Abstract
Background
Simple glycoside surfactants represent a class of chemicals that are produced from renewable raw materials. They are considered to be environmentally safe and, therefore, are increasingly used as pharmaceuticals, detergents, and personal care products. Although they display low to moderate toxicity in cells in culture, the underlying mechanisms of surfactant-mediated cytotoxicity are poorly investigated.
Results
We synthesized a series of triazole-linked (fluoro)alkyl β-glucopyranosides using the copper-catalyzed azide-alkyne reaction, one of many popular “click” reactions that enable efficient preparation of structurally diverse compounds, and investigate the toxicity of this novel class of surfactant in the Jurkat cell line. Similar to other carbohydrate surfactants, the cytotoxicity of the triazole-linked alkyl β-glucopyranosides was low, with IC50 values decreasing from 1198 to 24 μM as the hydrophobic tail length increased from 8 to 16 carbons. The two alkyl β-glucopyranosides with the longest hydrophobic tails caused apoptosis by mechanisms involving mitochondrial depolarization and caspase-3 activation.
Conclusions
Triazole-linked, glucose-based surfactants 4a-g and other carbohydrate surfactants may cause apoptosis, and not necrosis, at low micromolar concentrations via induction of the intrinsic apoptotic cascade; however, additional studies are needed to fully explore the molecular mechanisms of their toxicity.
Graphical Abstract.
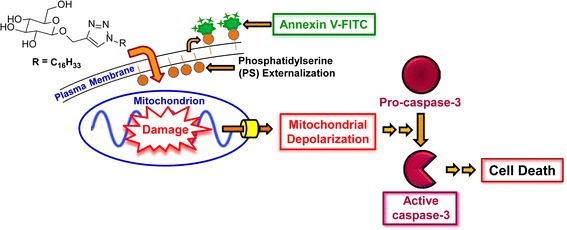
Triazole-linked, glucose-based surfactants cause apoptosis, and not necrosis, at low micromolar concentrations via induction of the intrinsic apoptotic cascade.
Electronic supplementary material
The online version of this article (doi:10.1186/s13065-014-0072-1) contains supplementary material, which is available to authorized users.
Background
Carbohydrate surfactants are an important class of surfactants that can be produced from renewable raw materials (e.g., starch, cellulose, hemicellulose, etc.) and are considered to be environmentally safe. Because of their interesting interfacial properties, carbohydrate surfactants with hydrocarbon tails are useful for a broad range of applications, such as pharmaceuticals, detergents, agrochemicals, food and personal care products [1-3]. Carbohydrate surfactants with partially fluorinated tails are of particular interest for biomedical applications, including blood substitutes and pulmonary drug delivery [4-7]. One feature of carbohydrate surfactants is the availability of an incredible number of structural motifs, including varied head groups, hydrophobic tails and linkers [2]. For example, the polar head group of carbohydrate surfactants can contain one or more mono- to polysaccharide moieties; be cyclic or linear; or differ in the stereochemistry of the hydroxyl groups. Furthermore, the carbohydrate head group can be linked by a variety of approaches, for example glycosylation, esterification and etherification, and using different linkers to one or more hydrophobic tails.
The copper-catalyzed azide-alkyne cycloaddition (CuAAC) [8] between an azide and an alkyne represents an attractive and straightforward approach to link a polar carbohydrate headgroup and a hydrophobic tail. Indeed, a considerable number of carbohydrate surfactants containing a 1,2,3-triazole linker have been described, including simple alkyl xylopyranoside [9] and glucopyranoside surfactants [10], structurally more complex glucose and maltose-based conjugates [11-14], alkyl and aryl O-xylosides and O-xylobiosides [15], 6-triazole-linked galacto- or glucolipids [16], branched fluorinated amphiphiles [17], bolaform surfactants with glucose, galactose and lactose head groups [18], mannitol-based gemini surfactants [19], and “star-like” carbohydrate surfactants [20]. Many triazole-linked carbohydrate surfactants can be synthesized by the reaction of a carbohydrate group containing an azide group with a suitable alkyne derivative, such as alkynes [18] or propargyl derivatives of alcohols [12,14] and fatty acids [11,13,16]. Alternatively, a carbohydrate group with a propargyl group can be reacted with alkyl azides to yield the desired triazole-linked carbohydrate surfactants [9,10,15,18,19].
The synthesis and physicochemical properties of carbohydrate surfactants have been investigated in considerable depths [3,21,22]. However, only limited structure-toxicity relationships of carbohydrate surfactants in general and triazole-liked carbohydrate surfactants in particular have been reported in mammalian systems. Typically, carbohydrate surfactants display low toxicity in cells in culture, with IC50 values in the micro- to millimolar concentration range [4-7,9,13,23-29]. For example, we observed IC50 values ranging from 26 to 890 μM for a series of triazole-linked alkyl β-D-xylopyranosides in several mammalian cell lines, with the Jurkat cell line being the most sensitive cell line [9]. Despite the potential use of carbohydrate surfactants in food and personal care products and biomedical applications, the mechanisms underlying the cytotoxicity of carbohydrate surfactants have not been explored systematically to date. Because of the potentially broad application of the triazole-linker in the synthesis of structurally diverse carbohydrate surfactants, we prepared a series of triazole-linked alkyl β-D-glucopyranosides with hydrocarbon and partially fluorinated hydrophobic tails, and performed a preliminary investigation of possible mechanisms of their toxicity in comparison to other carbohydrate surfactants in the Jurkat cell line.
Results and discussion
Synthesis of triazole-linked alkyl glucopyranosides
The synthesis of the desired glucose-based surfactants was analogous to our previously published synthesis of triazole-containing alkyl β-D-xylopyranosides [9]. These alkyl β-D-xylopyranosides contained a triazole ring incorporated through the CuAAC reaction [8], and possessed surface-active properties. This approach utilizes the ability of this so-called “click” reaction to quickly prepare a series of related molecules. Briefly, the synthesis began with a β-selective glycosylation of commercially-available β-D-glucose pentaacetate (Scheme 1A). This strategy was chosen to yield anomerically pure surfactants, as previous work has suggested the β-alkyl anomers may be more biocompatible [29]. Glycosylation with propargyl alcohol under Lewis-acid-promoted conditions afforded 2 as the β-anomer [30]. The CuAAC reaction between 2 and alkyl azides, which can easily be prepared from the corresponding alkyl bromides or iodides [31], was carried out using 0.1 equiv CuSO4 and 0.2 equiv sodium ascorbate in aqueous tert-butanol to generate 3a–g [8]. In the last step the acetate protecting groups were removed with sodium methoxide, followed by neutralization with Dowex 50 W × 8–100 ion exchange resin to yield the triazole-linked surfactants 4a-g. The final products were purified by recrystallization and provided satisfactory elemental analysis. 1H NMR spectroscopy confirmed the anomeric stereochemistry; the only previously reported synthesis of 4a-e used a Fisher glycosylation which resulted in a mixture of α and β anomers [10]. Overall, the synthetic approach outlined in Scheme 1 offers a facile approach to a large range of novel carbohydrate surfactants with well-defined stereochemistry at the anomeric carbon.
Scheme 1.
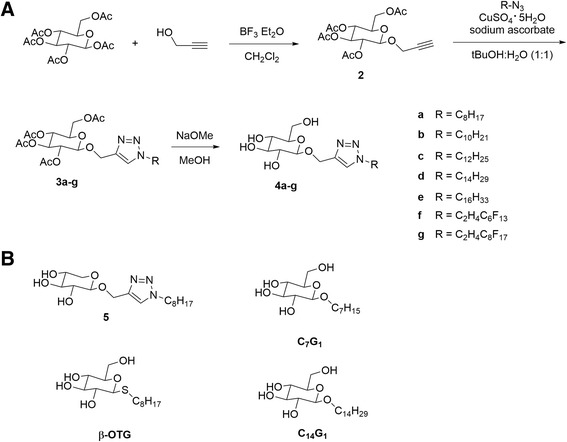
(A) Synthesis of triazole-containing alkyl β-D-glucopyranosides using the CuAAC reaction; (B) Chemical structure and abbreviations of reference surfactants used in the cell culture studies. 5, (1-octyl-1H-1,2,3-triazol-4-yl)methyl β-D-xylopyranoside; C7G1, heptyl-β-D-glucopyranoside; β-OTG, 1-S-octyl-β-D-thioglucopyranoside; C14G1, tetradecyl-β-D-glucopyranoside.
In vitro cytotoxicity of triazole-containing alkyl xyloside surfactants
Biocompatibility studies using mammalian cells in culture suggests that many carbohydrate surfactants with hydrocarbon and partially fluorinated hydrophobic tails are relatively non-toxic in vitro, with no cytotoxicity observable even at millimolar concentrations for some surfactants [4-7,9,13,16,23-29]. Growing experimental evidence suggests that many of these surfactants cause cell death by a mechanism involving apoptosis, not necrosis [9,23,25]. Here, we initially investigated the cytotoxicity of a series of seven triazole-containing alkyl glucopyranosides surfactants 4a-g (Scheme 1) with the MTS ([3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]) assay in the Jurkat cell line and, subsequently, explored possible mechanisms by which they cause cytotoxicity. Four structurally related carbohydrate surfactants (Scheme 1B) were also included in our initial cytotoxicity screening to facilitate the comparison with earlier studies [9,23-25].
The IC50 values of the triazole-containing alkyl β-D-glucopyranoside surfactants 4a-e decreased with increasing alkyl chain length (Table 1), i.e., their cytotoxicity increased with increasing chain-length. Similarly, the cytotoxicity of structurally related, hydrocarbon based carbohydrate surfactants, such as triazole-containing alkyl β-D-xylopyranosides, alkyl β-D-xylopyranosides, alkyl α- and β-D-glactopyranosides, alkyl α- and β-D-glucopyranoside surfactants, and 6-triazole-linked galacto- or glucolipids, increases from short to medium hydrophobic tails [9,16,23-25,29]. One likely explanation for this effect is an increased partitioning of the carbohydrate surfactants into the cell with increasing length of the hydrophobic tail. As a result, the intracellular concentration of homologous carbohydrate surfactants and, thus, their cytotoxicity increases as a function of hydrophobic tail length. Consistent with this observation, we have shown that the apparent membrane partitioning coefficient of carbohydrate surfactants is proportional to the hydrophobic tail length [24]. Unlike hydrocarbon surfactants 4a-e, many other carbohydrate surfactants investigated to date display a “cut-off” effect [32], i.e., carbohydrate surfactants with a long hydrophobic tail show a decrease in the cytotoxicity relative to medium length surfactant [9,23-25,29]. For example, triazole-containing alkyl β-D-xylopyranosides displayed large IC50 values for short chain (hexyl) and long chain surfactants (tetradecyl and hexadecyl), whereas the medium chain alkyl β-D-xylopyranosides (decyl and dodecyl) were the most toxic compounds in this series of surfactants [9]. Although we did not investigate longer hydrophobic tails due to the poor aqueous solubility of the corresponding surfactant (i.e., > hexadecyl), we propose that triazole-containing alkyl β-D-glucopyranoside surfactants 4 would display a “cut-off” effect for surfactants with long hydrophobic tails. The structure-dependent factors likely involved the “cut-off” effect, such as lipid and water solubility, critical aggregate concentration, binding to proteins in the cell and cell culture medium, and diffusion through the cell membrane, are poorly understood and warrant further investigation [32,33].
Table 1.
IC 50 values of hydrocarbon and fluorocarbon triazole-linked alkyl β-D-glucopyranosides 4a-g tested on Jurkat cells a
| Compound | Alkyl β-D-glucopyranosides b | Control surfactants b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | 4b | 4c | 4d | 4e | 4f | 4g | 5 | C14G1 | β-OTG | C7G1 | |
| Hydrophobic tail | C 8 H 17 | C 10 H 21 | C 12 H 25 | C 14 H 29 | C 16 H 33 | C 2 H 4 C 6 F 13 | C 2 H 4 C 8 F 17 | C 8 H 17 | C 14 H 29 | C 8 H 17 | C 7 H 15 |
| IC 50 value [μM] | 1198 | 171 | 89 | 53 | 24 | * | * | 663 | 67 | 163 | * |
aThe inhibitory concentration 50% (IC50) in μM is defined as the concentration of experimental compound required to inhibited 50% of the conversion of MTS to formazan, as compared with the absorbance produced by untreated cells after 16 h of incubation.
bPlease see Scheme 1 for the chemical structures and corresponding abbreviations for the alkyl β-D-glucopyranosides and the control surfactants.
*Cytotoxicity was <50% at the highest compound concentration tested (2000 μM) and therefore their IC50 values could not be determined.
Comparison of the IC50 values of the triazole-containing alkyl β-D-glucopyranosides surfactants 4 with structurally related surfactants reveals interesting structure-toxicity relationships (Table 1). For example, the IC50 values of the alkyl β-D-glucopyranosides C14G1 is comparable to the IC50 values of the structurally related triazole-containing alkyl glucoside 4d. This observations is consistent with our earlier findings that the triazole-linker does not markedly affect the cytotoxicity of triazole-containing alkyl xyloside [9] and the more general expectation that the introduction of a carbohydrate group renders drug molecules containing a triazole group less cytotoxic [34]. In contrast, the triazole-containing octyl glucoside compound 4a appeared to be more toxic compared to its structural analogue, C7G1, with IC50 values of 1198 μM and > 2,000 μM, respectively. It is also interesting to note that the IC50 value of the triazole-containing octyl xyloside 5 was significantly lower compared to its structural glucoside analog 4a, indicating that xyloside-based surfactants are more cytotoxic compared to glucose-based surfactants. This observation is remarkable because xylose and glucose differ only by a single hydroxymethyl group, but otherwise have the same stereochemistry in the pyranose ring system.
Consistent with this observation, we have previously reported that small changes in the structure of the carbohydrate head group of a surfactant can influence its toxicity [24]. Simple hexadecyl and octadecyl glucopyranoside surfactants, but not structurally related galactoside surfactants caused cytotoxicity at low millimolar concentrations in the B16F10 mouse melanoma cell line. A similar observation has been reported for partially fluorinated gluco- vs. galactopyranoside in the B16 melanoma cell line [28] and for 6-triazole-linked galacto- or glucolipids in the A549 human lung adenocarcinoma epithelial cell line [16]. Moreover, there is some evidence that the configuration at the anomeric center may play a role in the cytotoxicity of carbohydrate surfactants in different cancer cell lines [29]; however this effect of the stereochemistry on the anomeric center has not been observed in all studies [24], possibly due to differences in the carbohydrate surfactants, cell lines and/or experimental conditions employed. Since the stereochemistry of hydroxyl groups of some surfactants, such as uronic acid-based surfactants, is known to drastically alter their physicochemical properties [35,36], it is possible that small changes in the stereochemistry of the polar head-group result in differences in the cytotoxicity by either indirectly altering physicochemical properties of macromolecular structures, such as the cell membrane, and/or direct interaction with cellular targets.
The two partially fluorinated surfactants 4f (F-octyl) and 4g (F-decyl) displayed no cytotoxicity in the Jurkat cell line over the entire concentration range investigated (8 μM to 2 mM). The hydrocarbon surfactant 4a, which is the structural analog of partially fluorinated surfactant 4g, displayed low toxicity in the Jurkat cell line, with an IC50 value of 1,198 μM. Similarly, many other studies have reported that the introduction of a perfluoroalkyl group in a hydrocarbon surfactant is typically protective and significantly decreases its cytotoxicity in mammalian cells in culture [5-7,9,24,25,27,28]. However, a perfluoroalkyl group in the hydrophobic tail is not always protective, as we have shown for octyl versus F-octyl β-D-xylopyranosides [23]. These differences in the cytotoxicity of hydrocarbon and partially fluorinated carbohydrate surfactants likely reflect differences in the physicochemical properties of the respective carbohydrate surfactant caused by the introduction of varying degrees of fluorination in the hydrophobic tail.
Annexin V/PI apoptosis/necrosis assay
Our previous studies demonstrate that structurally diverse carbohydrate surfactants, including triazole-linked alkyl β-D-xylopyranosides cause cytotoxicity by apoptosis and not necrosis [9,23,25]. We therefore assessed whether triazole-linked alkyl β-D-glucopyranosides 4d (tetradecyl) and 4e (hexadecyl) cause cytotoxicity by apoptosis or necrosis. Because phosphatidylserine translocation from the inner leaflet to the outer membrane is an early event in apoptotic cell death [37], Annexin V-FITC, which has a high affinity for phosphatidylserine, was used to detect phosphatidylserine as a marker of apoptosis in live-cells by flow cytometry. As can be seen in Figure 1, a significant amount of phosphatidylserine is externalized when Jurkat cells were treated with the tetradecyl glycopyranoside C14G1 (~65%), while treatment with 4d and 4e resulted in lower (<20%) but significant Annexin-V staining after a 16 h incubation. These findings demonstrate that, similar to other carbohydrate surfactants, triazole-linked alkyl glucopyranosides 4d and 4e cause apoptosis in the Jurkat cell line.
Figure 1.
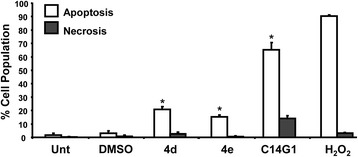
Triazole-containing alkyl β-D-glucopyranosides 4d and 4e and alkyl β-D-glucopyranosides C14G1 induced significant phosphatidylserine externalization in the Jurkat cell line. The mode of cell death induction, apoptosis or necrosis, was monitored via flow cytometric assay after co-staining of cells with Annexin V-FITC and PI. Cells were exposed to the ~ IC50 concentration of each compound as determined by MTS assay (see Table 1). The total percentage of apoptotic cells is expressed as the sum of percentages of both early and late stages of apoptosis (Annexin V-FITC positive; white bars), with green fluorescence signal. Cells that were stained only with PI due to the loss of plasma membrane integrity, but without FITC signal, are considered necrotic cells (gray bars). Analysis of the apoptotic populations using the two-tailed Student's paired t-test of 4d, 4e and C14G1-treated Jurkat cells against DMSO and untreated controls was P < 0.001 (*). Each bar represents the average of three independent measurement values, and error bars represent the standard deviation of the mean. Unt refers to untreated cells.
Surfactant-mediated cytotoxicity involves a mitochondria-dependent apoptosis pathway
Apoptosis can be caused by the activation of cysteine-aspartic acid proteases (caspases) through an intrinsic, mitochondria-mediated pathway or an extrinsic pathway involving cellular death receptors, such as FAS/CD95 or tumor necrosis factor receptor 1 (TNFR1). To gain further insights into the mechanisms involved in carbohydrate surfactant-mediated apoptosis, we investigated the dissipation of mitochondrial membrane potential (ΔΨm), an early facet in apoptosis that has been implicated in initiating the intrinsic pathway [38]. Briefly, Jurkat cells were treated for 6 h with carbohydrate surfactants 4d, 4e or C14G1, stained with the fluorophore 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) and analyzed via flow cytometry [39,40]. Results indicated that 4d and C14G1 compounds provoked preferential accumulation of JC-1 monomers (green), an indicator of mitochondrial depolarization, and interfered with the formation of JC-1 aggregates (red) (Figure 2). A similar distribution pattern was observed in cells treated with the positive control, H2O2. The most potent compound causing mitochondrial depolarization was C14G1 followed by 4d. These outcomes suggest that 4d- and C14G1-mediated cytotoxicity is initiated via ΔΨm depolarization, involving the intrinsic apoptotic pathway in the initiation of cell death. In contrast, 4e-mediated toxicity appeared to circumvent ΔΨm dissipation to induce cell death.
Figure 2.
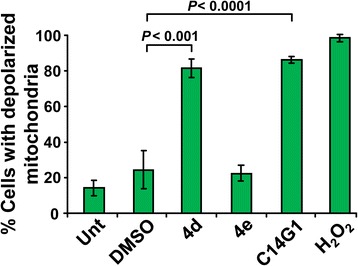
Mitochondrial depolarization mediated by triazole-containing alkyl β-D-glucopyranosides 4d and 4e and alkyl β-D-glucopyranosides C14G1. Jurkat cells were treated with triazole-containing alkyl β-D-glucopyranosides 4d and 4e and alkyl β-D-glucopyranosides C14G1 at their respective IC50 concentration and incubated for 6 h. Changes in the mitochondrial ΔΨm was determined by staining with 2 μM of JC-1. The IC50 concentration values that were used were the following: 4d = 53 μM; 4e = 24 μM; and C14G1 = 66.5 μM. After dissipation of ΔΨm, the JC-1 reagent emits a green fluorescence signal, while the compound in a polarizedmitochondrial membrane emits a red signal. Percentages of cells emitting green fluorescence signal (y-axis) are depicted. Each bar represents the mean ± SD of four independent replicates. The following controls were included: untreated cells as a negative control; cells treated with 0.1% v/v DMSO as a control for solvent effects; and cells exposed to 1 mM H2O2 as a positive control. Approximately 1x104 flow cytometry events were acquired and analyzed per sample using CXP software.
Surfactants inflict cytotoxicity via caspase-3 activation
Caspase-3 is activated by both the intrinsic and extrinsic apoptotic pathways. To examine whether caspase-3 activation was involved in the cytotoxicity provoked by the selected experimental compounds, a cell permeable fluorogenic reagent, NucView 488 Caspase-3 substrate, and Jurkat cells were utilized. This substrate allows the detection of caspase-3 activity in live cells via flow cytometry. Jurkat cells with active caspase-3 were significantly detected after 6 h of incubation with 4d, 4e and alkyl β-D-glucopyranoside C14G1, as compared with untreated and solvent controls (DMSO; P < 0.001; Figure 3) [39,40]. The most efficient carbohydrate surfactant eliciting caspase-3 activation was C14G1 (Figure 3). These observations suggest that the cytotoxicity induced by 4d, 4e and C14G1was indeed mediated via apoptosis as initially detected by phosphatidylserine externalization and corroborated by caspase-3 activation; both hallmarks of apoptosis.
Figure 3.
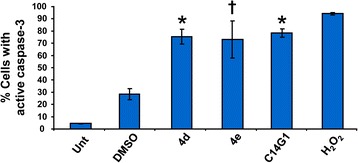
Treatment of Jurkat cells with triazole-containing alkyl β-D-glucopyranosides 4d and 4e and alkyl β-D-glucopyranosides C14G1 resulted in caspase-3 activation. Jurkat cells were treated with compounds at their respective IC50 concentration. The percentage of cells with activated caspase-3 as determined by emission of green fluorescence signal is indicated on y axis. Each bar represents average of three independent measurement values, and error bars their corresponding SD values. Data were analyzed using the two-tailed Student's paired t-test of compound treated cells vs. DMSO treated cells with P-values within statistically significant range of P < 0.0001 (*) and P < 0.008 (†).
Experimental
General procedures
The 1H and 13C NMR spectra were recorded on a Bruker DRX 400 Digital NMR spectrometer. 19F spectra were recorded using a Bruker Avance 300. NMR assignments were determined from a COSY spectrum of 3b. Representative 1H and 13C NMR spectra are included in Additional file 1. High resolution mass spectra were obtained at the University of California, Riverside Mass Spectrometry facility. Elemental analyses were obtained from Atlantic Micro Lab Microanalysis Service (Atlanta, Georgia, USA). All reactions were monitored by thin layer chromatography, followed by visualization with UV and anisaldehyde-H2SO4. Azides were prepared using a known method [31] and used without further purification. β-Propargyl 2,3,4,6-tetra-O-acetylglucopyranoisde was prepared from commercially available β-D-glucopyranoside pentaacetate as previously described. Compounds 3a-e and 4a-e have been reported in the literature [10]. Tetradecyl β-D-glucopyranoside (C14G1) was prepared as previously described [24]. 1-S-Octyl-β-D-thioglucopyranoside (β-OTG), propargyl alcohol and Dowex® 50W × 8-100 ion exchange resin were obtained from Acros Organics/Fisher Scientific (Pittsburgh, PA). Boron trifluoride diethyl ethereate and sodium methoxide were obtained from Alfa Aesar (Ward Hill, MA). Sodium azide and sodium ascorbate were obtained from Aldrich (St. Louis, MO). Cupric sulfate pentahydrate was obtained from Mallinckrodt (St. Louis, MO). All organic solvents were reagent grade or higher and were used without further purification. Flash chromatography was performed using 60 Å (40-63 μm, 230x400 mesh) silica gel.
General procedure for the CuAAC reaction
Triacetyl propargyl glucose (2) and n-alkyl azide (1.0 – 1.1 eq.) were combined with 2:1 tert-butanol: water (0.33 M) at room temperature. Sodium ascorbate (0.2 eq., 1.0 M in water) was added, followed by CuSO4 pentahydrate (0.1 eq., 75 mg/mL in water), and the mixture stirred at room temperature for 90 minutes. At this time the reactions often became homogeneous and faint blue. The reaction mixture was diluted with water and extracted three times with ethyl acetate. The combined extracts were washed with brine, dried over MgSO4 and concentrated. The crude residue was purified by silica gel column chromatography (hexanes/EtOAc), or used without further purification.
(1-Octyl-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3a)
The general procedure was used with propargyl glucose (512 mg, 1.32 mmol) and 1-azidooctane (208 mg, 1.32 mmol); after column chromatography using EtOAc:hexanes (2:1, v/v), 636 mg (89%) of 3a were obtained as a clear oil which solidified upon standing. 1H NMR (CDCl3, 400MHz): δ 7.49 (s, 1H, triazole-CH), 5.18 (app t, J = 9.4 Hz, 1H, H-3), 5.08 (app t, J = 9.9 Hz, 1H, H-4), 4.99 (dd, J = 9.5, 8.0 Hz, 1H, H-2), 4.92 (d, J = 12.6 Hz, 1H, H-1’a), 4.80 (d, J = 12.6 Hz, 1H, H-1’b), 4.66 (d, J = 7.9 Hz, 1H, H-1), 4.32 (t, J = 7.3 Hz, 2H, H-α), 4.26 (dd, J = 12.3, 4.8 Hz, 1H, H-6a), 4.14 (dd, J = 12.3, 2.3 Hz, 1H, H-6b), 3.72 (ddd, J = 9.9, 4.6, 2.3 Hz, 1H, H-5), 2.07 (s, 3H, OAc), 2.00 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.96 (s, 3H, OAc), 1.84 - 1.91 (m, 2H, H-β), 1.15 - 1.37 (m, 10H, 5 × CH2), 0.85 (t, J = 6.5 Hz, 3H, H-ω); 13C NMR (CDCl3, 100 MHz): δ 170.6, 170.2, 169.4, 169.3, 144.0, 122.5, 99.7, 72.7, 71.8, 71.1, 68.1, 63.0, 61.7, 50.4, 31.6, 30.2, 29.0, 28.8, 26.4, 22.5, 20.7, 20.61, 20.56 (2 × C), 14.0; HRESIMS calcd for C25H40N3O10 (M + H)+: 542.2708; found: 542.2710.
(1-Decyl-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3b)
The general procedure was used with propargyl glucose (507 mg, 1.31 mmol) and 1-azidodecane (240 mg, 1.31 mmol); after column chromatography using EtOAc:hexanes (2:1, v/v), 669 mg (90%) of 3b were obtained as a waxy solid. 1H NMR (CDCl3, 400MHz): δ 7.50 (s, 1H, triazole-CH), 5.19 (app t, J = 9.4 Hz, 1H, H-3), 5.09 (app t, J = 9.8 Hz, 1H, H-4), 5.01 (dd, J = 9.5, 8.0 Hz, 1H, H-2), 4.93 (d, J = 12.5 Hz, 1H, H-1’a), 4.82 (d, J = 12.5 Hz, 1H, H-1’b), 4.68 (d, J = 8.0 Hz, 1H, H-1), 4.33 (t, J = 7.3 Hz, 2H, H-α), 4.27 (dd, J = 12.3, 4.8 Hz, 1H, H-6a), 4.15 (dd, J = 12.3, 2.3 Hz, 1H, H-6b), 3.73 (ddd, J = 9.9, 4.7, 2.3 Hz, 1H, H-5), 2.09 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.98 (s, 2H, OAc), 1.84 - 1.94 (m, 2H, H-β), 1.18 - 1.37 (m, 14H, 7 × CH2), 0.87 (t, J = 6.5 Hz, 3H, H-ω); 13C NMR (CDCl3, 100 MHz): δ 170.5, 170.0, 169.3, 169.2, 143.3, 122.4, 99.5, 72.7, 71.8, 71.1, 68.2, 62.9, 61.7, 50.3, 31.7, 30.2, 29.35, 29.25, 29.1, 28.9, 26.4, 22.5, 20.6, 20.53, 20.47 (2 × C), 14.8; HRESIMS calcd for C27H44N3O10: 570.3021; found: 570.3034.
(1-Dodecyl-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3c)
The general procedure was used with propargyl glucose (526 mg, 1.36 mmol) and 1-azidododecane (286 mg, 1.36 mmol); after column chromatography using EtOAc:hexanes (2:1, v/v), 666 mg (82%) of 3c were obtained as a waxy solid. 1H NMR (CDCl3, 400MHz): δ 7.50 (s, 1H, triazole-CH), 5.20 (app t, J = 9.5 Hz, 1H, H-3), 5.09 (app t, J = 9.9 Hz, 1H, H-4), 5.01 (dd, J = 9.5, 7.9 Hz, 1H, H-2), 4.94 (d, J = 12.5 Hz, 1H, H-1’a), 4.82 (d, J = 12.5 Hz, 1H, H-1’b), 4.69 (d, J = 8.0 Hz, 1H, H-1), 4.33 (t, J = 7.2 Hz, 2H, H-α), 4.27 (dd, J = 12.3, 4.8 Hz, 1H, H-6a), 4.15 (dd, J = 12.3, 2.4 Hz, 1H, H-6b), 3.73 (ddd, J = 10.0, 4.8, 2.4 Hz, 1H, H-5), 2.09 (s, 3H, OAc), 2.03 (s, 3H, OAc), 2.00 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.85-1.95 (m, 2H, H-β), 1.19 - 1.36 (m, 18H, 9 x CH2), 0.88 (t, J = 6.7 Hz, 3H, H-ω); 13C NMR (CDCl3, 100 MHz) δ 171.7, 171.2, 170.5, 170.4, 145.1, 123.5, 101.0, 73.9, 73.0, 72.3, 69.4, 64.1, 62.9, 51.5, 32.9, 31.4, 30.63 (2 × C), 30.56, 30.43, 30.36, 30.0, 27.6, 23.7, 21.8, 21.7, 21.6 (2 × C), 15.2; HRESIMS calcd for C29H48N3O10: 598.3334; found: 598.3339.
(1-Tetradecyl-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3d)
The general procedure was used with propargyl glucose (526 mg, 1.36 mmol) and 1-azidotetradecane (371 mg, 1.55 mmol); after column chromatography using EtOAc:hexanes (3:2, v/v), 636 mg (75%) of 3d were obtained as a waxy solid. 1H NMR (CDCl3, 400MHz): δ 7.49 (s, 1H, triazole-CH), 5.19 (app t, J = 9.4 Hz, 1H, H-3), 5.09 (app t, J = 9.9 Hz, 1H, H-4), 5.01 (dd, J = 9.5, 8.0 Hz, 1H, H-2), 4.93 (d, J = 12.5 Hz, 1H, H-1’a), 4.81 (d, J = 12.1 Hz, 1H, H-1’b), 4.68 (d, J = 8.1 Hz, 1H, H-1), 4.33 (t, J = 7.3 Hz, 2H, H-α), 4.27 (dd, J = 12.3, 4.8 Hz, 1H, H-6a), 4.14 (dd, J = 12.3, 2.3 Hz, 1H, H-6b), 3.69 - 3.78 (m, 1H, H-5), 2.08 (s, 3H, OAc), 2.02 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.97 (s, 3H, OAc), 1.83 - 1.94 (m, 2H, H-β), 1.17 - 1.40 (m, 22H, 11 x CH2), 0.87 (t, J = 6.8 Hz, 3H, H-ω); 13C NMR (CDCl3, 100 MHz): δ 170.6, 170.1, 169.4, 169.3, 144.0, 122.4, 99.8, 72.7, 71.9, 71.2, 68.3, 63.0, 61.8, 50.4, 31.9, 30.3, 29.6, 29.6, 29.58 (2 × C), 29.54, 29.33, 29.29, 28.9, 26.5, 22.6, 20.7, 20.6, 20.5 (2 × C), 14.1; HRESIMS calcd for C31H52N3O10: 626.3647; found: 626.3670.
(1-Hexadecyl-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3e)
The general procedure was used with propargyl glucose (528 mg, 1.36 mmol) and 1-azidohexadecane (373 mg, 1.38 mmol); after column chromatography using EtOAc:hexanes (3:2, v/v), 692 mg (78%) of 3e were obtained as a white solid. 1H NMR (CDCl3, 400MHz): δ 7.49 (s, 1H, triazole-CH), 5.19 (app t, J = 9.5 Hz, 1H, H-3), 5.08 (app t, J = 9.8 Hz, 1H, H-4), 5.00 (dd, J = 9.5, 8.0 Hz, 1H, H-2), 4.93 (d, J = 12.6 Hz, 1H, H-1’a), 4.81 (d, J = 12.5 Hz, 1H, H-1’b), 4.68 (d, J = 7.9 Hz, 1H, H-1), 4.32 (t, J = 7.2 Hz, 2H, H-α), 4.27 (dd, J = 12.3, 4.8 Hz, 1H, H-6a), 4.14 (dd, J = 12.3, 2.3 Hz, 1H, H-6b), 3.73 (ddd, J = 9.9, 4.7, 2.4 Hz, 1H, H-5), 2.08 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.98 (s, 3H, OAc), 1.97 (s, 3H, OAc), 1.81 - 1.94 (m, 2H, H-β), 1.16 - 1.36 (m, 26H, 13 × CH2), 0.87 (t, J = 6.6 Hz, 3H, H-ω); 13C NMR (CDCl3, 100 MHz): δ 170.6, 170.1, 169.4, 169.3, 143.9, 122.5, 99.7, 72.7, 71.8, 71.1, 68.2, 62.9, 61.7, 50.3, 31.8, 30.9, 30.2, 29.59 (2 × C), 29.56 (2 × C), 29.52, 29.45, 29.3, 29.3, 28.9, 26.4, 22.6, 20.7, 20.6, 20.5, 14.1; HRESIMS calcd for C33H56N3O10: 654.3960; found: 654.3980.
(1-(3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluorooctyl)-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3f)
The general procedure was used with propargyl glucose (702 mg, 1.81 mmol) and 1-azido-3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctane (707 mg, 1.81 mmol); after column chromatography using EtOAc:hexanes (3:2, v/v), 846 mg (60%) of 3f were obtained as a white solid. 1H NMR 1H NMR (CDCl3, 400 MHz): δ 7.60 (s, 1H, triazole-CH), 5.20 (app t, J = 9.3 Hz, 1H, H-3), 5.10 (app t, J = 9.8 Hz, 1H, H-4), 5.01 (dd, J = 9.4, 8.1 Hz, 1H, H-2), 4.94 (d, J = 12.4 Hz, 1H, H-1’a), 4.83 (d, J = 12.7 Hz, 1H, H-1’b), 4.66-4.69 (m, 3H, H-1, H-α), 4.25 (dd, J = 12.4, 4.6 Hz, 1H, H-6a), 4.15 (dd, J = 12.4, 2.1 Hz, 1H, H-6b), 3.73 (ddd, J = 10.0, 4.4, 2.3 Hz, 1H, H-5), 2.83 (tt, J = 18.0, 7.5 Hz, 1H, H-β); 13C NMR (CDCl3, 100 MHz): δ 170.6, 170.2, 169.4, 169.4, 144.6, 123.4, 100.0, 72.6, 71.9, 71.1, 68.2, 63.0, 61.6, 42.3, 31.6, 20.7, 20.6 (3 × C); 19F NMR (282 MHz, CDCl3) δ -81.23, -114.70, -122.35, -123.37, -123.96, -126.64; HRESIMS calcd for C25H27N3O10F13: 776.1483; found: 776.1470.
(1-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl)-1H-1,2,3-triazol-4-yl)methyl 2,3,4-tri-O-acetyl-β-glucopyranoside (3g)
The general procedure was used with propargyl glucose (702 mg, 1.81 mmol) and 1-azido-3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorooctane (707 mg, 1.81 mmol); after column chromatography using EtOAc:hexanes (3:2, v/v), 846 mg (60%) of 3g were obtained as a white solid. 1H NMR (CDCl3, 400 MHz): δ 7.59 (s, 1H, triazole-CH), 5.20 (app t, J = 9.3 Hz, 1H, H-3), 5.09 (app t, J = 9.8 Hz, 1H, H-4), 5.01 (dd, J = 9.4, 8.1 Hz, 1H, H-2), 4.93 (d, J = 12.4 Hz, 1H, H-1’a), 4.83 (d, J = 12.7 Hz, 1H, H-1’b), 4.66-4.70 (m, 3H, H-1, H-α), 4.25 (dd, J = 12.4, 4.6 Hz, 1H, H-6a), 4.18 (dd, J = 12.4, 2.1 Hz, 1H, H-6b), 3.73 (ddd, J = 10.0, 4.4, 2.3 Hz, 1H, H-5), 2.83 (tt, J = 18.0, 7.5 Hz, 1H, H-β); 13C NMR (CDCl3, 100 MHz): δ 170.6, 170.2, 169.4, 169.4, 144.6, 123.4, 100.0, 72.6, 71.9, 71.1, 68.2, 63.0, 61.6, 42.3, 31.6, 20.7, 20.6 (3 × C); 19F NMR (282 MHz, CDCl3) δ -81.23, -114.68, -122.13, -122.44 (2 × CF2), -123.22, -123.94, -126.62; HRESIMS calcd for C27H27N3O10F17: 867.1420; found: 876.1421.
General procedure for acetate deprotection
Triazole peracetates 3 were stirred in dry methanol. NaOMe (1 eq.) was added and the solution stirred at room temperature for 2-4 hr. Dowex® 50W × 8-100 ion exchange resin was added and the reaction mixture stirred for another 30 min. The resin was filtered and the solvent concentrated. The crude residue was purified by recrystallization or column chromatography to yield pure 1-alkyl-1H-1,2,3-triazol-4-ylmethyl β-D-glucopyranosides 4.
(1-Octyl-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4a)
Following the general procedure for acetate deprotection, 902 mg (1.66 mmol) of 3a and 90 mg (1.66 mmol) NaOMe were stirred in 6 mL MeOH. The crude product purified by column chromatography, yielding 434 mg (70%) of 4a as a white solid. 1H NMR (MeOD, 400 MHz): δ 8.03 (s, 1H, triazole-CH), 4.98 (d, J = 12.3 Hz, 1H, H-1’a), 4.79 (d, J = 12.4 Hz, 1H, H-1’b), 4.37 - 4.43 (m, 3H, H-1, H-α), 3.91 (dd, J = 11.9, 1.7 Hz, 1H, H-6a), 3.69 (dd, J = 11.9, 5.6 Hz, 1H, H-6b), 3.18 - 3.41 (m, 4H, H-2, H-3, H-4, H-5), 1.85 - 1.98 (m, 2H, H-β), 1.22 - 1.42 (m, 10H, 5 × CH2), 0.90 (t, J = 6.7 Hz, 3H, H-ω); 13C NMR (MeOD, 100 MHz): δ 145.8, 125.4, 103.7, 78.2, 78.1, 75.1, 71.7, 63.1, 62.9, 51.5, 33.1, 31.5, 30.4, 30.2, 27.6, 23.8, 14.6; HRESIMS calcd for C17H32N3O6 (M + H)+: 374.2286; found: 374.2289; Anal calcd for C17H31N3O6: C 54.68, H 8.37, N 11.25; found: C 54.43, H 8.20, N 10.99.
(1-Decyl-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4b)
Following the general procedure for acetate deprotection, 597 mg (1.05 mmol) of 3b and 56 mg (1.05 mmol) NaOMe were stirred in 4 mL MeOH for 4 hours. The crude product was purified by column chromatography (5:4:1 CH2Cl2:acetone:MeOH), yielding 266 mg (62%) of 4b as a white solid. 1H NMR (MeOD, 400 MHz): δ 8.03 (s, 1H, triazole-CH), 4.78 (d, J = 12.4 Hz, 1H, H-1’a), 4.78 (d, J = 12.4 Hz, 1H, H-1’b), 4.36-4.44 (m, 3H, H-1, H-α), 3.90 (dd, J = 11.9, 1.6 Hz, 1H, H-6a), 3.68 (dd, J = 11.9, 5.6 Hz, 1H, H-6b), 3.11 - 3.44 (m, 4H, H-2, H-3, H-4, H-5), 1.81 - 2.00 (m, 2H, H-β), 1.17 - 1.44 (m, 14H, 7 × CH2), 0.90 (t, J = 6.7 Hz, 3H, H-ω); 13C NMR (MeOD, 100 MHz) δ 145.8, 125.4, 103.7, 78.2, 78.1, 75.1, 71.7, 63.1, 62.9, 51.5, 33.2, 31.4, 30.8, 30.7, 30.6, 30.3, 27.6, 23.9, 14.6; HRESIMS calcd for C19H36N3O6 (M + H)+: 402.2599; found: 402.2599; Anal cald for C19H35N3O6 (H2O)0.4: C 55.84, H 8.83, N 10.28; found: C 55.90, H 8.77, N 10.09.
(1-Dodecyl-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4c)
Following the general procedure for acetate deprotection, 540 mg (0.904 mmol) of 3c and 48 mg (0.904 mmol) NaOMe were stirred in 4 mL MeOH for 4 hours. The crude product was purified by recrystallization from acetone/hexane, yielding 266 mg (62%) of 4c as a white solid. 1H NMR (MeOD, 400 MHz): δ 8.01 (s, 1H, triazole-CH), 4.97 (d, J = 12.3 Hz, 1H, H-1’a), 4.78 (d, J = 12.4 Hz, 1H, H-1’b), 4.38-4.1 (m, 3H, H-1, H-α), 3.90 (dd, J = 11.7, 1.5 Hz, 1H, H-6a), 3.66 (dd, J = 11.9, 4.9 Hz, 1H, H-6b), 3.17 - 3.37 (m, 4H, H-2, H-3, H-4, H-5), 1.78 - 2.04 (m, 2H, H-β), 1.19 - 1.42 (m, 18H, 9 x CH2), 0.90 (t, J = 6.7 Hz, 3H, H-ω); 13C NMR (MeOD, 100 MHz): δ 145.8, 125.4, 103.8, 78.2, 78.1, 75.2, 71.8, 63.2, 62.9, 51.5, 33.2, 31.4, 30.9 (2 × C), 30.8, 30.7, 30.6, 30.2, 27.6, 23.9, 14.6; HRESIMS calcd for C21H40N3O6 (M + H)+: 430.2912; found: 430.2917; Anal calcd for C21H39N3O6(H2O)0.25: C 58.11, H 9.17, N 9.68: Found: C 58.33, H 9.14, N 9.37.
(1-Tetradecyl-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4d)
Following the general procedure for acetate deprotection, 394 mg (0.630 mmol) of 3d and 34 mg (0.63 mmol) NaOMe were stirred in 2.4 mL MeOH for 2 hours. The crude product was purified by column chromatography (5:4:1 CH2Cl2:acetone:MeOH), yielding 177 mg (61%) of 4d as a white solid. 1H NMR (MeOD, 400MHz): δ 8.01 (s, 1H, triazole-CH), 4.97 (d, J = 12.4 Hz, 1H, H-1’a), 4.78 (d, J = 12.4 Hz, 1H, H-1’b), 4.35 - 4.44 (m, 3H, H-1, H-α), 3.90 (dd, J = 11.8, 1.6 Hz, 1H, H-6a), 3.65 - 3.72 (m, 1H, H-6b), 3.19 - 3.34 (m, 4H, H-2, H-3, H-4, H-5), 1.83 - 1.99 (m, 2H, H-β), 1.18 - 1.43 (m, 22H, 11 × CH2), 0.90 (t, J = 6.6 Hz, 3H, H-ω); 13C NMR (MeOD, 100 MHz): δ 145.8, 125.4, 103.8, 78.2, 78.1, 75.2, 71.8, 63.2, 62.9, 51.5, 33.2, 31.4, 30.92, 30.90, 30.89, 30.87, 30.8, 30.7, 30.6, 30.2, 27.6, 23.9, 14.6; HRESIMS calcd for C24H44N3O6 (M + H)+: 458.3225; found: 458.3246; Anal calcd for C24H43N3O6: 60.37, H 9.47, N 9.18; found: C 59.97, H 9.29, N 8.91.
(1-Hexadecyl-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4e)
Following the general procedure for acetate deprotection, 800 mg (1.22 mmol) of 3e and 66 mg NaOMe (1.22 mmol) were stirred in 5 mL MeOH for 3.5 hours. The crude product was purified by recrystallization from methanol, yielding 176 mg (30%) of 4e as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 8.10 (s, 1H, triazole-CH), 4.83 (d, J = 12.1 Hz, 1H, H-1’a), 4.62 (d, J = 12.4 Hz, 1H, H-1’b), 4.32 (t, J = 7.1 Hz, 2H, H-α), 4.25 (d, J = 7.7 Hz), 3.71 (dd, J = 11.7, 1.8 Hz, 1H, H-6a), 3.46 (dd, J = 11.8, 6.4 Hz, 1H, H-6b), 3.08 - 3.18 (m, 3H, H-3, H-4, H-5), 2.98 (app t, J = 7.8 Hz, H-2), 1.73 - 1 .86 (m, 2H, H-β), 1.15 - 1.39 (m, 26H, 13 × CH2), 0.85 (t, J = 7.3 Hz, H-ω); 13C NMR (DMSO-d6, 100 MHz): δ 143.7, 124.0, 102.1, 76.9, 76.7, 73.4, 70.1, 61.5, 61.2, 49.2, 31.3, 29.7, 29.01 (4 × C), 28.98 (2 × C), 28.93, 28.85, 28.7, 28.4, 25.8, 22.0, 13.9; HRESIMS calcd for C25H48N3O6 (M + H)+: 486.3538; found: 486.3530; Anal calcd for C25H47N3O6 : C 61.83, H 9.75, N 8.65; found: C 61.66, H 9.61, N 8.44.
(1-(3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluorooctyl)-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4f)
Following the general procedure for acetate deprotection, 397 mg of 3f and 28 mg NaOMe were stirred in 2 mL MeOH for 3.5 hours. The crude product was purified by recrystallization from acetone/hexane, yielding 128 mg (38%) of 4f as a white solid. 1H NMR (MeOD, 400 MHz): δ 8.10 (s, 1H, triazole-CH), 4.97 (d, J = 12.5 Hz, 1H, H-1’a), 4.75 - 4.83 (m, 3H, H-1’a, H-α), 4.38 (d, J = 7.7 Hz, 1H, H-1), 3.89 (dd, J = 11.9, 2.0 Hz, 1H, H-6a), 3.67 (dd, J = 11.8, 5.3 Hz, 1H, H-6b), 3.26 - 3.41 (m, 3H (1H buried under solvent signal), H-3, H-4, H-5), 3.21 (dd, J = 8.9, 7.8 Hz, 1H, H-2), 2.95 (tt, J = 19.0, 7.1 Hz, 2H, H-β); 13C NMR (MeOD 100 MHz): δ 146.2, 126.0, 103.8, 78.2, 78.1, 75.2, 71.8, 63.1, 62.9, 43.6, 32.3; 19F NMR (MeOD, 282 MHz) δ -80.7, -113.68, -121.17, -122.16, -122.85, -125.61; HRESI MS calcd for C17H19N3O6 (M + H)+: 608.1061; found: 608.1064; Anal calcd for C17H18N3O6: C 33.62, H 2.99, N 6.92; found: C 33.62, H 2.91, N 6.85.
(1-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl)-1H-1,2,3-triazol-4-yl)methyl β-D-glucopyranoside (4g)
Following the general procedure for acetate deprotection, 425 mg (0.48 mmol) of 3g and 28 mg (0.48 mmol) NaOMe were stirred in 2 mL MeOH for 3.5 hours. The crude product was purified by recrystallization from methanol, yielding 138 mg (41%) of 4g as a white solid. 1H NMR (MeOD, 400MHz): δ (ppm) 8.11 (s, 1H), 4.98 (d, J = 12.5 Hz, 1H), 4.75 - 4.83 (m, 3H, H-1’a, H-α), 4.39 (d, J = 7.7 Hz, 1H), 3.90 (dd, J = 11.9, 1.8 Hz, 3H), 3.68 (dd, J = 11.9, 5.6 Hz, 4H), 3.25 - 3.39 (m, 3H (1H buried under solvent signal), H-3, H-4, H-5), 3.21 (dd, J = 9.0, 7.8 Hz, 1H), 2.95 (tt, J = 18.7, 7.1 Hz, 8H); 13C NMR (MeOD, 100 MHz): δ (ppm) 146.2, 126.0, 103.8, 78.2, 78.1, 75.2, 71.8, 63.1, 62.9, 43.6, 32.3; 19F NMR (282 MHz, MeOD) δ ppm -80.64, -113.67, -120.97, -121.14 (2 × CF2), -122.00, -122.79, -125.56 (br. s.); HRESIMS calcd for C19H19N3O6F17: 708.0997; found: 708.1006; Anal calcd for C19H18N3O6: C 32.26, H 2.56, N 5.94; found: 31.92, H 2.57, N 5.66.
Cell culture experiments
Dilutions of experimental chemical compounds
Chemical compounds stock solutions and their dilutions were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO) and as necessary aliquots were added directly to 24- and 96-wells plates containing cells in complete media.
Cell line & culture conditions
The human acute leukemia T-lymphocytes Jurkat cell line (Jurkat; ATCC, Manassas, VA) was used for the cytotoxicity assay [41]. Jurkat cells were derived from a 14 years old male donor afflicted with non-Hodgkin T-lymphoma. The culture medium for Jurkat cells was Roswell Park Memorial Institute medium (RPMI; HyClone, Logan, UT) with 10% heat inactivated fetal bovine serum (FBS; HyClone). The medium was supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Lonza, Walkersville, MD). Cells growing exponentially around 60–75% confluence were counted and seeded into 96-well plate format (Greiner Bio-One, Monroe, NC) at a density of 25,000 cells in 100 μL culture media per well. All the incubation conditions were 37°C in a humidified 5% CO2 atmosphere. To guarantee high viability, cells were prepared as previously detailed [23]. All tests were assessed in quadruplicate.
MTS colorimetric assay for cell viability
Jurkat cells were incubated with a gradient of the experimental compounds from 8 μM to 2000 μM. After 12 h of incubation, 20 μL of the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] reagent (CellTiter 96 AQueousOne Solution Cell Proliferation Assay; Promega, Madison, WI) were added to each well and subsequently incubated for an additional 4 h for a total incubation period of 16 h. The colored formazan product was measured by absorbance at 490 nm with a reference wavelength of 650 nm using a microplate reader (SpectraMax 190 Absorbance Microplate Reader, Molecular Devices, Sunnyvale, CA). Control wells, containing the same volumes of culture medium and MTS reagent, were utilized to subtract background absorbance [9]. In addition, 1 mM of hydrogen peroxide (H2O2; Sigma-Aldrich, St Louis, MO) was used as a positive control for cytotoxicity. DMSO treated cells as solvent control and untreated (Unt) cells were also included in each experimental plate. Data are expressed as the cell viability percentage relative to DMSO treated control cells. Each experimental point was performed in quadruplicate to obtain the mean and standard deviation values.
Inhibitory concentration 50% (IC50) in μM is defined as the concentration of experimental compound required to inhibit 50% of the conversion of MTS to formazan, as compared with the absorbance produced by untreated cells after 16 h of incubation. Data derived from the MTS assay was used to determine the IC50. The two absorbance values closest to the 50% point were plotted with its corresponded chemical compound concentration and the equation of the regression line was utilized to calculate the IC50 as described previously [42].
Annexin V/PI apoptosis/necrosis assay
The triazole-containing alkyl β-D-glucopyranosides 4d and 4e were selected because of their comparatively high toxicity (Table 1) to gain further insights into the mode and mechanism of cell death caused by this class of surfactants. The structural analog of 4d, alkyl β-D-glucopyranosides C14G1, was selected as a control surfactant because its IC50 value is comparable to 4d and 4e. Briefly, Jurkat cells were seeded in a 24-well flat bottom tissue culture plate (Becton Dickinson, Franklin Lakes, NJ) at a cell density of 100,000 cells per well in 1 mL of culture media as described above. Triazole-containing alkyl β-D-glucopyranosides 4d and 4e and C14G1 were added to the cells at their respective IC50 followed by additional incubation of 16 h. The following controls were included in each experimental plate: (1) H2O2 (1 mM) was used as a positive control for apoptosis; (2) DMSO (1% v/v) was used as a solvent control; and (3) untreated (Unt) cells that were not exposed to DMSO or compound. All treatments including controls were run in quadruplicates. Cells from each individual well were collected in a pre-chilled ice-water cytometric tube, washed and processed essentially as detailed previously [40]. Briefly, cells were stained with a solution containing a mix of Annexin V-FITC and PI in 100 μL of binding buffer (Beckman Coulter, Miami, FL). After 15 min of incubation on ice in the dark, 300 μL of ice-cold binding buffer was added to the cell suspensions and immediately examined via flow cytometry (Cytomics FC 500; Beckman Coulter, Miami, FL). The total percentage of apoptotic cells was interpreted as the sum of both early and late stages of apoptosis (Annexin V-FITC positive), bottom and top right quadrants in a flow cytometric dot plots, respectively. Cells undergoing necrosis only stain with PI and not with Annexin V-FITC. For each sample, approximately 5,000 individual events were acquired per sample and analyzed with CXP software (Beckman Coulter, Miami, FL). Prior to data acquisition, the flow cytometer was set up and calibrated utilizing unstained, single- (PI or Annexin V-FITC) and double- (PI and Annexin V-FITC) stained cells. FL1 and FL2 detectors were plotted at x-axis versus y-axis, respectively.
Mitochondrial membrane potential (ΔΨm) polychromatic analysis
Jurkat cells, plated in a 24 well format, were treated for 6 h [39] with IC50 concentration values of compounds and stained with 2 μM of JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide) fluorophore following the manufacturer’s instructions (MitoProbe; Life Technologies, Grand Island, NY). Cells with intact polarized mitochondria allow JC-1 aggregation that emits a red fluorescence signal; whereas cells with depolarized mitochondria result in the formation of JC-1 monomers that emit a green fluorescence signal. The same controls described in the previous section were also included in these analyses. Data acquisition and analysis was accomplished by using CXP software (Beckman Coulter). Each data point was obtained from four independent replicates.
Live-cell detection of intracellular caspase-3 activation
Cysteine-aspartic protease-3 (caspase-3) activation was verified by using a fluorogenic NucView 488 Caspase-3/7 substrate for live cells, following the vendor’s protocol (Biotium, Hayward, CA). This substrate diffuses easily into cells with intact plasma membrane and permits the detection of caspase-3 activation in live cells. Jurkat cells were seeded on a 24-well plate format and treated with the IC50 concentration of experimental compounds for 6 h. Cells exhibiting a green fluorescence signal, revealing of caspase-3 activation, were monitored via flow cytometry (Cytomics FC500, Beckman Coulter). The same three controls were also analyzed in parallel as described in previous sections. Each data point was obtained from three replicates. Approximately 5,000 events were collected and analyzed per sample using CXP software as described above.
Statistical analysis
Every experimental test was accomplished in quadruplicate. To denote experimental variability, all data are plotted with the standard deviation of the mean. The statistical importance of differences between two experimental samples was achieved via two-tailed paired Student's t-tests. To define whether comparisons of two independent samples have statistical significance, P < 0.01 value was considered significant.
Conclusions
The synthetic approach employed allows the rapid synthesis of novel triazole-linked, glucose-based surfactants 4a-g with well-defined stereochemistry at the anomeric carbon and hydrocarbon or fluorocarbon hydrophobic tails. An initial toxicity assessment revealed that selected triazole-containing alkyl β-D-glucopyranosides (4c-e) and the structurally related tetradecyl β-D-glucopyranoside (i.e., C14G1) cause cytotoxic effects on Jurkat cells at low micromolar concentrations. Jurkat cells treated with triazole-containing alkyl β-D-glucopyranosides 4d and 4e and alkyl β-D-glucopyranoside C14G1 exhibited phosphatidylserine externalization, an early biochemical event of apoptosis. Furthermore, selected compounds induced mitochondria depolarization and caspase-3 activation that are features of induction of the intrinsic apoptotic cascade. Additional studies are needed to explore the impact of triazole-containing alkyl β-D-glucopyranosides 4 and other carbohydrate surfactants to better understand the molecular mechanisms of their toxicity.
Acknowledgements
We thank Gladys Almodovar for critical review of the manuscript and cell culture expertise. We also thank the Cytometry, Screening and Imaging Core Facility at the University of Texas at El Paso (UTEP), supported by RCMI program Grant No. 2G12MD007592, to the Border Biomedical Research Center (BBRC) at UTEP, from the National Center on Minority Health and Health Disparities, a component of National Institutes of Health. The synthesis of the triazole-containing alkyl β-D-glucoyranosides 4a-g was supported by grants from the National Science Foundation (CBET-0967381/0967390) and the U.S. Department of Agriculture Biomass Research and Development Initiative (Grant Agreement 68-3A75-7-608) to HJL. The cell culture work was supported by NIGMS SCORE Grant 1SC3GM103713-01 to RJA.
Additional file
The following additional data are available with the online version of this paper. Additional data file 1 contains copies of 1H and 13C NMR spectra.
Footnotes
Edward Davis Oldham and Larissa M Nunes contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EDO carried out the synthesis, purification and characterization of the compounds; LMN and AVR performed the cell culture experiments and analyzed the results; AVR, SER, BLK, RJA and HJL conceived the study, participated in its design and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Edward Davis Oldham, Email: eoldham@umw.edu.
Larissa M Nunes, Email: larissa2rh@gmail.com.
Armando Varela-Ramirez, Email: avarela2@utep.edu.
Stephen E Rankin, Email: stephen.rankin@uky.edu.
Barbara L Knutson, Email: bknutson@engr.uky.edu.
Renato J Aguilera, Email: raguilera@utep.edu.
Hans-Joachim Lehmler, Email: hans-joachim-lehmler@uiowa.edu.
References
- 1.Hill K, LeHen-Ferrenbach C. Sugar-Based Surfactants for Consumer Products and Technical Applications. In: Ruiz CC, editor. Surfactant Sci Ser. Boca Raton, FL: CRC Press; 2008. p. 1. [Google Scholar]
- 2.Dembitsky VM. Astonishing diversity of natural surfactants: 1. Glycosides of fatty acids and alcohols. Lipids. 2004;39(10):933–53. doi: 10.1007/s11745-004-1316-1. [DOI] [PubMed] [Google Scholar]
- 3.Queneau Y, Chambert S, Besset C, Cheaib R. Recent progress in the synthesis of carbohydrate-based amphiphilic materials: the examples of sucrose and isomaltulose. Carbohydr Res. 2008;343(12):1999–2009. doi: 10.1016/j.carres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Riess JG, Greiner J. Carbohydrate- and related polyol-derived fluorosurfactants: an update. Carbohydr Res. 2000;327(1–2):147–68. doi: 10.1016/S0008-6215(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 5.Milius A, Greiner J, Riess JG. Synthesis of F-alkylated glycosides as surfactants for in vivo uses. Effects of the length of the hydrocarbonated spacer in the aglycone on Königs-Knorr reaction, surface activity and biological properties. New J Chem. 1991;15:337–44. [Google Scholar]
- 6.Zarif L, Greiner J, Pace S, Riess JG. Synthesis of perfluoroalkylated xylitol ethers and esters: new surfactants for biomedical uses. J Med Chem. 1990;33(4):1262–9. doi: 10.1021/jm00166a028. [DOI] [PubMed] [Google Scholar]
- 7.Zarif L, Greiner J, Riess JG. Perfluoroalkylated monoesters of 1,4-D-sorbitan, isosorbide, and isomannide: new surfactants for biomedical applications. J Fluorine Chem. 1989;44:73–85. doi: 10.1016/S0022-1139(00)84372-9. [DOI] [Google Scholar]
- 8.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41(14):2596–9. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Oldham ED, Seelam S, Lema C, Aguilera RJ, Fiegel J, Rankin SE, et al. Synthesis, surface properties, and biocompatibility of 1,2,3-triazole-containing alkyl β-D-xylopyranoside surfactants. Carbohydr Res. 2013;379:68–77. doi: 10.1016/j.carres.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sani FA, Heidelberg T, Hashim R. Farhanullah: Alkyl triazole glycosides (ATGs)–a new class of bio-related surfactants. Colloids Surf, B. 2012;97:196–200. doi: 10.1016/j.colsurfb.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Clemente MJ, Fitremann J, Mauzac M, Serrano JL, Oriol L. Synthesis and characterization of maltose-based amphiphiles as supramolecular hydrogelators. Langmuir. 2011;27(24):15236–47. doi: 10.1021/la203447e. [DOI] [PubMed] [Google Scholar]
- 12.Paul KJV, Loganathan D. Synthesis of novel glycolipids derived from glycopyranosyl azides and N-(β-glycopyranosyl)azidoacetamides. Tetrahedron Lett. 2008;49(44):6356–9. doi: 10.1016/j.tetlet.2008.08.073. [DOI] [Google Scholar]
- 13.Song S-X, Zhang H-L, Kim C-G, Sheng L, He X-P, Long Y-T, et al. Expeditious preparation of triazole-linked glycolipids via microwave accelerated click chemistry and their electrochemical and biological assessments. Tetrahedron. 2010;66(52):9974–80. doi: 10.1016/j.tet.2010.10.033. [DOI] [Google Scholar]
- 14.Zhang H-L, He X-P, Deng Q, Long Y-T, Chen G-R, Chen K. Research on the structure–surface adsorptive activity relationships of triazolyl glycolipid derivatives for mild steel in HCl. Carbohydr Res. 2012;354:32–9. doi: 10.1016/j.carres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Brusa C, Ochs M, Remond C, Muzard M, Plantier-Royon R. Chemoenzymatic synthesis of "click" xylosides and xylobiosides from lignocellulosic biomass. RSC Advances. 2014;4(18):9330–8. doi: 10.1039/c3ra46173d. [DOI] [Google Scholar]
- 16.Zhang H-L, He X-P, Sheng L, Yao Y, Zhang W, Shi X-X, et al. Synthesis of novel 6-triazologlycolipids via click chemistry and their preliminary cytotoxicity assessments. Mol Divers. 2011;15(4):889–900. doi: 10.1007/s11030-011-9318-1. [DOI] [PubMed] [Google Scholar]
- 17.Schuster T, Schellenberger S, Friedrich R, Klapper M, Müllen K. Branched fluorinated amphiphiles based on carbohydrates. J Fluorine Chem. 2013;154:30–6. doi: 10.1016/j.jfluchem.2013.06.005. [DOI] [Google Scholar]
- 18.Neto V, Granet R, Krausz P. Novel class of non-ionic monocatenary and bolaform alkylglycoside surfactants. Synthesis by microwave-assisted glycosylation and olefin cross-metathesis or by ‘click-chemistry’: physicochemical studies. Tetrahedron. 2010;66(25):4633–46. doi: 10.1016/j.tet.2010.03.115. [DOI] [Google Scholar]
- 19.Mohammed AI, Abboud ZH, Alghanimi AHO. Synthesis of D-mannitol substituted ether-linked bis-1,2,3-triazoles as models of gemini surfactants. Tetrahedron Lett. 2012;53(38):5081–3. doi: 10.1016/j.tetlet.2012.07.014. [DOI] [Google Scholar]
- 20.Neto V, Granet R, Mackenzie G, Krausz P. Efficient synthesis of “star‐like” surfactants via “click chemistry” [3 + 2] copper (I)‐catalyzed cycloaddition. J Carbohydr Chem. 2008;27(4):231–7. doi: 10.1080/07328300802105365. [DOI] [Google Scholar]
- 21.Stubenrauch C. Sugar surfactants - aggregation, interfacial, and adsorption phenomena. Curr Opin Colloid Interface Sci. 2001;6(2):160–70. doi: 10.1016/S1359-0294(01)00080-2. [DOI] [Google Scholar]
- 22.Goodby JW, Goertz V, Cowling SJ, Mackenzie G, Martin P, Plusquellec D, et al. Thermotropic liquid crystalline glycolipids. Chem Soc Rev. 2007;36(12):1971–2032. doi: 10.1039/b708458g. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Osei-Prempeh G, Lema C, Davis Oldham E, Aguilera RJ, Parkin S, et al. Synthesis, thermal properties, and cytotoxicity evaluation of hydrocarbon and fluorocarbon alkyl β-D-xylopyranoside surfactants. Carbohydr Res. 2012;349:12–23. doi: 10.1016/j.carres.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Turanek J, Knoetigova P, Kudlackova H, Masek J, Parkin S, et al. Hydrophobic tail length, degree of fluorination and headgroup stereochemistry are determinants of the biocompatibility of (fluorinated) carbohydrate surfactants. Colloids Surf, B. 2009;73(1):65–74. doi: 10.1016/j.colsurfb.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Turánek J, Knötigová P, Kudlácková H, Mašek J, Pennington DB, et al. Synthesis and biocompatibility evaluation of fluorinated, single-tailed glucopyranoside surfactants. New J Chem. 2008;32:2169–79. doi: 10.1039/b805015e. [DOI] [Google Scholar]
- 26.Söderlind E, Wollbratt M, von Corswant C. The usefulness of sugar surfactants as solubilizing agents in parenteral formulations. Int J Pharm. 2003;252(1–2):61–71. doi: 10.1016/S0378-5173(02)00599-9. [DOI] [PubMed] [Google Scholar]
- 27.Kasuya MCZ, Cusi R, Ishihara O, Miyagawa A, Hashimoto K, Sato T, et al. Fluorous-tagged compound: a viable scaffold to prime oligosaccharide synthesis by cellular enzymes. Biochem Biophys Res Commun. 2004;316(3):599–604. doi: 10.1016/j.bbrc.2004.02.094. [DOI] [PubMed] [Google Scholar]
- 28.Kasuya MCZ, Ito A, Cusi R, Sato T, Hatanaka K. Cellular uptake and saccharide chain elongation of "fluoro-amphiphilic" glycosides. Chem Lett. 2005;34(6):856–7. doi: 10.1246/cl.2005.856. [DOI] [Google Scholar]
- 29.Song S-X, Wu M-L, He X-P, Zhou Y-B, Sheng L, Li J, et al. The anomeric mixture of some O-galactolipid derivatives is more toxic against cancer cells than either anomer alone. Bioorg Med Chem Lett. 2012;22(5):2030–2. doi: 10.1016/j.bmcl.2012.01.069. [DOI] [PubMed] [Google Scholar]
- 30.Rowan AS, Nicely NI, Cochrane N, Wlassoff WA, Claiborne A, Hamilton CJ. Nucleoside triphosphate mimicry: a sugar triazolyl nucleoside as an ATP-competitive inhibitor of B. anthracis pantothenate kinase. Org Biomol Chem. 2009;7(19):4029–36. doi: 10.1039/b909729e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez SG, Alvarez MT. A practical procedure for the synthesis of alkyl azides at ambient temperature in dimethyl sulfoxide in high purity and yield. Synthesis. 1997;4:413–4. doi: 10.1055/s-1997-1206. [DOI] [Google Scholar]
- 32.Balgavy P, Devinsky F. Cut-off effects in biological activities of surfactants. Adv Colloid Interf Sci. 1996;66:23–63. doi: 10.1016/0001-8686(96)00295-3. [DOI] [PubMed] [Google Scholar]
- 33.Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):149–59. doi: 10.1016/j.plefa.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Zhou CH, Wang Y. Recent researches in triazole compounds as medicinal drugs. Curr Med Chem. 2012;19(2):239–80. doi: 10.2174/092986712803414213. [DOI] [PubMed] [Google Scholar]
- 35.Razafindralambo H, Richel A, Wathelet B, Blecker C, Wathelet JP, Brasseur R, et al. Monolayer properties of uronic acid bicatenary derivatives at the air-water interface: effect of hydroxyl group stereochemistry evidenced by experimental and computational approaches. Phys Chem Chem Phys. 2011;13(33):15291–8. doi: 10.1039/c1cp21365b. [DOI] [PubMed] [Google Scholar]
- 36.Razafindralambo HL, Richel A, Paquot M, Lins L, Blecker C. Liquid crystalline phases induced by the hydroxyl group stereochemistry of amphiphilic carbohydrate bicatenary derivatives. J Phys Chem B. 2012;116(13):3998–4005. doi: 10.1021/jp209765j. [DOI] [PubMed] [Google Scholar]
- 37.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–27. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 39.Robles-Escajeda E, Lerma D, Nyakeriga AM, Ross JA, Kirken RA, Aguilera RJ, et al. Searching in mother nature for anti-cancer activity: anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS One. 2013;8(9):e73508. doi: 10.1371/journal.pone.0073508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robles-Escajeda E, Martinez A, Varela-Ramirez A, Sanchez-Delgado RA, Aguilera RJ. Analysis of the cytotoxic effects of ruthenium-ketoconazole and ruthenium-clotrimazole complexes on cancer cells. Cell Biol Toxicol. 2013;29(6):431–43. doi: 10.1007/s10565-013-9264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider U, Schwenk HU, Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977;19(5):621–6. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 42.Varela-Ramirez A, Costanzo M, Carrasco YP, Pannell KH, Aguilera RJ. Cytotoxic effects of two organotin compounds and their mode of inflicting cell death on four mammalian cancer cells. Cell Biol Toxicol. 2011;27(3):159–68. doi: 10.1007/s10565-010-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


