marker | Immature DCs (%) | Mature DCs (%) |
|---|---|---|
| CD11c+MHC-II+ | 45.77±4.53 | 95.50±4.07** |
| CD80 | 20.07±2.66 | 64.00±10.66** |
| CD86 | 16.37±6.91 | 29.77±4.48* |
| CD83 | 86.83±2.89 | 98.37±0.97** |
All data presented as mean±SD; n=3.
p<0.05; **p<0.01 mature vs. immature DCs.
DC, dendritic cells.
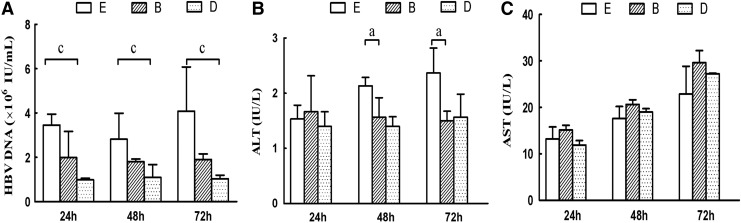
Comparison of supernatant HBV DNA levels between HepG2.2.15 cells cultured with lymphocytes and mature or immature DCs
Supernatant HBV DNA levels of group D (HepG2.2.15+mature DCs+LCs) at 24 (0.98±0.07×106 IU/mL), 48 (1.10±0.58×106 IU/mL), and 72 h (1.04±0.16×106 IU/mL) were significantly less than those of group E (HepG2.2.15) at the same respective time-points—24 h: 3.45±0.50×106 IU/mL; 48 h: 2.82±1.17×106 IU/mL; and 72 h 4.09±1.99×106 IU/mL (p<0.01). The supernatant HBV DNA of group B (HepG2.2.15+immature DCs+LCs) at 24 (1.99±1.18×106 IU/mL), 48 (1.80±0.12×106 IU/mL), and 72 h (1.90±0.25×106 IU/mL) were not statistically different compared with group E (p>0.05). These results suggested that lymphocytes stimulated by mature DCs could effectively inhibit HBV release in vitro, and the inhibitory effect was more significant in group D than in group B (Fig. 2).
FIG. 2.
Effect of co-culturing mature and immature DCs on hepatitis B virus (HBV) DNA levels and markers of liver function (n=3). The supernatant concentration of HBV DNA (A), alanine transaminase (ALT) (B), and aspartate aminotransferase (AST) (C) is presented. Group B: HepG2.2.15+immature DCs+LC; group D: HepG2.2.15+mature DCs+LC; group E: HepG2.2.15. ap<0.05, group B vs. group E; cp<0.05, group D vs. group E.
Comparison of supernatant liver enzyme levels between HepG2.2.15 cells cultured with lymphocytes and mature or immature DCs
The supernatant ALT levels of group B and group D at 48 and 72 h were significantly less than those in group E. The supernatant AST levels showed an increasing trend in groups B and D compared to group E, which was not statistically significant. These results suggested that immune cells co-cultured with xenogeneic hepatocytes did not result in rejection between the cells, but may have protective effects on the liver cell membrane.
Effect of immune effector lymphocytes on intracellular HBV DNA and cccDNA of HepG2.2.15 cells
When co-cultured with mature DCs and lymphocytes (group D), the intracellular HBV DNA and cccDNA levels of HepG2.2.15 cells at 24, 48, and 72 h significantly decreased compared with those of group E (HepG2.2.15 cells). The intracellular HBV DNA and cccDNA of HepG2.2.15 cells co-cultured with immature DCs and LCs (group B) at 24, 48, and 72 h were not statistically different from group E (Table 2). These results suggested that autologous lymphocytes from HBV transgenic mice, which were stimulated by HBV-related antigen-induced mature DCs, showed significant inhibitory effects on ex vivo HBV replication of HepG2.2.15 cells. Furthermore, they also showed inhibitory effects on intracellular HBV cccDNA replication and HBV DNA secretion.
Table 2.
Intracellular Hepatitus B Virus DNA and cccDNA Levels in HepG2.2.15 Cells (n=3)
| 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|
| Group | HBVDNA (×106) | cccDNA (×102) | HBVDNA (×106) | cccDNA (×102) | HBVDNA (×106) | cccDNA (×102) |
| HepG2.2.15(E) | 46.93±2.56 | 88.73±28.82 | 47.93±1.24 | 77.03±7.35 | 41.13±0.85 | 79.70±8.23 |
| HepG2.2.15+immature DCs+LC (B) | 33.27±12.74 | 59.27±6.60 | 34.73±13.20 | 64.80±16.59 | 31.57±14.79 | 57.13±12.26 |
| HepG2.2.15+mature DCs+LC (D) | 13.45±3.21* | 26.17±4.39* | 11.82±10.26* | 18.87±7.70* | 9.11±6.32* | 19.44±12.93* |
p<0.05, group D vs. group E.
HBV, hepatitis B virus.
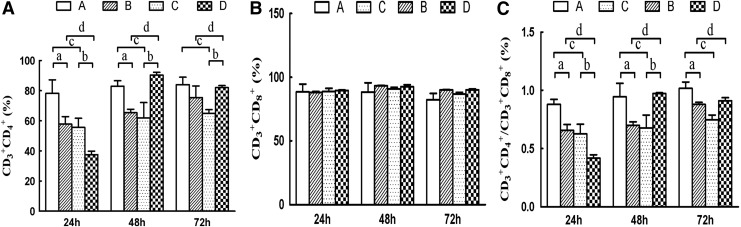
Analysis of lymphocyte subsets
Potential changes in the CD3+CD4+, CD3+CD8+, and ratio of CD3+CD4+/CD3+CD8+ surface markers were checked when lymphocytes (stimulated by either mature or immature DCs) were co-cultured with HepG2.2.15 cells (Fig. 3). The results showed that CD3+CD4+ cells, and the ratio of CD4+/CD8+ declined at first and then increased as the DCs matured, although the change of CD3+CD8+ was not statistically significant. This suggested that lymphocytes activated by mature DCs mediated their immune effects primarily by CD3+CD8+ cells at 24 h, whereas this changed to primarily CD3+CD4+ cells after 48 h.
FIG. 3.
Effect of co-culturing mature and immature DCs on lymphocyte cell surface makers (n=3). The percentage of CD3+CD4+ (A), CD3+ CD8+ (B), and CD3+CD4+/CD3+ CD8+ (C) cells is presented. Group A: immature DCs+LC; group B: immature DCs+LC+HepG2.2.15; group C: mature DCs+LC; group D: mature DCs+LC+HepG2.2.15. ap<0.05, group B vs. group A; bp<0.05, group D vs. group C; cp<0.05, group C vs. group A; dp<0.05, group D vs. group B.
Groups B, C, and D showed statistically significant differences compared to group A in terms of CD3+CD4+ expression at 24, 48, and 72 h respectively (p<0.01; Fig. 3). The differences between group A and groups B, C, and D were statistically significant (p<0.01), which decreased at 24 h and increased after 48 h. But CD3+CD8+ expression showed no significant difference between any groups at any time. The ratio of CD4+/CD8+ in group C decreased significantly compared with group A. The ratio of CD4+/CD8+ in group D decreased significantly at 24 h, but increased at 48 and 72 h when compared with group C (p<0.01). Compared with group B, the ratio of CD4+/CD8+ in group D decreased significantly at 24 h (p<0.01), and increased at 48 and 72 h, with statistical significance only at 48 h (p<0.05).
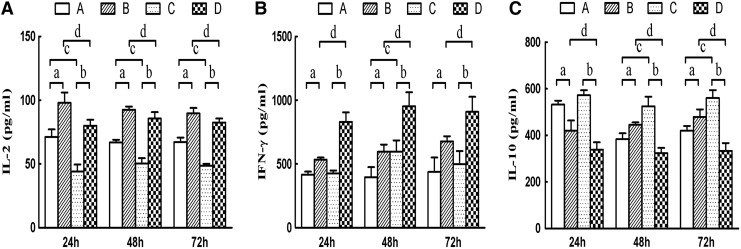
Change in supernatant cytokine levels in HepG2.2.15 and IE cell co-cultures
The supernatant levels of IL-2 and IFN-γ in group B increased at 24, 48, and 72 h compared with those of group A (pa<0.05; Fig. 4). Secretion of IL-2 and IFN-γ in group D also increased at 24, 48, and 72 h compared with group C (pb<0.05). Levels of IL-2 in group D and group B at different times also significantly decreased (pd<0.05), and levels of IFN-γ in group B and group D at different times also significantly increased (pd<0.05), but this change was not time dependent. Compared with group A, IFN-γ in group C at 24 h and 72 h showed no significant difference, but the levels of IFN-γ in group C increased significantly at 48 h compared with those of group A. IL-2 levels in group A increased significantly at 24, 48, and 72 h compared with those of group C (pc<0.05).
FIG. 4.
Effect of co-culturing mature and immature DCs on cytokine secretion (n=3). The supernatant concentrations of IL-2 (A), IFN-γ (B), and IL-10 (C) are presented. Group A: immature DCs+LC; group B: immature DCs+LC+HepG2.2.15; group C: mature DCs+LC; group D: mature DCs+LC+HepG2.2.15. ap<0.05, group B vs. group A; bp<0.05, group D vs. group C; cp<0.05, group C vs. group A; dp<0.05, group D vs. group B.
In contrast, secretion of IL-10 in group A increased compared to group B at 24 h, and decreased at 48 and 72 h (pa<0.05; Fig. 4). Levels of IL-10 in group C and group D all decreased at 24, 48, and 72 h (pb<0.05). Compared with group A, IL-10 in group C at 24 h was significantly different, but not at 48 and 72 h. Compared with group B, IL-10 levels in group D were significantly different (pd<0.01), which was not related with time. Taken together, these results suggested that DC-stimulated lymphocytes co-cultured with HepG2.2.15 could induce higher levels of IL-2 and IFN-γ secretion, as well as lower IL-10 secretion. These effects of IFN-γ and IL-10 were significantly more pronounced in mature DCs compared with immature DCs (group B vs. group D).
Discussion
The primary findings of this study are that IECs co-cultured with xenogeneic hepatocytes in vitro do not result in rejection between these cells. We also found that DC-stimulated IECs from HBV transgenic mice showed significant inhibitory effects on HBV replication in vitro, and that the Th1/Th2 cell ratio may be in a dynamic equilibrium to regulate the replication and secretion of HBV in vitro. Finally, our data also suggest that cytokines may play an important role in these anti-HBV effects, and may be an important factor in the protection from liver cell injury. The significance of these findings is discussed below in the context of the broader literature.
It is widely believed that chronic viral hepatitis and persistent virus infection depend not only on the biological characteristics of the virus itself, but more importantly the state of the host's immune function (9,28). Using the body's immune cells to identify target-specific antigens of lymphocytes and promoting functional lymphocyte proliferation in a specific way could make functional IECs able to recognize and kill target cells specifically (e.g., cancer and virus-infected cells). For example, studies have shown that isolation of a patient's DCs, and then retransfusing them after they were stimulated with HBcAg in vitro could promote T-cell immunity in the body, leading to inhibition of viral replication and improvement of liver function (4). Similarly, it has been shown that DCs activated by HBcAg produce specific action to HBV-specific CD8+ cells (5).
In this study, we isolated DCs cells and lymphocytes from HBV transgenic mice, stimulated and matured the DCs with different HBV proteins, and induced IECs by co-culture of mature DCs and lymphocytes. We found that when IECs were co-cultured in vitro with HepG2.2.15 cells containing the full-length HBV genome, no xenogeneic rejection occurred between the cells, and the supernatant ALT showed a downward trend compared with that of the control group. This is in agreement with the notion that cellular immunotherapy does not cause severe damage to hepatocytes (15), and even may have reparative effects on the hepatocyte membrane, which suggested that our ex vivo treatment for HBV exposure was feasible. We also found that when co-cultured with HBV-related antigen-stimulated mature DCs, the supernatant HBV DNA of HepG2.2.15 cells, intracellular HBV DNA, and cccDNA all decreased significantly compared with that of those co-cultured with immature DCs (p<0.01). These results suggested that lymphocytes activated by mature DCs had significant inhibitory effects on HBV cccDNA synthesis and secretion.
HBV cccDNA plays a key role in HBV replication, and is a prominent marker of HBV replication. HBV cccDNA is difficult to be cleared, so the elimination of HBV cccDNA-positive hepatocytes following antiviral therapy is a major therapeutic goal in chronic hepatitis B (3). Currently HBV cccDNA is considered to be immune eliminated in the body by: (a) cell lysis that eliminates the infected hepatocytes, with the HBV phagocytized by macrophages or combined with anti-HBs antibodies to form immune complexes themselves phagocytized by macrophages and excreted in the urine, with the damaged hepatocytes replaced by regenerated cells; and (b) clearance mediated by Th1 cytokine-dependent non-cytolytic mechanisms, such as interferon, tumor necrosis factor, and interleukin-2, which mediate non-cytolytic mechanisms to eliminate HBV cccDNA in the nuclei or block the synthesis of new HBV cccDNA (10,27). It has also been reported that HBV elimination was achieved by adoptive transfer of immunity from a donor to the liver transplant recipient (26).
In order to explain the mechanism of HBV elimination, we measured changes in the surface markers of sensitized lymphocyte subsets, and found that the effects of specific IECs on HBV at the beginning were primarily mediated by CD8+ cells, while CD4+ cells decreased significantly (p<0.05), and the ratio of CD4+/CD8+ also decreased significantly (p<0.01). After 48 h, the number of CD4+ cells increased significantly, revealing that the early anti-HBV effects of IECs were primarily mediated by CD8+ cells, and then later effects were mediated by CD4+ T-cells. Similarly, with increasing time, the ratio of CD4+/CD8+ cells increased, whereas intracellular HBV DNA levels decreased significantly (p<0.01), which may be because of the direct involvement of CD4+ and CD8+ cells in the anti-HBV process (11). We also observed that HBV DNA levels decreased while the ratio of CD4+/CD8+ cells increased significantly (p<0.01), suggesting that the change in IEC subsets was initially a decreased ratio of CD4+/CD8+ cells, which then increased after the effect of HBV, and was not related to rejection between xenogenic cells. Studies have shown that IEC CD8+ lymphocytes were important to determine HBV elimination, and that cells lysis or non-cytolytic mechanisms (such as those mediated by IFN-γ or TNF-α) were the primary way CD8+ T-cells eliminate HBV (7,19).
We found that when HBV DNA was highly expressed, IFN-γ and IL-2 levels decreased while IL-10 increased, and when HBV DNA was expressed at a low level, IFN-γ and IL-2 increased and IL-10 decreased. Increased secretion of IFN-γ could promote CD4+ T-cells to differentiate into Th1 cells and suppress Th2 formation, thereby activating HBV-specific cytotoxic lymphocyte clone proliferation to eliminate HBV (13).
We observed that IL-10 concentration increased with higher viral load, suggesting that HBV pathogenicity was associated with higher humoral immune activity mediated by Th2 cells. It has been shown that elevated levels of IL-l0 inhibit the function of Th1 cells, and reduce the secretion of IFN-γ and IL-2 from Th1 cells, which reduces the cellular immune effects and the body's ability to remove the virus, leading to viral persistence (29). It has also been reported that serum IL-10 concentrations in HBV-infected individuals are significantly higher than the normal control group, and that serum concentration of IL-10 with high HBV load was significantly higher than the normal control group and the group with low HBV load (6). Taken together, this suggests that cytokines can correlate with intrahepatocellular HBV DNA levels, especially with HBV cccDNA in hepatocytes.
Acknowledgments
This study was supported by Natural Science Foundation of China (Nos. 81270528 and 81170444), the Natural Science Foundation of Tianjin, China (Nos. 08JCYBJC08400, 11JCZDJC27800, and 12JCZDJC25200), and the Technology Foundation of Health Bureau in Tianjin, China (Nos. 05KYZ24, 2011KY11, and 10KG101).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alter MJ. Epidemiology and prevention of hepatitis B. Semin Liver Dis 2003;23:39–46 [DOI] [PubMed] [Google Scholar]
- 2.Avolio AW, Nure E, Pompili M, et al. Liver transplantation for hepatitis B virus patients: long-term results of three therapeutic approaches. Transplant Proc 2008;40:1961–1964 [DOI] [PubMed] [Google Scholar]
- 3.Bohne F, Chmielewski M, Ebert G, et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology 2008;134:239–247 [DOI] [PubMed] [Google Scholar]
- 4.Carotenuto P, Artsen A, Niesters HG, et al. In vitro use of autologous dendritic cells improves detection of T cell responses to hepatitis B virus (HBV) antigens. J Med Virol 2009;81:332–339 [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Zhang Z, Shi M, et al. Activated plasmacytoid dendritic cells act synergistically with hepatitis B core antigen-pulsed monocyte-derived dendritic cells in the induction of hepatitis B virus-specific CD8 T-cell response. Clin Immunol 2008;129:295–303 [DOI] [PubMed] [Google Scholar]
- 6.Das A, Ellis G, Pallant C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol 2012;189:3925–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao YQ, Yao Y, and Li M. Expression characteristics of some immune effector molecules in CD8+T lymphocytes from patients with chronic hepatitis B. Nan Fang Yi Ke Da Xue Xue Bao 2010;30:1606–1609 [PubMed] [Google Scholar]
- 8.Gao YT, Han T, Li Y, et al. Enhanced specificity of real-time PCR for measurement of hepatitis B virus cccDNA using restriction endonuclease and plasmid-safe ATP-dependent DNase and selective primers. J Virol Methods 2010;169:181–187 [DOI] [PubMed] [Google Scholar]
- 9.Godon O, Fontaine H, Kahi S, et al. Immunological and antiviral responses after therapeutic DNA immunization in chronic hepatitis B patients efficiently treated by analogues. Mol Ther 2014;22:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti LG, Rochford R, Chung J, et al. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999;284:825–829 [DOI] [PubMed] [Google Scholar]
- 11.Han YP, Li J, Jiang LF, et al. Hepatitis B e antigen from chronic hepatitis B patients induces Th1/Th2 cytokine imbalance in vitro. Zhonghua Gan Zang Bing Za Zhi 2013;21:584–589 [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Yan L, Wen T, et al. Hepatitis B prophylaxis using lamivudine and individualized low-dose hepatitis B immunoglobulin in living donor liver transplantation. Transplant Proc 2013;45:2326–2330 [DOI] [PubMed] [Google Scholar]
- 13.Kakumu S, Okumura A, Ishikawa T, et al. Serum levels of IL-10, IL-15 and soluble tumor necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin Exp Immunol 1997;109:458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy M, and Alexopoulos SP. Hepatitis B virus infection and liver transplantation. Curr Opin Organ Transplant 2010;15:310–315 [DOI] [PubMed] [Google Scholar]
- 15.Krebs K, Böttinger N, Huang LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology 2013;145:456–465 [DOI] [PubMed] [Google Scholar]
- 16.Kubo S, Hirohashi K, Tanaka H, et al. Usefulness of viral concentration measurement by transcription-mediated amplification and hybridization protection as a prognostic factor for recurrence after resection of hepatitis B virus-related hepatocellular carcinoma. Hepatol Res 2003;25:71–77 [DOI] [PubMed] [Google Scholar]
- 17.McKenna K, Beignon AS, and Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol 2005;79:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrakis A, Fortsch T, Del MA, et al. Liver transplantation for hepatitis B-induced liver disease: long-term outcome and effectiveness of antiviral therapy for prevention of recurrent hepatitis B infection. Transplant Proc 2013;45:1953–1956 [DOI] [PubMed] [Google Scholar]
- 19.Phillips S, Chokshi S, Riva A, et al. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol 2010;184:287–295 [DOI] [PubMed] [Google Scholar]
- 20.Ren J, Wang L, Chen Z, et al. Gene expressionprofile of transgenic mouse kidney reveals pathogenesis of hepatitis B virus associated nephropathy. J Med Virol 2006;78:551–560 [DOI] [PubMed] [Google Scholar]
- 21.Rosenau J, Hooman N, Hadem J, et al. Failure of hepatitis B vaccination with conventional HbsAg vaccine in patients with continuous HBIG prophylaxis after liver transplantation. Liver Transplant 2007;13:367–373 [DOI] [PubMed] [Google Scholar]
- 22.Rosendahl HS, van Beek J, de Jonge J, et al. T cell responses to viral infections—opportunities for peptide vaccination. Front Immunol 2014;5:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel D, Feray C, and Bismuth H. Hepatitis viruses and liver transplantation. J Gastroenterol Hepatol 1997;12:S335–S341 [DOI] [PubMed] [Google Scholar]
- 24.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 2003;15:138–147 [DOI] [PubMed] [Google Scholar]
- 25.Shen ZY, Zheng WP, Deng YL, et al. Variations in the S and P regions of the hepatitis B virus genome under immunosuppression in vitro and in vivo. Viral Immunol 2012;25:368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shouval D. Adoptive transfer of immunity to HBV in liver transplant patients: a step forward toward the proof of concept for therapeutic vaccination or a transient immunologic phenomenon? Liver Transpl 2007;13:14–17 [DOI] [PubMed] [Google Scholar]
- 27.Thimme R, Wieland S, Steiger C, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitisB virus infection. J Virol 2003;77:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trehanpati N, Hissar S, Shrivastav S, et al. Immunological mechanisms of hepatitis B virus persistence in newborns. Indian J Med Res 2013;138:700–710 [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai SL, Liaw YF, Chen MH, et al. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology 1997;25:449–458 [DOI] [PubMed] [Google Scholar]
- 30.Wursthorn K, Wedemeyer H, Manns MP. Managing HBV in patients with impaired immunity. Gut 2010;59:1430–1445 [DOI] [PubMed] [Google Scholar]
- 31.Yamashiki N, Sugawara Y, Tamura S, et al. Double-dose double-phase use of second generation hepatitis B virus vaccine in patients after living donor liver transplantation: not an effective measure in transplant recipients. Hepatol Res 2009;39:7–13 [DOI] [PubMed] [Google Scholar]
- 32.Yuan CH, Xiu DR, Jiang B, et al. HBV recurrence lowered by lamivudine/HBIG combination therapy in liver transplant patients: ten-year experience. Hepatobiliary Pancreat Dis Int 2013;12:149–153 [DOI] [PubMed] [Google Scholar]