Abstract
Severe pathoanatomical and mechanical injuries compromise patient recovery and survival following penetrating brain injury (PBI). The realization that the blood–brain barrier (BBB) plays a major role in dictating post-PBI events has led to rising interests in possible therapeutic interventions through the BBB. Recently, the choroid plexus has also been suggested as a potential therapeutic target. The use of biocompatible scaffolds for the delivery of therapeutic agents, but little is known about their interaction with cerebral tissue, which has important clinical implications. Therefore, the authors have sought to investigate the effect of polycaprolactone (PCL) and PCL/tricalcium phosphate (PCL/TCP) scaffolds on the maintenance of BBB phenotype posttraumatic brain injury. Cranial defects of 3 mm depth were created in Sprague Dawley rats, and PCL and PCL/TCP scaffolds were subsequently implanted in predetermined locations for a period of 1 week and 1 month. Higher endothelial barrier antigen (EBA) expressions from PCL-based scaffold groups (p>0.05) were found, suggesting slight advantages over the sham group (no scaffold implantation). PCL/TCP scaffold group also expressed EBA to a higher degree (p>0.05) than PCL scaffolds. Importantly, higher capillary count and area as early as 1 week postimplantation suggested lowered ischemia from the PCL/TCP scaffold group as compared with PCL and sham. Evaluation of interlukin-1β expression suggested that the PCL and PCL/TCP scaffolds did not cause prolonged inflammation. BBB transport selectivity was evaluated by the expression of aquaporin-4 (AQP-4). Attenuated expression of AQP-4 in the PCL/TCP group (p<0.05) suggested that PCL/TCP scaffolds altered BBB selectivity to a lower degree as compared with sham and PCL groups, pointing to potential clinical implications in reducing cerebral edema. Taken together, the responses of PCL-based scaffolds with brain tissue suggested safety, and encourages further preclinical evaluation in PBI management with these scaffolds.
Introduction
The blood–brain barrier (BBB) helps to maintain neurological function. Shlosberg et al. cited that the high incidence of BBB breakdown is commonly associated with traumatic brain injury (TBI).1 The consequences following BBB dysfunction are severe, with BBB breakdown leading to transcriptional changes in the neurovascaular network that cause neuronal dysfunction and degeneration.1 In addition, severe pathoanatomical (hematoma, subarachnoid hemorrhage, diffuse axonal injury2) and mechanical consequences (cerebral edema and ischemia3,4) may result. Primary BBB damage may be a result of traumatic injury,1 which may be inflicted through closed head injuries or penetrating brain injuries (PBI). Despite the severity of PBI, their relative complexity,5 compounded with our lack of understanding has led to nonideal clinical management (typically defaulted to that closed head injuries).6 It was suggested that the paracrine signaling effects among the astrocytes, glial, and cerebrovascular endothelium play a role in maintaining the function and phenotype of the BBB.7–9
Cerebral inflammation that arises after injury is also a major concern in the regulation of the BBB. On this note, Schwartz and Baruch has made significant contributions to our understanding of the brain-immunity relationship, stating that the choroid plexus (CP) is an on-alert gate for the recruitment of leukocytes capable of resolving inflammation, going further to suggest that the CP might also be a target for therapeutics.10,11 More importantly, they have also suggested that circulating immune cells are critical to the normal functioning of the central and peripheral nervous system.12
To prevent and/or restore neurological function after PBI, targeted delivery of biomolecules may be necessary. As such, one proposed strategy for targeted delivery could be attained either through the development of cytoprotective genes13 or proteins.14 However, these biomolecules require suitable carriers that prolong their bioavailability for effective therapeutic effects.15,16 To address this, synthetic methylcellulose-based gel constructs,17 freeze-dried hyaluronic acid and polylysine hydrogels,18 and fibrin scaffolds for the transplantation of bone marrow stromal cells19 have all been evaluated for their use with brain tissue. Wong et al.20 recently demonstrated in a murine model of PBI that polycaprolactone (PCL) sponges elicited a lower immune response than poly(lactic-co-glycolic acid) (PLGA) and that both lowered the scarring and secondary cell death as compared to not having an implant. We have also previously demonstrated that PCL-based scaffolds produced through fused deposition modeling did not worsen inflammation when placed in direct contact with brain tissue.21
Apart from their potential use as biomolecule delivery vehicles, bioactive scaffolds may also be used to assist in the regeneration of the cranium,22 which is important for preventing neurological deterioration and significant depression after PBI.23,24 For this purpose, we have developed PCL/tricalcium phosphate (PCL/TCP) scaffolds that are capable of promoting bone regeneration.25–27 In addition, our previous report suggested that PCL/TCP scaffolds have reduced inflammation as compared with PCL scaffolds, possibly due to the localized increase in pH to combat clinical acidosis.
To the authors' knowledge, there is no previous report on the investigation of PCL and PCL/TCP scaffolds placed in direct contact with brain tissue, and thus this study aimed to investigate the effects of these scaffolds on the BBB and cerebral inflammation. We hypothesized that PCL-based scaffolds will not further compromise the integrity of the BBB, and will not lead to higher inflammation when placed in direct contact with cerebral tissue. The potential benefit of the results now, taken together with our previous findings, will provide more evidence for the safe use of PCL and PCL/TCP scaffolds as potential biomolecule delivery vehicles in the future.
Materials and Methods
Surgical procedure
The animal studies in this work were approved by the animal ethics committee Institutional Animal Care and Use Committee (IACUC, protocol: IACUC 096/11) at the National University of Singapore. Female Sprague Dawley rats (∼250 g) were anesthetized with isofluorane, before surgical exposure of their skulls. Three millimeter anterior to the bregma (sham) and two other positions were marked: 3.5 mm left (PCL/TCP) and right (PCL). Holes of 3 mm depth were drilled into the cerebral cortex at these three locations with a 3 mm outer diameter trephine. All animals were closely monitored after surgery and all of them recovered without any neurological deficits.
PCL and PCL/TCP scaffolds
PCL and PCL/TCP scaffolds (Osteopore International Pte Ltd.) were fabricated using a solvent-free approach of fused deposition modeling19,20 to achieve a lay-down pattern of 0°/60°/120° with a porosity of 70%, as reported earlier. The incorporation of TCP could be visualized by scanning electron microscopy (SEM 6390LA; JEOL) (white arrows, Fig. 1A). To fit the defects created in the rat cranium, the scaffolds were designed in a plug-like manner. PCL plugs with a diameter of 3 mm were fitted snugly into the cavity left of the bregma, whereas PCL/TCP scaffolds of similar geometry were fitted snugly into the cavity right of the bregma. Sham (3 mm anterior, superior sagittal sinus) was left without scaffold implantation (Fig. 1B).
FIG. 1.
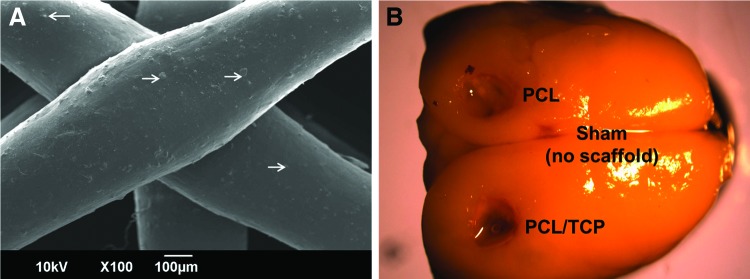
(A) Visualization of the morphology of the implanted PCL-based scaffolds taken by scanning electron microscopy, at 100× magnification. TCP particles (white arrows) were observed distributed within each PCL filament. (B) Representative image of a harvested Sprague Dawley rat brain with the locations of the scaffolds and sham (control) clearly indicated. PCL, polycaprolactone; TCP, tricalcium phosphate. Color images available online at www.liebertpub.com/tea
Histopathology and immunohistochemistry
Following perfusion, the brains were harvested and postfixed in 10% buffered formalin. The brains were then dehydrated in an ascending series of alcohol, cleared with xylene, and then embedded in paraffin wax. The brain tissue was dehydrated in an ascending series of alcohol, cleared with xylene, and embedded in paraffin wax. 10 μm thick coronal serial sections were cut. The sections were dewaxed in xylene and hydrated with descending series of alcohol. Paraffin sections of 4 μm thickness were cut and microwaved in citrate buffer for antigen retrieval and blocked with peroxidase blocking reagent (S2023; DAKO UK Ltd.). For immunohistochemistry, sections were incubated with rat blood–brain barrier (SMI-71) monoclonal antibody (SMI-71R; Covance) diluted 1:500 in phosphate buffered saline (PBS); polyclonal rabbit anti-IL-1β (ab9722; Abcam) diluted 1:500; mouse monoclonal anti-glial fibrillary acidic protein (GFAP, MAB360; Chemicon International, Inc.) diluted 1:1800 in PBS; rabbit monoclonal anti-aquaporin (AQP)-4 affinity (5582-1; Epitomics, Inc.) diluted 1:200 in PBS for the detection of SMI-71, IL-1β, AQP-4, and GFAP, respectively. Subsequent antibody detection was carried out using anti-mouse (rat absorbed), anti-goat, or anti-rabbit IgG (ImPRESS Ig reagent kit; Vector Laboratories). For fluorescence staining, FITC-conjugated and Cy3-conjugated secondary antibodies were used instead. For brightfield microscopy, all samples were visualized using 3,3′-diaminobenzidine (DAB). All samples were examined using a brightfield and fluorescence slide scanner (SCN400; Leica).
Determination of pixel area and intensity
Pixel areas and intensity for SMI-71 and AQP-4, respectively, were calculated using ImageJ (version 1.46r; National Institute of Health, http://imagej.nih.gov/ij). Colored images were first converted into 8-bit images followed by thresholding, leaving the regions of interest black. Subsequently, noise was removed by removing outliers, and the final binary image was compared to the original image carefully to ensure a good representation of the original. Cell counting and area measurement were then conducted on the final binary image. At least three regions were chosen for analysis for each image. A total of three images per anatomical location were used in the calculation of the pixel area (thus n=9). In this study, the defect (R1) and periphery (R2) region are as defined in Figure 2.
FIG. 2.
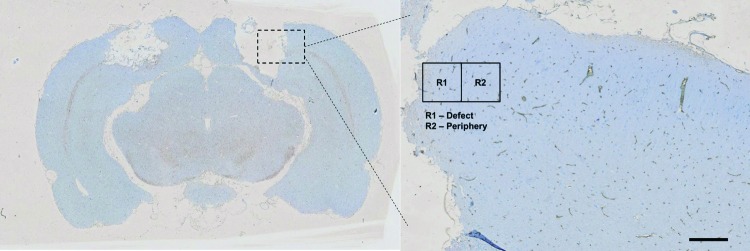
Illustration of the selected regions used: R1 indicating the defect region; R2 refers to the periphery (scale bar represents 1 mm). Color images available online at www.liebertpub.com/tea
Statistical analysis
For determining statistical significance, two-tailed Student's t-test was conducted, and p<0.05 was considered statistically significant.
Results
Expression of endothelial proteins (SMI-71)
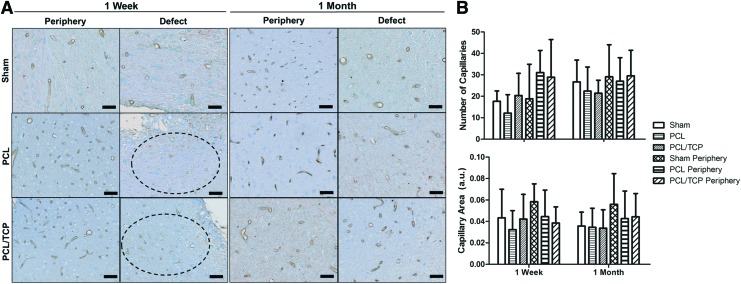
The expression of SMI-71 could be clearly observed in the peripheries of the sham, PCL, and PCL/TCP groups (Fig. 3A, 1 week and 1 month), with most taking on a circular cross-sectional morphology. At the defect region, however, PCL and PCL/TCP groups exhibited lower expression of SMI-71. At endpoint (Fig. 3A, 1 month), general recovery in the expression of endothelial barrier antigens (EBAs) could be observed at the defect region across all groups.
FIG. 3.
(A) SMI-71 immunohistochemistry and (B) ImageJ quantification of total capillary number and average capillary area. SMI-71 was clearly expressed in the peripheral region of all groups. In the defect region (dark circled), morphological observations that the capillary count and area were superior in the PCL/TCP group as compared with the PCL group was confirmed by ImageJ analysis, with the results presented in (B). Notable superiority in total capillary count and average capillary area in PCL/TCP could be observed. Scale bar represents 500 μm. Color images available online at www.liebertpub.com/tea
Capillary area
The number of capillaries and their individual areas were determined, and by comparing against the peripheries of each individual group (i.e., defect vs. periphery), the average capillary area in the PCL/TCP scaffold group was 30% smaller, whereas the PCL scaffold group was 60% smaller (Fig. 3B). Between PCL and PCL/TCP scaffold groups at the defect site, capillary area in the PCL/TCP group was 23% larger (Fig. 3B, p>0.05). Interestingly, the number of capillaries and their area in the PCL/TCP group remained at approximately the same levels between 1 week and 1 month, whereas an increase was observed in the PCL groups.
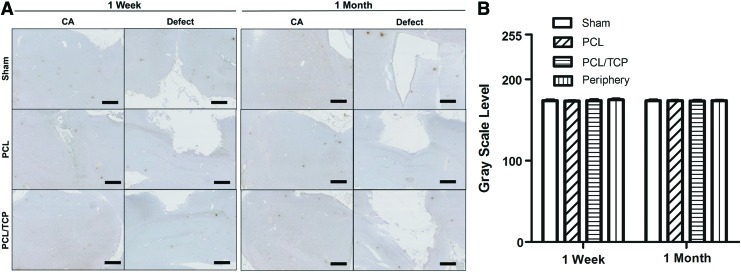
IL-1β expression
Proinflammatory cytokine IL-1β was evaluated after PBI. Gross observations (Fig. 4A) indicated that IL-1β expression levels were similar across all groups, as compared with the peripheral region of the hippocampus (Fig. 4B, p>0.05).
FIG. 4.
(A) IL-1β expression over 1 week and 1 month and (B) ImageJ analysis of stain intensity. Scale bar represents 1 mm. Color images available online at www.liebertpub.com/tea
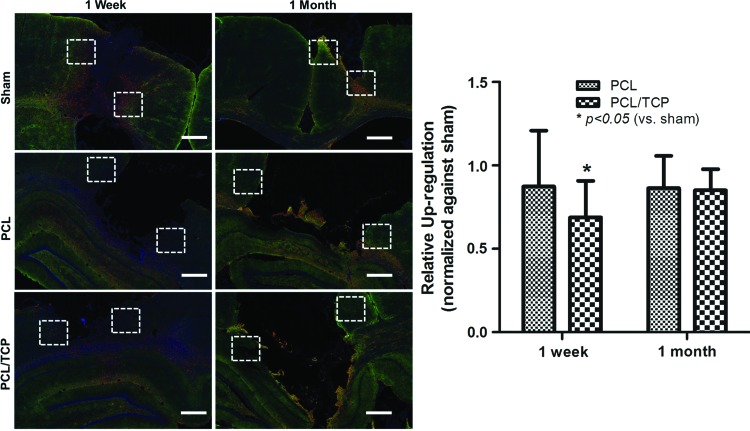
AQP-4
The AQP-4 expression was observed across all groups (Fig. 5), as evidenced by the appearance of AQP-positive astrocytes. Compared to sham (no scaffolds), the intensity of AQP-positive astrocytes in the PCL/TCP scaffolds was upregulated to a lower extent (Fig. 5, p<0.05). Conversely, PCL scaffolds attenuated BBB alterations less when compared to PCL/TCP (Fig. 5, p>0.05).
FIG. 5.
AQP-4 immunohistochemistry. Expression of AQP-4 in PCL/TCP scaffold group appeared to be lower than that of PCL and sham, and was confirmed by ImageJ analysis for stain intensity in the selected regions (white dotted squares). Scale bar represents 1 mm. Color images available online at www.liebertpub.com/tea
Discussion
Presently, scaffolds in tissue engineering and regenerative medicine have bifunctionality, as structural supports as well as biomolecule delivery vehicles. In this study, we evaluated PCL-based scaffolds that may function as biomolecule delivery vehicles20 and for the regeneration of the cranium. PCL is a biomaterial that has traditionally been used as a drug delivery device,28 and more recently as a scaffold for promoting bone regeneration.29 Previously, we have also demonstrated that PCL and PCL-based scaffolds were able to deliver recombinant human bone morphogenetic protein-2, as well as provide sufficient structural support to promote bone healing.30 Given the bifunctionality of PCL-based scaffolds, they may have potential for use in PBI, both as biomolecule delivery vehicles as well as for the regeneration of the cranium. As a preliminary study to evaluate this, PCL-based scaffolds were evaluated for their compatibility with brain tissue, by investigating their effects on the BBB, and cerebral inflammation, which plays a significant role in disrupting/maintaining the BBB.
Cerebral ischemia has severe clinical complications that will affect patient mortality rates.31 Therefore, it is important to maintain or promote regeneration of the vascular supply to injured brain tissue after PBI. To ascertain this, we have chosen to evaluate the expression of EBAs, which are proteins located within the plasma membrane of microvascular endothelium, and are selectively expressed in the normal nervous system.32 In this study, we observed that PCL and PCL/TCP scaffolds were comparable in the expression of EBAs as compared with the sham group (no scaffolds), suggesting that they do not cause further changes to vascular supply in and around the defect.
The use of scaffolds for the treatment of TBI is not unprecedented, with Mahmood et al.33 showing that collagen scaffolds may be used as vehicles for transplanting human mesenchymal stem cells. In addition, Wong et al.20 illustrated the use of PCL and PLGA sponges as potential biomolecule delivery vehicles. Common to both these reports would be the similarities in terms of stiffness to brain tissue, which is reportedly between (1.0–3.5×103 dyn/cm2).34 While the stiffness of both PCL and PCL/TCP scaffolds are considerably higher than that of brain tissue,35 tissue ingrowth into the bioresorbable scaffold is still possible, owing to its highly porous structure.36 In fact, a highly porous structure is a necessary requirement to facilitate cellular and vascular infiltration.37 Our results suggested that both PCL and PCL/TCP did not obtund vascularization, which is a significant finding.
Cerebral vascularization and inflammation are key events that determine the extent of neurological dysfunction. Our previous findings21 demonstrated that cerebral inflammation was not further aggravated in PCL and PCL/TCP groups. The attenuated expression of IL-1β was an expected outcome, as it is known from previous studies that its expression does not persist more than a few days.38 However, due to foreign body reaction, it was of interest to ensure that inflammation was not prolonged further. In this study, our results demonstrated that prolonged inflammation did not occur in both scaffold groups.
AQP-4 is a water channel protein that allows for the transmembrane transport of water.39 It is strongly expressed in the brain, and has been postulated to be the main membrane protein regulating water flux.40 The need for AQP-4 stems from its involvement in brain edema,41–44 the swelling of tissue due to increased water and sodium loading.39 Previously, AQP-4-deficient mice have been reported to have poorer neurological deficit scores and poorer survival rate, a direct consequence of increased tissue water content and astrocytic swelling.42 Therefore, it is important that the transcellular transport of water is regulated tightly by the aquaporins, specifically AQP-4 in the brain. This has clear, potential, clinical implications such as reduction in cerebral edema, which may improve patient prognosis, as suggested by Ding et al. recently.4
Conclusion
In this study, we studied the effect of PCL and PCL/TCP scaffolds on the BBB and cerebral inflammatory response to PBI. EBA expressions were recovered by 1 month across all groups, suggesting that PCL-based scaffolds did not cause further changes to the vascular supply in and around the defect region. In terms of cerebral inflammation, prolonged inflammatory responses were not present despite the presence of PCL-based scaffolds for 1 month, demonstrating that these scaffolds do not induce undesired and prolonged inflammation in the long run. Finally, AQP-4 expression was downregulated initially in the PCL/TCP group, suggesting that interleukin-induced AQP-4 expression was reduced, potentially leading to better clinical prognosis. Taken together, the results of this study provide evidence that PCL-based scaffolds may be used in direct contact with cerebral tissue safely.
Acknowledgments
This work was funded by the National University Health System Clinician's Research Grant. The authors would like to express their gratitude to Mary for her contribution in histopathology, Kian Chye who was the perfusionist, Dr. Enoka for providing assistance in attaining IACUC approval, and Dr. Yang Ming for helping out in the procedure.
Disclosure Statement
Dr. S.H. Teoh declares his conflict of interest as he is a shareholder of Osteopore Int. The remaining authors declare no conflicts of interest.
References
- 1.Shlosberg D., Benifla M., Kaufer D., and Friedman A.Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6,393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saatman K.E., Duhaime A.-C., Bullock R., Maas A.I., Valadka A., and Manley G.T.Classification of traumatic brain injury for targeted therapies. J Neurotrauma 25,719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chodobski A., Zink B.J., and Szmydynger-Chodobska J.Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2,492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding Z., Zhang J., Xu J., Sheng G., and Huang G.Propofol administration modulates aqp-4 expression and brain edema after traumatic brain injury. Cell Biochem Biophys 67,615, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Kazemi H., Hashemi-Fesharaki S., Razaghi S., Najafi M., Kolivand P.H., Kovac S., et al. Intractable epilepsy and craniocerebral trauma: analysis of 163 patients with blunt and penetrating head injuries sustained in war. Injury 43,2132, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Santiago L.A., Oh B.C., Dash P.K., Holcomb J.B., and Wade C.E.A clinical comparison of penetrating and blunt traumatic brain injuries. Brain Inj 26,107, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Wolburg H., Noell S., Mack A., Wolburg-Buchholz K., and Fallier-Becker P.Brain endothelial cells and the glio-vascular complex. Cell Tissue Res 335,75, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Abbott N.J., Rönnbäck L., and Hansson E.Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7,41, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Lassmann H., Zimprich F., Vass K., and Hickey W.Microglial cells are a component of the perivascular glia limitans. J Neurosci Res 28,236, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M., and Baruch K.The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J 33,7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokaia Z., Martino G., Schwartz M., and Lindvall O.Cross-talk between neural stem cells and immune cells: the key to better brain repair[quest]. Nat Neurosci 15,1078, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz M., and Kipnis J.A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun 25,817, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardridge W.M.Blood-brain barrier genomics. Stroke 38,686, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Cucullo L., Marchi N., Marroni M., Fazio V., Namura S., and Janigro D.Blood-brain barrier damage induces release of alpha2-macroglobulin. Mol Cell Proteomics 2,234, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Baldwin S.P., and Mark Saltzman W.Materials for protein delivery in tissue engineering. Adv Drug Deliv Rev 33,71, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Werle M., and Bernkop-Schnürch A.Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 30,351, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Tate M.C., Shear D.A., Hoffman S.W., Stein D.G., and LaPlaca M.C.Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials 22,1113, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Tian W.M., Hou S.P., Ma J., Zhang C.L., Xu Q.Y., Lee I.S., et al. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng 11,513, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Yasuda H., Kuroda S., Shichinohe H., Kamei S., Kawamura R., and Iwasaki Y.Effect of biodegradable fibrin scaffold on survival, migration, and differentiation of transplanted bone marrow stromal cells after cortical injury in rats. J Neurosurg 112,336, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Wong D.Y., Hollister S.J., Krebsbach P.H., and Nosrat C.Poly(epsilon-caprolactone) and poly (L-lactic-co-glycolic acid) degradable polymer sponges attenuate astrocyte response and lesion growth in acute traumatic brain injury. Tissue Eng 13,2515, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Choy D.K., Nga V.D., Lim J., Lu J., Chou N., Yeo T.T., et al. Brain tissue interaction with three-dimensional, honeycomb polycaprolactone-based scaffolds designed for cranial reconstruction following traumatic brain injury. Tissue Eng Part A 22,22, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schantz J.-T., Lim T.-C., Ning C., Teoh S.H., Tan K.C., Wang S.C., et al. Cranioplasty after trephination using a novel biodegradable burr hole cover: technical case report. Neurosurgery 58, ONS-E176, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Stula D.The problem of the “sinking skin-flap syndrome” in cranioplasty. J Maxillofac Surg 10,142, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Han P.Y., Kim J.H., Kang H.I., and Kim J.S.“Syndrome of the sinking skin-flap” secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc 43,51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita N., Matsushita T., Ishida K., Sasaki K., Kubo S., Matsumoto T., et al. An analysis of bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein 2 from a biodegradable sponge composed of gelatin and β-tricalcium phosphate. J Tissue Eng Regen Med 6,291, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Thakare K., Bhongade M., Charde P., Jaiswal P., Shah N., and Deshpande A.Periodontal regeneration using platelet-derived growth factor in infrabony defects: a series of three cases. Case Rep Dent 2013,849823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busuttil Naudi K., Lappin D., McMahon J., Ayoub A., and Di Silvio L.The viability of rabbit mesenchymal stem cells on tricalcium phosphate scaffold for potential use in bone regeneration. J Appl Biomater Biomech, 40,e461, 2012 [Google Scholar]
- 28.Cha Y., and Pitt C.The acceleration of degradation-controlled drug delivery from polyester microspheres. J Controlled Release 8,259, 1989 [Google Scholar]
- 29.Lowry K., Hamson K., Bear L., Peng Y., Calaluce R., Evans M., et al. Polycaprolactone/glass bioabsorbable implant in a rabbit humerus fracture model. J Biomed Mater Res 36,536, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Rai B., Teoh S.H., Hutmacher D.W., Cao T., and Ho K.H.Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials 26,3739, 2005 [DOI] [PubMed] [Google Scholar]
- 31.The global burden of cerebrovascular disease, The World Health Organisation, 2000 [Google Scholar]
- 32.Lin B., Ginsberg M.D., Zhao W., Alonso O.F., Belayev L., and Busto R.Quantitative analysis of microvascular alterations in traumatic brain injury by endothelial barrier antigen immunohistochemistry. J Neurotrauma 18,389, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Mahmood A., Wu H., Qu C., Xiong Y., and Chopp M.Effects of treating traumatic brain injury with collagen scaffolds and human bone marrow stromal cells on sprouting of corticospinal tract axons into the denervated side of the spinal cord. J Neurosurg 118,381, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Metz H., McElhaney J., and Ommaya A.K.A comparison of the elasticity of live, dead, and fixed brain tissue. J Biomech 3,453, 1970 [DOI] [PubMed] [Google Scholar]
- 35.Lam C.X.F., Savalani M.M., Swee-Hin T., and Hutmacher D.W.Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomed Mater 3,034108, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Chiu Y.-C., Cheng M.-H., Engel H., Kao S.-W., Larson J.C., Gupta S., et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 32,6045, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Thomson K.S., Korte F.S., Giachelli C.M., Ratner B.D., Regnier M., and Scatena M.Prevascularized microtemplated fibrin scaffolds for cardiac tissue engineering applications. Tissue Eng Part A 19,967, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinoshita K., Chatzipanteli K., Vitarbo E., Truettner J.S., Alonso O.F., and Dietrich W.D.Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery 51,195; discussion 203, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Venero J.L., Vizuete M.L., Machado A., and Cano J.Aquaporins in the central nervous system. Prog Neurobiol 63,321, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Takumi Y., Nagelhus E.A., Eidet J., Matsubara A., Usami Si, Shinkawa H., et al. Select types of supporting cell in the inner ear express aquaporin-4 water channel protein. Eur J Neurosci 10,3584, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Vajda Z., Promeneur D., Doczi T., Sulyok E., Frøkiaer J., Ottersen O., et al. Increased aquaporin-4 immunoreactivity in rat brain in response to systemic hyponatremia. Biochem Biophys Res Commun 270,495, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Manley G.T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A.W., et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6,159, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi M., Yamashita T., Kumura E., Tamatani M., Kobayashi A., Yokawa T., et al. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Mol Brain Res 78,131, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Vizuete M., Venero J., Vargas C., Ilundain A., Echevarría M, Machado A., et al. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol Dis 6,245, 1999 [DOI] [PubMed] [Google Scholar]