Abstract
Stem cells may represent an excellent strategy to improve the healing of skin ulcers. Today the administration mode of stem cells to skin defects remains unsatisfactory. Delivering stem cells with topical treatments represents a new strategy and answering the patients' need. Mesenchymal stromal cells (MSC) have been shown to improve wound healing of cutaneous lesions and amniotic membrane (AM) is known to represent a natural scaffold for cells. The aim of this study is to develop a tissue-engineered product combining MSC and AM for clinical use. In this work we investigated whether the stromal matrix of intact human AM could constitute a scaffold for human MSC derived from either bone marrow (BM) or adipose tissue (AT). For this purpose, clinical-grade AM, MSC, and culture medium were used. We performed experiments of short-term adherence and proliferation for 15 days after the seeding of the cells. Morphological aspects and secretion profiles of MSC onto AM were studied, respectively, by scanning electron microscopy and Luminex analysis. Results demonstrated that the stromal matrix allow the adherence in much greater amount of MSC from BM or AT compared to 2D material. Experiments of proliferation showed that both kinds of MSC could proliferate on the stromal matrix and remain viable 15 days after the seeding of the cells. The 3D analysis of MSC culture demonstrated that both types of MSC invaded the stromal matrix and grew in multiple layers while retaining their fibroblastic morphology. By studying the secretion profile of MSC onto the stromal matrix, we found that both kinds of MSC secrete important cytokines and growth factors for wound healing of cutaneous lesions, such as vascular endothelial growth factor, hepatocyte growth factor, and basic fibroblast growth factor. In conclusion, these results suggest that the stromal matrix of AM seeded with MSC represents a bioactive scaffold that should be evaluated in patients with a nonhealing cutaneous wound.
Introduction
Despite the existence of several guidelines for the treatment of chronic ulcers,1 healing of chronic ulcers remains a major clinical concern. Innovative strategies that could improve healing time would be of great interest to reduce patient morbidity, hospitalization, and the consequences with regard to healthcare spending. Mesenchymal stromal cells (MSC) are multipotent cells that can differentiate into osteogenic, chondrogenic, and adipogenic lineages and produce trophic factors for tissue repair.2 Bone marrow (BM) and adipose tissue (AT) are the two main sources of MSC for clinical application. MSC derived from bone marrow (BM-dMSC) or adipose tissue (ASC) share the same capacity of in vitro multilineage differentiation, but cells differ in their expression of particular markers (expression of CD34+ and CD49d on ASC, whereas CD106 is expressed on BM-dMSC, but not on ASC),3,4 immunomodulatory and functional capacities.5 BM-dMSC6,7 and ASC8 have been demonstrated to be effective in animal models of cutaneous wound when they are injected into the wound. The therapeutic effect of MSC occurs by promoting reepithelialization and angiogenesis through either differentiation or secretion of growth factors such as vascular endothelial growth factor (VEGF). However, there are several inconveniences to the injection of MSC directly into skin ulcers in humans. Injections are invasive, painful and human ulcers require numerous injections to cover the entire surface to be treated. After surrounding injections, contact of MSC with the wound is not optimal and after intravenous injection MSC are distributed mainly in the lung.9 Interestingly, only a small number of studies have investigated the administration of MSC into wounded human skin.10,11
Human amniotic membrane (AM) is a fetal membrane that is widely used as a wound dressing in ophthalmic surgery.12 It improves the corneal reepithelialization and reduces the anti-inflammatory acute phase through the release of soluble factors.13 It is constituted of a single layer of epithelial cells that lie on the basement membrane, which is adjacent to a compact layer of collagen. AM is easily isolated from the placenta after a cesarean delivery and can be cryopreserved for several months.14 AM availability is unlimited and the processing cost is low. For these reasons, AM could be considered as a promising biomedical scaffold for the delivery of MSC directly into skin ulcers. Indeed, AM has been used to construct tissue-engineered products with amnion epithelial cells and amnion mesenchymal cells for prematurely ruptured fetal membrane repair,15 with fibroblasts and keratinocytes for skin repair,16 with limbal epithelial cells for corneal damage,17 with chondrocytes for cartilage repair.18 In all these studies, amniotic epithelium has been removed to use AM as a scaffold. However, protocols for decellularization of AM are long, complicated, required manual scrapping, and lack standardization.14 To overcome these inconvenience, we aimed to evaluate the feasibility of the stromal matrix of AM as a MSC scaffold for human skin repair. In this work, we studied the adherence, proliferation, maintenance of phenotype of BM-dMSC and ASC after seeding onto the stromal matrix of AM. We investigated the secretion profile of MSC on AM related to skin wound healing. To constitute a biocompatible patch of MSC for human cutaneous wound, we used clinical-grade MSC, AM, and culture medium.
Materials and Methods
MSC culture
Clinical-grade human MSC from BM or AT were kindly provided by Markus T Rojewski from the University of Ulm (Ulm, Germany) and Philippe Bourin from the Etablissement Français du Sang (Toulouse, France) (7th Framework Program of EU Cascade project), respectively. For all experiments, MSC were cultured in clinical-grade α-modified Eagle's medium (α-MEM) (PAA) supplemented with 5% of clinical-grade human platelet lysate kindly provided by Markus T. Rojewski,19 0.5% of ciprofloxacin (Bayer Pharma) and 1 U/mL of heparin (Sanofi-Aventis). Cells were cultured in a humidified atmosphere at 37°C and 5% CO2. Culture medium was changed twice a week and cells were used at passage 2–4. For each experiment, the passage number of BM-dMSC and ASC was identical. Three donors from each source were used interchangeably between experiments.
Flow cytometry and differentiation
The phenotype of BM-dMSC (passage 2) and ASC (passage 3) was analyzed by a Canto II flow cytometer (BD Biosciences) using the Diva software. Cells were stained at 4°C for 30 min with the following antibodies (BD Biosciences): anti-CD73 phycoerythrin (PE), anti-CD90 fluoro isothiocyanate, anti-105 PE, anti-CD34 phycoerythrin-cyanin 7 (PC7), anti-CD45 allophycocyanin H7 (APC H7), anti-CD146 PE, and their respective isotypes in accordance with the manufacturer's instructions (BD Biosciences). Then, cells were washed in PBS 1×, pelleted, and resuspended in PBS 1×before analysis. Expression of surface molecules were analyzed by the FlowJo software.
MSC were seeded in six-well plates to perform the in vitro multilineage differentiation assays. For osteogenic differentiation, the medium was replaced at 25% confluence by α-MEM 10% fetal bovine serum (FBS) (StemCell Technologies) supplemented with 50 μM ascorbic acid-2 phosphate, 10 mM α-glycerophosphate, and 0.1 μM dexamethasone (Sigma). On day 21, the monolayers were fixed in 70% ethanol for 1 h at 4°C and stained for 15 min with Alizarin Red-S (Sigma) at room temperature. For adipogenic differentiation, the medium was replaced at 80% confluence by a DMEM high glucose (Gibco by Life Technologies) supplemented with 10% FBS, 0.1 μM dexamethasone, 0.2 mM indomethacin, 0.01 mg/mL insulin, and 0.5 mM IBMX. On day 21, the monolayers were fixed using paraformaldehyde 4% for 5 min at room temperature, and then stained for 15 min with 0.3% Oil Red O (Sigma)/60% isopropanol. For chondrogenic differentiation, the medium was replaced at 80% confluence by DMEM high glucose medium (Gibco by Life Technologies) supplemented with 10% FBS, 1× ITS+ (Sigma), 0.1 mg/mL sodium pyruvate (Sigma), 0.04 mg/mL L-proline (Sigma), 0.05 mg/mL ascorbic acid-2 phosphate (Sigma), 1×10−7 M dexamethasone, 0.1 μg/mL transforming growth factor beta-3 (TGF-β3) (Sigma). Controls were performed with differentiation medium without TGF-β3. On day 21, the monolayers were fixed in 4% paraformaldehyde and stained with Alcian Blue 8GX (Sigma).
Amniotic membrane
Clinical-grade human AM were prepared by the Tissue Bank of the Etablissement Français du Sang Ile de France from three placentas obtained after cesarean deliveries after the signature of an informed consent by the donor. The AM were used interchangeably between experiments. AM were cryopreserved by the Tissue Bank at −80°C in glycerol and RPMI at a ratio of 1/1 following a good manufacturing practice (GMP) process. Before use in experiments, all AM were thawed at 37°C and washed two times with HBSS 1×(PAA). AM from the same placenta were specially prepared and cryopreserved at −80°C in either glycerol/RPMI at a ratio of 1/1, HBSS 1× or dimethyl sulfoxide/RPMI at a ratio of 1/9 to study the role of storage solution.
Adherence and proliferation of MSC
Custom-made steel rings were placed on the stromal side of thawed AM to define the area of loading (1.8 cm2) and MSC were seeded at various doses (10,000/cm2, 30,000/cm2, 90,000/cm2, and 180,000/cm2) in triplicates. To compare adherence of MSC onto the plastic, MSC were seeded in triplicates in wells of 24-well plate (each well representing a surface of 1.8 cm2). MSC loaded onto AM were maintained in culture in α-MEM supplemented with 5% platelet lysate. Adherence and proliferation of MSC on thawed AM were determined by measuring the metabolic activity of the cells using an Alamar Blue assay (Life Technologies SAS) according to the manufacturer's protocol (10% v/v). Alamar Blue assays were performed by measuring the fluorescence (λex=543 nm and λem=590 nm) of two points on each sample. The percentage of cell adherence was calculated as the ratio of Alamar Blue results after seeding on Alamar Blue results before seeding.
Scanning electronic microscopy
Specimens were fixed by immersion in 2.5% glutaraldehyde buffer at 4°C overnight. Scaffolds were then dehydrated in increasing concentrations of ethanol (70–100%) and dehydration was completed using a hexamethyldisilazane treatment (Sigma Aldrich). Finally, the samples were air dried, sputtered with a nanogold film and analyzed with a scanning electron microscope JSM-6301F (Jeol) at the LaboratoireInteruniversitaire des SystèmesAtmosphériques from the University of Paris-Est.
Secretion profile
The secretion profile of MSC was determined by measuring the concentration of 14 proteins (interleukin [IL]-8, IL-10, IL-13, IL-1-RA, platelet-derived growth factor [PDGF-BB], granulocyte-macrophage colony-stimulating factor [GM-CSF], granulocyte-colony-stimulating factor [G-CSF], transforming growth factor alpha [TGFα], tumor necrosis factor alpha [TNFα], tumor necrosis factor beta [TNFβ], basic fibroblast growth factor [FGF], vascular endothelial growth factor A [VEGF-A], hepatocyte growth factor [HGF], and EGF) in the supernatants of culture with a Luminex assay according to the manufacturer's protocol (Affymetrix). MSC derived from one BM and one AT were seeded at 180,000 cells/cm2 on the stromal side of thawed AM and maintained in the culture for 48 h in α-MEM supplemented with 5% platelet lysate as described above. Supernatants were harvested and cell number was determined using an Alamar Blue assay. Supernatants were cryopreserved at −80°C until Luminex assay. The concentration of proteins (pg/mL) in each well was obtained from duplicates of the Luminex assay and standardized with the number of cells in each well. The presence of trophic factors in AM and in the medium was deducted before standardization with the number of cells. Analysis of IL-8, VEGF-A, HGF, TGFα, TNFα, TNFβ, and EGF proteins were obtained from triplicate (for the first experiment) and duplicate (for the second experiment) of two independent experiments. Analysis of IL-10, IL-13, IL-1-RA, PDGF-BB, GM-CSF, G-CSF, and basic FGF were obtained from triplicate of one experiment.
Statistical analysis
Comparison between two experimental sources of cells (BM-dMSC vs. ASC) was performed using the unpaired nonparametric Mann–Whitney U-test with the Graphpad software. When three groups were compared, data were statistically analyzed using one-way analysis of variance (ANOVA) and post hoc Bonferroni test. Differences between the groups with a p-value≤0.05 were considered to be significant.
Results
Characterization of MSC
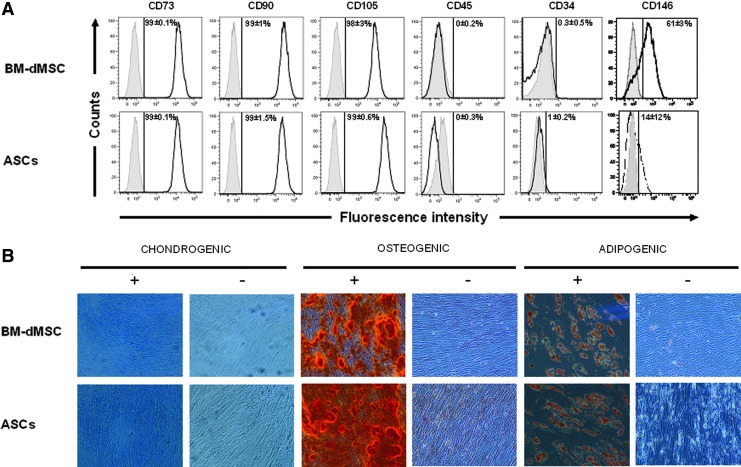
BM-dMSC and ASC had a typical fibroblastic aspect when cultured in α-MEM supplemented with 5% of platelet lysate. Analysis of the phenotype of MSC as described by Horwitz et al.20 for BM-dMSC and Bourin et al.21 for ASC showed that both cell types expressed strong CD73, CD90, and CD105 surface markers and remained CD45 negative. Cells differed by their CD146 expression with 61% on BM-dMSC versus 14% on ASC.3 CD34+ expression was not expressed on ASC at passage 3 (Fig. 1A).4
FIG. 1.
Characterization of mesenchymal stromal cells (MSC). (A) Phenotype of MSC. MSC derived from bone marrow (BM-dMSC) (passage 2) and adipose tissue (ASC) (passage 3) were analyzed by fluorescence-activated cell sorting after staining with conjugated monoclonal antibodies against surface markers (black line) or IgG control isotypes (gray line). Results were representative of three independent experiments. The mean percentage of fluorescence above the threshold for isotype controls is indicated. (B) Differentiation potential of MSC for mesodermic lineage: MSC were cultured in a medium with (+) or without (−) differentiation cocktails for 3 weeks. Chondrogenic, osteogenic, and adipogenic differentiation were observed, respectively by coloration with Alcian Blue, which detects proteoglycans of chondroblasts, Alizarin Red, which detects calcium mineralization of osteoblasts and Oil red O, which detects lipid vesicles in adipocytes. Results depicted the differentiation of MSC derived from the bone marrow (BM-dMSC) or ASC from one donor out of three. Color images available online at www.liebertpub.com/tea
Culture of MSC in a conditioned medium for the differentiation toward osteoblastic, chondrogenic, and adipogenic lineages showed that both kinds of MSC were able to differentiate into osteoblasts, chondrocytes, and adipocytes as demonstrated in Figure 1 by a positive staining with Alizarin Red, Alcian Blue and Oil Red O (Fig. 1B).
MSC adherence on the surface of human AM
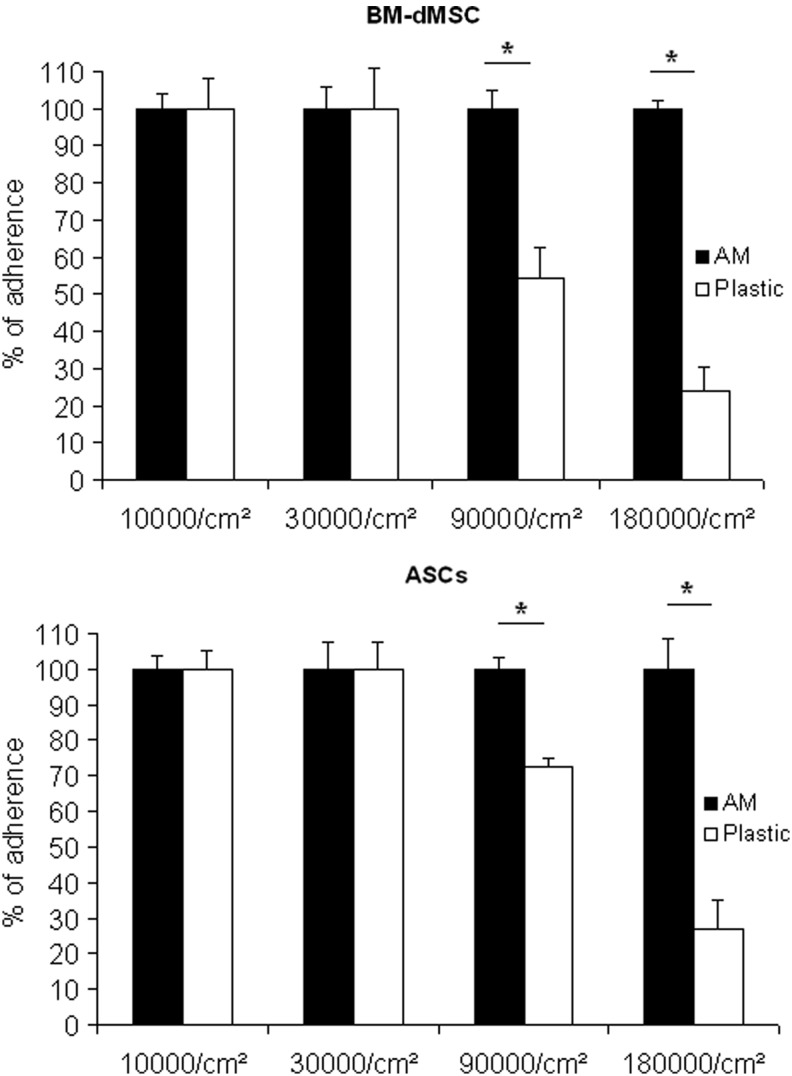
In the context of tissue engineering, scaffolds provide a mechanical structure for cell adherence, migration, and proliferation. BM-dMSC and ASC that reach a confluence of 100% in 2D culture on plastic have a cell density of about 20,000 cells/cm2 (data not shown). Consequently, to investigate MSC adherence on 3D stromal matrix, we decided to start MSC loading with the dose corresponding to less than 100% of confluence on plastic. We evaluated adherence of cells at short term (6 h) to allow firm cell attachment while avoiding proliferation. MSC were seeded from 10,000 cells/cm2 to 180,000 cells/cm2 on the stromal side of AM or on the plastic treated for cell culture. The Figure 2 shows that 100% of MSC from BM or AT were able to adhere onto AM whatever the seeding dose. Additionally, 100% of MSC from BM or AT were able to adhere onto the plastic when cells were seeded at 10,000 cells/cm2 and 30,000 cells/cm2. However, adherence onto the plastic at doses as high as 90,000 cells/cm2 and 180,000 cells/cm2 was only 54% and 24% for BM-DMSC and 73% and 27% for ASC, respectively (Fig. 2). Considering these data, the stromal side of AM could support the adherence of a huge amount of MSC compared to 2D material.
FIG. 2.
MSC adherence on amniotic membrane (AM). MSC were allowed to adhere on the stromal side of AM or on culture plastic at doses from 10,000 cells/cm2 to 180,000 cells/cm2. An Alamar Blue assay was performed 6 h after loading. Results are representative of two independent experiments performed in triplicate and mean data±SEM are presented (*p<0.05).
MSC proliferation on human AM
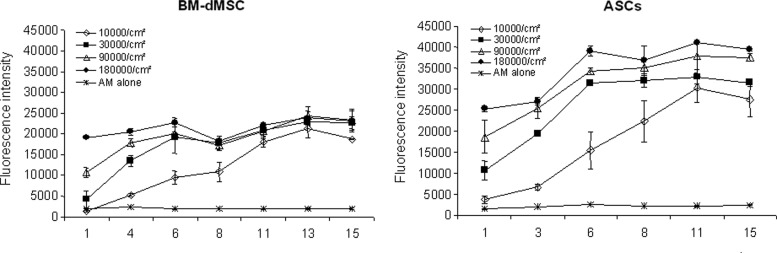
To investigate the viability and the proliferation of MSC on the stromal side of AM over a period of 15 days, MSC were loaded from 10,000 cells/cm2 to 180,000 cells/cm2 and an Alamar Blue assay was performed at several time points. Results showed that both BM-dMSC and ASC proliferated on AM and remained viable after loading for 15 days (Fig. 3). MSC loaded at 10,000 cells/cm2 increased over the 15 days. In contrast, MSC loaded at 30,000, 90,000, and 180,000 cells/cm2 increased over the first 6 days and no significant increase could be observed between 6 and 15 days. In the control group without cells (Fig. 3), we observed that no cells inside the AM could proliferate, demonstrating that the data are the results of loaded MSC proliferation. Likewise, we observed by an Alamar Blue assay that no epithelial cells remained viable during the 15 days following AM thawing (data not shown). These results demonstrated that the stromal side of AM is a suitable scaffold for the proliferation of MSC. In clinical practice, a cell density of 180,000 MSC/cm2 preserved the viability and the proliferation capacity of MSC without reaching 100% of confluence onto the AM during the first 6 days of the culture. This dose could be chosen.
FIG. 3.
MSC proliferation on AM. MSC were loaded at various doses from 10,000 cells/cm2 to 180,000 cells/cm2 on the stromal side of AM and cultured for a period of 15 days. Cell viability was assessed using an Alamar Blue assay performed in triplicate. Results are representative of two independent experiments performed in duplicate and mean data±SEM are presented.
Impact of the conditions of AM cryopreservation on MSC adherence
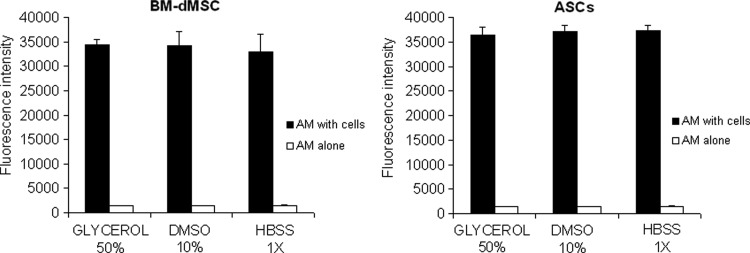
Given that AM can be prepared with various storage medium, including glycerol/DMEM,22 dimethyl sulfoxide/RPMI,14,23,24 and Hank's solution,17 we sought to evaluate the influence of the storage solution on MSC adherence on the stromal side of AM. To develop a tissue-engineered product for the clinic, the process of production shall be as simple as possible. With the aim to avoid maintaining MSC culture for 15 days, we decided to culture MSC on AM for a limited time of 2 days with the dose of MSC that would deliver the higher dose of trophic factors into the wound (180,000 cells/cm2). Figure 4 show that BM-dMSC and ASC can be loaded on the stromal side whatever the method of AM conservation without changing their capacity of adherence. There was no significant difference between the three types of AM preparations on MSC adherence and proliferation (The ANOVA test to compare the three preparations of AM: pBM-dMSC=0.71; pASC=0.56). Comparison between BM-dMSC and ASC groups showed no significant difference for the three types of AM preparations (The Mann–Whitney U-test to compare MSC for both source of cells: pGlycerol 50%=0.20; pDMSO 10%=0.40; pHBSS 1×=0.20). AM are routinely cryopreserved in glycerol/RPMI in the Tissue Bank of the Etablissement Français du Sang Ile de France and this process is approved by the regulatory authorities. Thus, we selected AM preserved in glycerol/RPMI to perform the subsequent experiments.
FIG. 4.
Effect of AM preparation on MSC adherence. MSC seeded at 180,000 cells/cm2 were allowed to adhere on the stromal side of AM cryopreserved in glycerol 50%, DMSO 10% or HBSS 1×. An Alamar Blue assay was performed 48 h after the loading. Results are representative of two independent experiments performed in triplicate and mean data±SEM are presented. The ANOVA test was used to compare the three preparations of AM: pBM-dMSC=0.71; pASC=0.56. The Mann–Whitney U-test was performed to compare MSC for both sources of cells: pGlycerol 50%=0.20; pDMSO 10%=0.40; pHBSS 1×=0.20.
Morphology of adherent MSC to the surface of AM
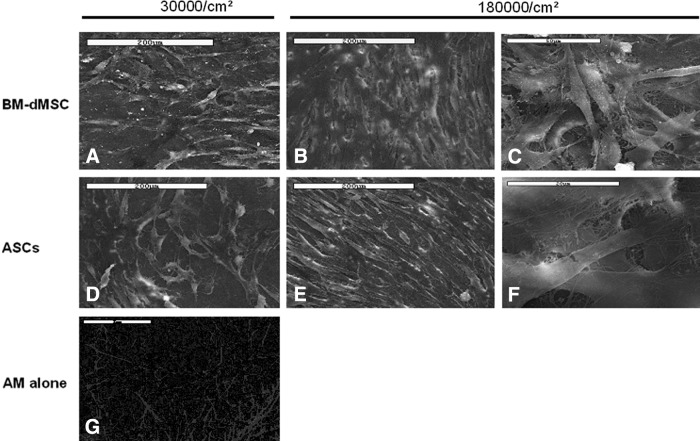
Morphology and distribution of seeded MSC loaded on the stromal side of AM were studied using the technique of scanning electronic microscopy performed at 48 h after the loading. Results showed that both BM-dMSC and ASC retained a fibroblastic morphology at 30,000 cells/cm2 and 180,000 cells/cm2 and adhere to the entire surface of the AM (Fig. 5). At the concentration of 180,000 cells/cm2, we could observe that MSC were forming multilayers, whereas at the concentration of 30,000 cells/cm2, MSC were not confluent and were forming monolayers. Despite a high level of loading (180,000 cells/cm2), MSC retained their typical morphology after a culture period of 2 days.
FIG. 5.
MSC morphology on AM. BM-dMSC were cultured at 30,000 cells/cm2 (A) or 180,000 cells/cm2 (B and C) and ASC were cultured at 30,000 cells/cm2 (D) or 180,000 cells/cm2 (E and F) on the stromal side of AM in duplicate. MSC morphology was observed at 48 h of culture by scanning electron microscopy. The stromal side of AM without MSC is presented on (G). Scale bars: (A–F) 200 μm; (G) 2 μm.
Study of the secretion profile
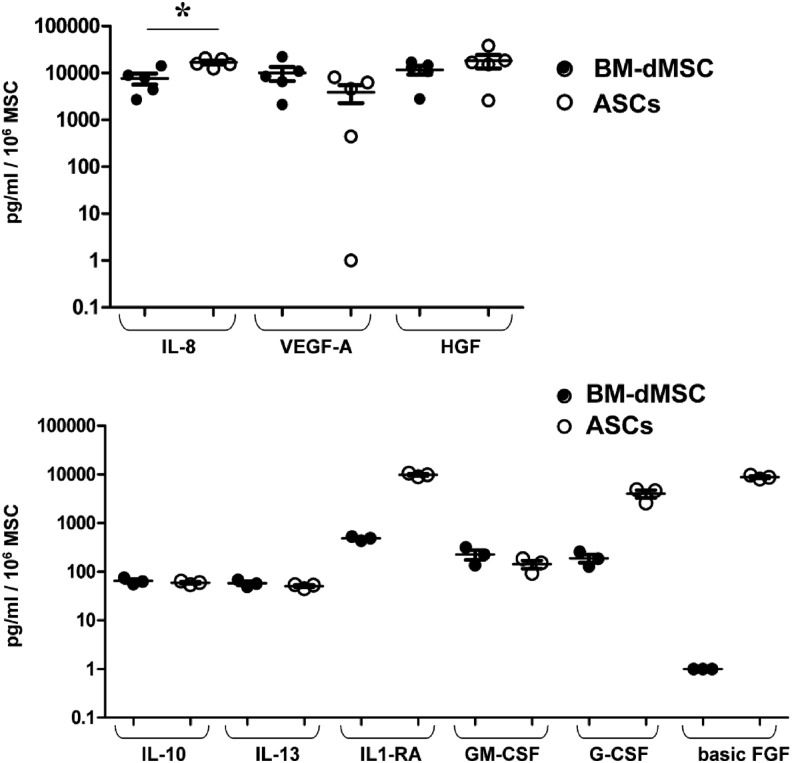
We next investigated the secretion profile of MSC related to wound healing. For this purpose, MSC were cultured on the stromal side of AM for 48 h and supernatants were collected to study the secretion of angiogenic proteins (VEGF-A, IL-8), anti-inflammatory cytokines (IL-10, IL-13, and IL-1-RA) and growth factors (GM-CSF, G-CSF, TGFα, TNFα, TNFβ, basic FGF, HGF, PDGF-BB, and EGF).
Results (Fig. 6) demonstrated that both BM-dMSC and ASC secrete high amounts of VEGF-A, IL-8 (*p≤0.05), HGF and low amounts of IL-10, IL-13, and GM-CSF. Moreover, ASC secreted high amounts of IL-1-RA, G-CSF, and basic FGF compared with BM-dMSC that secreted low amounts of these proteins. TGFα, TNFα, TNFβ, PDGF-BB, EGF were not secreted by BM-dMSC nor by ASC (Data not shown).
FIG. 6.
Study of MSC secretion profile on AM. MSC derived from one BM and one adipose tissue were incubated at 180,000 cells/cm2 on the stromal side of AM. Supernatants were harvested at 48 h to study the secretion of proteins related to wound healing. Protein concentration was normalized with the quantity of cells determined using an Alamar Blue assay. Values represent the mean±SEM of triplicate (for the first experiment) and duplicate (for the second experiment) of two independent experiments for interleukin (IL)-8, vascular endothelial growth factor (VEGF)-A, and hepatocyte growth factor (HGF) or triplicate of one experiment for IL-10, IL-13, IL-1-RA, platelet-derived growth factor (PDGF-BB), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte-colony-stimulating factor (G-CSF), and basic fibroblast growth factor (FGF) (*p<0.05 between BM-dMSC and ASC).
Discussion
In this study, we aimed to evaluate AM as a biocompatible patch for MSC culture and paracrine activities for wound healing. We demonstrated that AM support the adherence, proliferation, viability, morphology, and the secretion of trophic factors related to wound healing of MSC derived from BM or AT.
To enhance skin repair, MSC have to interact with the entire wound site. For this purpose, MSC delivery onto skin wounds through a scaffold has several advantages compared to direct or intravenous injection. Direct injection or adjacent injection into the wound concentrates MSC only at the site of injection. However, after intravenous injection, MSC are trapped in organs such as the lung, kidney, or spleen and only a small amount of cells reach the wound.9 Thus, interactions of MSC with the wound are more likely to be optimal when a scaffold recovers the entire surface of the wound.
The choice of the scaffold is a critical issue for constructing a skin patch. Many natural or synthetic scaffolds have been developed to deliver cells in target sites.25–27 However, only a small number have been tested in humans and consist of MSC incorporated into a fibrin spray10 or collagen sponge.11 Dermal substitute of animal origin may induce inflammatory reactions.28 Therefore, further developments are warranted to lead to more clinical studies on tissue-engineered products. To create a biocompatible scaffold, low risk of inflammation and immunogenicity are important factors. Several studies reported an anti-inflammatory effect of AM by reducing the expression of IL-1α and IL-1β proinflammatory cytokines29 or by decreasing polymorphonuclear cell infiltration.30 Immunorejection of AM has not been observed in clinical studies of AM23,31 and cells of cryopreserved AM are expected to be dead after cryopreservation at −80°C in glycerol.32 This suggests that AM immunogenicity seems to be low. In our study, no viable cells in the epithelium could be detected (data not shown) nor in the stroma (Fig. 2) of AM during the 15 days studied suggesting that our results are similar with the results published by Kruse et al.32 Considering these properties, the risk of inflammatory or antigenic reaction to the cryopreserved AM scaffold would be limited in the context of allogeneic use.
In our study, we used AM of human origin with the aim of developing a product for the clinical application. Preparation of AM from placenta from tissue banks is simple, rapid and fragments of various sizes can be prepared to treat ulcers of different areas. As a biological dressing, AM has many qualities such as low cost, close adherence and handling properties, oxygen permeability,33 antimicrobial effect,34 finesse, and elasticity.35 AM has been used successfully to construct tissue-engineered products with various cell types for cutaneous repair,16 corneal surface wounds,17 or cartilage repair.18 These results provide evidence that AM is a suitable scaffold for adherence and proliferation of different cells as we have demonstrated in this study for both types of MSC (Figs. 2 and 3).
For cell seeding, AM has been used either intact with amniotic epithelium or denuded without it. Reepithelialization of AM requires several steps, including enzymatic treatment, washing, manual scraping, DNA and RNA degradation that make the process of AM preparation more difficult. These treatments could degrade basal membrane if the technique is not controlled36 and it does not ensure complete cell removal. In the same way, procedures for denuding AM differ among studies,14,17,18,37 suggesting that biological properties of AM could be variable. The advantage of our procedure is that MSC were loaded directly on the stromal side of intact AM without any treatment technique. The stromal layer is composed of materials of the extracellular matrix (ECM) such as collagen, fibronectin, and proteoglycans38 and, therefore, represents a natural scaffold compared with synthetic scaffolds.26 Walker et al.26 recently reported that 85% of MSC from a synthetic scaffold (silicone) were capable to migrate to a reepithelialized human dermis. After a period of contact of 24 h, we observed a transfer of only 3.7% for BM-dMSC and 5.2% for ASC from an AM (stromal matrix) seeded with 180,000 cells/cm2 to another receiving AM (stromal matrix) (data not shown). This discrepancy between the two scaffolds illustrates that MSC attachment is extremely strong on natural ECM compared to a synthetic scaffold. Our type of biological scaffold limited cell delivery in vitro and likely in vivo and, therefore, may facilitate the validation of our tissue-engineered product as clinical grade. The AM patches that we proposed in this study should be mainly considered with its MSC paracrine activities.
In this study, we developed a one-step method that is simple to implement for clinical use. AM can be preserved in French tissue banks with a medium containing 50% glycerol or 10% dimethyl sulfoxide. By comparing the two methods of conservation, we ensured that AM preparation has no impact on MSC adherence at the concentration of 180,000 cells/cm2 (Fig. 5).
In this study, we investigated the biocompatibility of MSC loaded onto the stromal matrix of AM. It is now established that cells are sensitive to the nature of the ECM and that ECM influences the functional properties of cells.39,40 By evaluating the secretion profile of MSC on AM, we were able to characterize the final product that will be applied onto the wound. We demonstrated that both BM-dMSC and ASC adherent to the AM secrete high amounts of VEGF-A, IL-8, and HGF, which are known to be important for neovascularization.6,41–43 Furthermore, adherent MSC secrete anti-inflammatory cytokines such as IL-10, IL-13, and IL-1-RA that would reduce chronic inflammation observed in nonhealing wounds.44 Interestingly, we observed that adherent BM-dMSC and ASC secrete GM-CSF which is likely to have significant patient benefit for chronic wounds when applied locally.45 In this study, adherent ASC secrete a high amount of basic FGF that would restore the decreased levels of basic FGF observed in chronic wounds46 to promote granulation tissue formation, reepithelialization, and tissue remodeling. Our findings suggest that MSC secretome on AM are well suited for skin repair. In vivo, Hong et al. demonstrated that ASC were superior to BM-dMSC in improving granulation tissue.47 In this study, ASC secreted high levels of basic FGF and IL-1-RA compared to BM-dMSC (Fig. 6). Therefore, we would expect that our skin patch constituted with ASC would be more efficient in vivo for improving granulation tissue formation and reducing inflammation in wounded skin.
Developing tissue-engineered product for therapeutic purpose requires production process compliant with GMP. As recommended by the European Medicine Agency (EMA),48 media for cell culture should be devoid of animal components to avoid transmission of zoonotic infections or xenogenic immune reaction. Materials should be biocompatible and suitable for clinical use to avoid inflammatory disorders.28 Moreover, the medium and the process of MSC culture are critical as these parameters could influence MSC properties.49 In this study, we used (1) human AM agreed for clinical use, (2) GMP culture medium supplemented with human platelet lysate produced in GMP condition and extensively studied by Fekete and colleagues19 for ex vivo MSC expansion, (3) clinical-grade MSC from two different sources: BM19 and AT.21 Overall, our data showed that this construction is feasible and releases important cytokines and growth factors that are important in regulating wound healing and tissue regeneration. Animal studies comparing the efficacy of these two products constituted either with BM-dMSC or ASC are ongoing. This therapeutic strategy should represent an alternative treatment for improving cutaneous wound in humans.
Acknowledgments
This work was supported by grant from the 7th FP of EU Cascade Project (no. 223236). We acknowledge Aline Piquemal and Céline De Sousa from the tissue bank of Etablissement Français du Sang Ile de France for preparing AM, Patrick Ausset from the Laboratory of Atmospheric Systems of the University Paris-Est for the analysis of scanning electron microscopy samples. We acknowledge Markus Rojewski and Hubert Schrezenmeier from Ulm University and IKT Ulm in Germany for preparing human platelet lysate and BM-dMSC and Philippe Bourin from Etablissement Français du Sang of Toulouse for preparing ASC.
Disclosure Statement
No competing financial interests exist.
References
- 1.Simon D.A., Dix F.P., and McCollum C.N.Management of venous leg ulcers. BMJ 328,1358, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. . Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Delorme B., Ringe J., Gallay N., Le Vern Y., Kerboeuf D., Jorgensen C., et al. . Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 111,2631, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell J.B., McIntosh K., Zvonic S., Garrett S., Floyd Z.E., Kloster A., et al. . Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24,376, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Strioga M., Viswanathan S., Darinskas A., Slaby O., and Michalek J.Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21,2724, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Wu Y., Chen L., Scott P.G., and Tredget E.E.Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25,2648, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., and Shimizu H.Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180,2581, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Ebrahimian T.G., Pouzoulet F., Squiban C., Buard V., Andre M., Cousin B., et al. . Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol 29,503, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kidd S., Spaeth E., Dembinski J.L., Dietrich M., Watson K., Klopp A., et al. . Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27,2614, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falanga V., Iwamoto S., Chartier M., Yufit T., Butmarc J., Kouttab N., et al. . Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13,1299, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T., Mitsuno H., Nonaka I., Sen Y., Kawanishi K., Inada Y., et al. . Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 121,860, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fernandes M., Sridhar M.S., Sangwan V.S., and Rao G.N.Amniotic membrane transplantation for ocular surface reconstruction. Cornea 24,643, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Oh J.Y., Kim M.K., Shin M.S., Lee H.J., Ko J.H., Wee W.R., et al. . The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 26,1047, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Riau A.K., Beuerman R.W., Lim L.S., and Mehta J.S.Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 31,216, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Portmann-Lanz C.B., Ochsenbein-Kolble N., Marquardt K., Luthi U., Zisch A., and Zimmermann R.Manufacture of a cell-free amnion matrix scaffold that supports amnion cell outgrowth in vitro. Placenta 28,6, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Wilshaw S.P., Kearney J., Fisher J., and Ingham E.Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A 14,463, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Shortt A.J., Secker G.A., Lomas R.J., Wilshaw S.P., Kearney J.N., Tuft S.J., et al. . The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials 30,1056, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Jin C.Z., Park S.R., Choi B.H., Lee K.Y., Kang C.K., and Min B.H.Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng 13,693, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Fekete N., Gadelorge M., Furst D., Maurer C., Dausend J., Fleury-Cappellesso S., et al. . Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy 14,540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., et al. . Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7,393, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., et al. . Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15,641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.H., and Tseng S.C.Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 123,303, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Mermet I., Pottier N., Sainthillier J.M., Malugani C., Cairey-Remonnay S., Maddens S., et al. . Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen 15,459, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kubo M., Sonoda Y., Muramatsu R., and Usui M.Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42,1539, 2001 [PubMed] [Google Scholar]
- 25.Ehrenreich M., and Ruszczak Z.Update on tissue-engineered biological dressings. Tissue Eng 12,2407, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Walker N.G., Mistry A.R., Smith L.E., Eves P.C., Tsaknakis G., Forster S., et al. . A chemically defined carrier for the delivery of human mesenchymal stem/stromal cells to skin wounds. Tissue Eng Part C Methods 18,143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman A.M., Yan Y., Matthias N., Bai X., Rios C., Mathur A.B., et al. . IFATS collection: human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 27,250, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Klein B., Schiffer R., Hafemann B., Klosterhalfen B., and Zwadlo-Klarwasser G.Inflammatory response to a porcine membrane composed of fibrous collagen and elastin as dermal substitute. J Mater Sci Mater Med 12,419, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Solomon A., Rosenblatt M., Monroy D., Ji Z., Pflugfelder S.C., and Tseng S.C.Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol 85,444, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Y., Ma D.H., Hwang D.G., Kim W.S., and Zhang F.Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19,348, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Akle C.A., Adinolfi M., Welsh K.I., Leibowitz S., and McColl I.Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2,1003, 1981 [DOI] [PubMed] [Google Scholar]
- 32.Kruse F.E., Joussen A.M., Rohrschneider K., You L., Sinn B., Baumann J., et al. . Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol 238,68, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Yoshita T., Kobayashi A., Sugiyama K., and Tseng S.C.Oxygen permeability of amniotic membrane and actual tear oxygen tension beneath amniotic membrane patch. Am J Ophthalmol 138,486, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Robson M.C., and Krizek T.J.The effect of human amniotic membranes on the bacteria population of infected rat burns. Ann Surg 177,144, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hieber A.D., Corcino D., Motosue J., Sandberg L.B., Roos P.J., Yu S.Y., et al. . Detection of elastin in the human fetal membranes: proposed molecular basis for elasticity. Placenta 18,301, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Hopkinson A., Shanmuganathan V.A., Gray T., Yeung A.M., Lowe J., James D.K., et al. . Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng Part C Methods 14,371, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Wilshaw S.P., Kearney J.N., Fisher J., and Ingham E.Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng 12,2117, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Parry S., and Strauss J.F., 3rd.Premature rupture of the fetal membranes. N Engl J Med 338,663, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Stadler G., Hennerbichler S., Lindenmair A., Peterbauer A., Hofer K., van Griensven M., et al. . Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy 10,743, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Belperio J.A., Keane M.P., Arenberg D.A., Addison C.L., Ehlert J.E., Burdick M.D., et al. . CXC chemokines in angiogenesis. J Leukoc Biol 68,1, 2000 [PubMed] [Google Scholar]
- 42.Cai L., Johnstone B.H., Cook T.G., Liang Z., Traktuev D., Cornetta K., et al. . Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 25,3234, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Roubelakis M.G., Tsaknakis G., Pappa K.I., Anagnou N.P., and Watt S.M.Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS One 8,e54747, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mast B.A., and Schultz G.S.Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 4,411, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Cianfarani F., Tommasi R., Failla C.M., Viviano M.T., Annessi G., Papi M., et al. . Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol 154,34, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Robson M.C.The role of growth factors in the healing of chronic wounds. Wound Repair Regen 5,12, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Hong S.J., Jia S.X., Xie P., Xu W., Leung K.P., Mustoe T.A., et al. . Topically delivered adipose derived stem cells show an activated-fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS One 8,e55640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.EMA (The European Agency for the Evaluation of Medicinal Products). Note for Guidance on Minimizing the Risk of Transmitting Animal Spongiform Encephalopathy Agents via Human and Veterinary Medicinal Products. EMEA/410/01 revision 2, 2003
- 49.Sotiropoulou P.A., Perez S.A., Salagianni M., Baxevanis C.N., and Papamichail M.Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24,462, 2006 [DOI] [PubMed] [Google Scholar]