Abstract
Epigenetic asymmetry has been shown to be associated with the first lineage allocation event in preimplantation development, that is, the formation of the trophectoderm (TE) and inner cell mass (ICM) lineages in the blastocyst. Since parthenogenesis causes aberrant segregation between the TE and ICM lineages, we examined several development-associated histone modifications in parthenotes, including those involved in (i) transcriptional activation [acetylated histone H3 lysine 9 (H3K9Ac) and lysine 14 (H3K14Ac), trimethylated histone H3 lysine 4 (H3K4Me3), and dimethylated histone H3 arginine 26 (H3R26Me2)] and (ii) transcriptional repression [trimethylated histone H3 lysine 9 (H3K9Me3) and lysine 27 (H3K27Me3), and mono-ubiquitinated histone H2A lysine 119 (H2AK119u1)]. Here, we report that in parthenotes, H3R26Me2 expression decreased from the morula stage, while expression patterns and levels of H3K9Ac, H3K27Me3, and H2AK119u1 were unchanged until the blastocyst stage; whereas H3K14Ac, H3K4Me3, and H3K9Me3 showed normal patterns and levels of expressions. Relative to the decrease of H3K9Ac in the ICM and increase in the TE of parthenotes, we detected reduced expression of TAT-interactive protein 60 acetyltransferase and histone deacetylase 1 deacetylase in the ICM and TE of parthenotes, respectively. Relative to the decrease of H3R26Me2, we also observed decreased expression of coactivator-associated arginine methyltransferase 1 methyltransferase and increased expression of the Wnt effector transcription factor 7L2 and miR-181c microRNA in parthenotes. Furthermore, relative to the decrease in H3K27Me3 and H2AK119u1, we found increased phosphorylation of Akt1 and enhancer of zeste homolog 2 in parthenogenetic TE. Therefore, our findings that histone signatures are impaired in parthenotes provide a mechanistic explanation for aberrant lineage segregation and TE defects.
Introduction
Parthenogenesis is a process by which unfertilized oocytes undergo embryogenesis in the absence of male gametes. This phenomenon naturally occurs in several non-mammalian species, but does not take place spontaneously in mammals [1–4]. It has been suggested that parthenogenesis may be a suitable means of generating histocompatible embryonic stem (ES) cells for transplantation without ethical concerns [5,6]. However, previous studies have reported three major genomic anomalies associated with parthenogenetic ES cells: (i) aberrant genome-wide methylation patterns; (ii) loss of genomic imprinting; and (iii) X chromosome instability [7–18]. In addition, our previous study demonstrated aberrant expression of lineage-associated genes in parthenogenetic embryos, including dramatically increased Fgf3 and Gata4 and decreased Nanog and Sox2 expression in the parthenote inner cell mass (ICM), as well as ectopic Gata4 and decreased Elf5 and Tbr2 expression in the parthenote trophectoderm (TE) [19]. Furthermore, in vitro embryo cultures that have not undergone parthenogenesis are also associated with altered DNA/histone methylation and loss of imprinting patterns [10,20–26].
It has been demonstrated that normal development of fetuses and placentae requires the proper expression of several imprinted genes [11,27–29]. In addition, it was recently reported that the presence of paternal chromatin is essential for repressing nascent RNA synthesis during the two-cell to four-cell transition stage in the preimplantation mouse embryo, and this, in turn, is required for normal development [30]. Parthenogenetic embryos do not express paternal genes, but express maternal genes at twice the normal level [11]; such an imbalance of maternal–paternal gene dosage may be associated with the developmental defects observed in parthenotes [31–33].
In contrast to the well-documented aberrations of gene expression in parthenogenetic embryos, histone remodeling and modifications during parthenogenetic development are less well understood. Epigenetic asymmetries have been demonstrated between paternal and maternal genomes at the pronuclear and two-cell stages, and between the ICM and TE in blastocysts during preimplantation development [34–36]. Diploid parthenotes, which have two sets of maternal chromosomes and no paternal chromosomes, are known to lose the epigenetic asymmetry of DNA methylation and histone H3 lysine methylation between the two pronuclei, because both pronuclei inherit the same epigenetic profile from the mother [8,37]. It was previously reported that pronuclei of parthenotes at the one- and two-cell stages do not exhibit asymmetric distributions of heterochromatin protein 1β (Hp1β), trimethylated histone H3 lysine 9 (H3K9), or trimethylated histone H3 lysine 4 (H3K4) [37]. Interestingly, a separate study demonstrated that levels of Hp1 mRNA were elevated by almost four-fold in parthenotes as compared with fertilized embryos cultured in vitro to the zygote stage, but not to other developmental stages [22]. Parthenotes at the zygote stage exhibited perturbed expression of several histones and histone variants, including increased expression of histone H2B, H2afx, and H3.3b, and decreased expression of H2afz [22]. At later preimplantation stages (ie, the morula and blastocyst stages), only the mRNA levels of histone H2A variants H2afx and H2afz were affected in parthenotes, with both exhibiting an increase as compared with fertilized embryos [22]. Moreover, both DNA methylation and histone H4 acetylation were significantly decreased in the parthenote ICM as compared with the control ICM [38]. In addition to histone signatures, several histone-modifying proteins showed increased expression in parthenotes, including histone deacetylase 1 (HDAC1; at the two-cell and morula stages), DNA (cytosine-5)-methyltransferase 3B (at the two-cell stage), histone acetyltransferase 1 (at the blastocyst stage), and the histone-lysine N-methyltransferases SUV39H1 (at the morula and blastocyst stages) and G9A (at the blastocyst stage) [22,39].
Given that (i) the establishment of epigenetic asymmetry between the morula and blastocyst stages has been speculated to be associated with lineage allocation and commitment in mammalian embryos [34,35], and (ii) lineage segregation is impaired in mouse parthenogenetic embryos [19], we hypothesized that fertilized and parthenogenetic mouse embryos may exhibit different patterns of asymmetrical histone modifications at the morula and blastocyst stages. Since previous studies have reported differential expression of several histone variants, histone-modifying enzymes, or associated proteins between in vitro-fertilized and in vivo-fertilized mouse and bovine embryos [22,39], we chose to use in vivo- instead of in vitro-fertilized mouse embryos as controls in this study, to reflect on the authentic in vivo situation of epigenetic regulation. As compared with in vivo-fertilized control embryos, we observed (i) significantly decreased dimethylation of histone H3 arginine 26 (H3R26) in parthenogenetic morulae and ICM; (ii) altered levels of acetylation at histone H3 lysine 9 (H3K9) in the parthenote (decreased in the ICM and increased in the TE); (iii) loss of punctate signals of histone H3 lysine 27 (H3K27) trimethylation in the parthenote TE; and (iv) reduced mono-ubiquitination of histone H2A lysine 119 (H2AK119) in parthenogenetic blastocysts. We also found perturbed expression of several histone acetyltransferases, methyltransferases, and related signaling factors regulating the histone signatures mentioned earlier, including decreased expression of general control of nucleotide synthesis 5 (GCN5) and TAT-interactive protein 60 (Tip60) acetyltransferases, HDAC1 deacetylase and coactivator-associated arginine methyltransferase 1 (CARM1) methyltransferase, increased expression of CARM1 inhibitor miR-181c microRNA and its upstream Wnt effector transcription factor 7L2 (TCF7L2) [40–42], and increased phosphorylation of enhancer of zeste homolog 2 (Ezh2) methyltransferase and its upstream Akt1 serine/threonine protein kinase [43].
Materials and Methods
Parthenogenetic activation and in vitro culture of preimplantation embryos
Animals used in this study were purchased from BioLASCO Taiwan, and approval was received from the Academia Sinica Institutional Animal Care and Utilization Committee. B6DBA female mice at 10–14 weeks old were superovulated by an intraperitoneal (i.p.) injection of 5 IU pregnant mare serum gonadotropin (Calbiochem®; Merck Millipore 367222), followed by an i.p. injection of 5 IU human chorionic gonadotropin (hCG) (Sigma-Aldrich C1063) at 48–50 h later; the mice were subsequently separated into two groups: One group was unmated, while mice of the other group were individually mated with B6DBA males of proven fertility. Hence, both unmated and mated female mice had been superovulated. At 14–15 h after the hCG injection mentioned earlier (counted as embryonic day 0.5 or E0.5), the oviducts of both unmated and mated females were collected into droplets of pre-equilibrated M2 media (EmbryoMax® M2 medium from Millipore; purchased from Level Biotechnology). Zygotes or unfertilized oocytes enclosed in cumulus masses were released from the ampullae, and cumulus cells were removed by pipetting using a mouth-controlled pipette (with an inner diameter of 200–300 μm) after 5 min of treatment with M2 media containing 50−100 U/mL hyaluronidase (EmbryoMax M2 medium with hyaluronidase; Millipore). Zygotes and oocytes were then washed and incubated in 35 μL droplets of potassium simplex optimized medium (KSOM) (EmbryoMax KSOM w/1/2 amino acids, glucose, and phenol red from Millipore), and covered with mineral oil at 37°C in a humidified atmosphere of 5% CO2 in air.
Parthenogenesis was activated after incubation of the unfertilized oocytes in KSOM for 1 h. Oocytes were then washed and incubated in droplets of CZBG medium containing 10 mM strontium chloride, which would stimulate the IP3-PIP2-medicated calcium oscillation mimicking fertilization [44,45], and 5 μg/mL cytochalasin B, which would inhibit the first cytokinesis and maintain the diploid chromosome set [46], at 37°C with 5% CO2 in air, as previously described [47]. After 5–6 h, successful parthenogenetic activation was confirmed by the presence of two pronuclei in the unfertilized oocytes, as previously described [48,49]. These parthenogenetic embryos at the pronuclei stage were then transferred to KSOM and incubated until they reached the developmental stage of interest (ie, the morula or blastocyst stage in this study).
Embryos usually cleave to two cells at E1.0–1.5, and form morulae at E2.5–3.0 (48–60 h in culture). Early blastocysts are formed at E3.5 (70–72 h in culture), blastocysts are formed at E4.0 (80–84 h in culture), and late expanded blastocysts are formed at E4.5 (92–96 h in culture). In this study, the embryos developed from fertilized oocytes that were extracted from mated superovulated female mice are defined as control embryos, while the embryos developed from unfertilized and strontium chloride-activated oocytes which were extracted from unmated superovulated female mice are defined as parthenogenetic embryos. The numbers of sacrificed female mice and embryos used as the control or parthenogenetic group for each analyzed marker were listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd).
Immunofluorescence staining
For immunostaining, embryos were washed for 5–10 s in droplets of acidic Tyrode's solution (prepared by Sigma-Aldrich; purchased from Uni-onward) to remove the zona pellucida, and then fixed in 4% paraformaldehyde (Sigma-Aldrich) in 1× phosphate-buffered saline (PBS) for 15 min at room temperature. Embryos were then permeabilized with 0.25% Triton X-100 for 15 min, followed by washing and blocking for 1 h in blocking solution containing 0.05% Tween-20, 3% bovine serum albumin (BioXtra BSA suitable for mouse embryo cell culture; Sigma A3311), and 5% normal goat serum (Gibco®; Life Technologies PCN5000) in 1× PBS. After blocking, embryos were incubated at 4°C overnight with the following primary antibodies diluted in blocking solution: anti-acetylated histone H3 lysine 9 (rabbit polyclonal, 1:200 dilution; Abcam), anti-CARM1 (both mouse monoclonal and rabbit polyclonal, 1:200 dilution; Abcam), anti-Cdx2 (mouse monoclonal, 1:100 dilution; BioGenex), anti-dimethylated histone H3 arginine 26 (rabbit polyclonal, 1:200 dilution; Abcam), anti-Ezh2 (rabbit polyclonal, 1:200 dilution; Millipore), anti-mono-ubiquitinated histone H2A lysine 119 (mouse monoclonal, 1:200 dilution; Millipore), anti-phosphorylated Akt1 (phospho-S473) (rabbit polyclonal, 1:100 dilution; Abcam), anti-phosphorylated Ezh2 (phospho-S21) (rabbit polyclonal, 1:100 dilution; Abcam), anti-TCF7L2 (rabbit polyclonal, 1:100 dilution; Abcam), anti-trimethylated histone H3 lysine 4 (rabbit polyclonal, 1:200 dilution; Abcam), anti-trimethylated histone H3 lysine 9 (rabbit polyclonal, 1:200 dilution; Millipore), or anti-trimethylated histone H3 lysine 27 (rabbit polyclonal, 1:200 dilution; Millipore). On the second day, the embryos were washed and blocked for 1 h in blocking solution, followed by incubation at room temperature for 1 h with the following secondary antibodies conjugated to fluorophores: goat anti-mouse AlexaFluor 488 (green fluorescence) or goat anti-rabbit AlexaFluor 555 (red fluorescence) (Invitrogen Taiwan). After incubation, the embryos were washed for 10 min in washing solution containing 0.2% Triton X-100 in 1× PBS, and then counter-stained with 0.2 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) in washing solution for 10 min, followed by mounting in VectaShield (Vector Laboratories) on glass slides.
In situ hybridization of miR-181c
An LNA (Locked Nucleic Acid)™-modified DNA oligonucleotide complementary to the mature microRNA miR-181c was used as a probe for in situ hybridization (Exiqon). The sequence of the LNA-miR181c probe was 5′-ACTCACCGACAGGTTGAATGTT-3′, while the sequence of the LNA-scrambled negative control probe was 5′-GCGTTATGACTGATGCTACTTACA-3′. Probes were labeled enzymatically using a 3′-end digoxigenin (DIG) labeling kit (Roche) according to the manufacturer's instructions, and purified using illustra® MicroSpin® G-25 Columns (GE Healthcare). The miRNA in situ hybridization procedure was performed using the DIG-AP Rembrandt® Universal RISH and Detection Kit (Life Technologies), in accordance with the manufacturer's protocol.
Confocal microscopy and statistical analyses
A series of confocal sections through three-dimensional (3D) preserved embryo nuclei were collected using a Leica TCS SP5 confocal microscope (Genomics Research Center, Academia Sinica) equipped with a Super Z galvanometer stage and Plan Apo 63×/1.4 NA oil immersion objectives. Fluorochromes were visualized using an argon laser with an excitation wavelength of 488 nm (for AlexaFluor 488), a diode-pumped solid-state laser with a laser line of 561 nm (for AlexaFluor 555), and a diode laser with a laser line of 405 nm (for DAPI). For each optical section, images were sequentially collected using the XYZ mode for two or three fluorochromes. The pinhole was set to 1–1.5 Airy units, with a 1.5× scan zoom. In order to compare the relative intensities of immunostaining between control and parthenogenetic embryos, identical scanning parameters (including the strength of laser emissions) were maintained for embryos stained with the same antibodies. Images of optical sections were then analyzed using Leica Application Suite, and 3D and maximum projections were constructed from serial stacks of sections for each embryo.
The image files of optical sections of each embryo were input into the Count Nuclei/Cell Sorting Application Module for MetaMorph (MetaMorph Offline vers. 7.0; Universal Imaging Corporation™) for quantitative analyses and a comparison between control and parthenogenetic embryos. Briefly, for cell counting, the numbers of blastomeres or nuclei stained with different colors of fluorescence were counted on the maximum projection pictures (which were stacked from all consecutive optical sections) from controls and parthenotes, respectively. For a comparison of fluorescent intensities, a specified unit of area (eg, a blastomere or a nucleus) in the control embryo was set up to quantify the fluorescent intensity within the area, which was recorded as a standard value, and the same unit of area was picked up from the parthenogenetic embryo, followed by quantification of fluorescent intensity within the area and comparison with the standard value. The relative intensity of an embryo was calculated as the summation of relative values obtained from all units of areas within the single embryo, which were quantified as a fold change of the standard value.
For statistical analyses, Student's t-tests were performed using Microsoft® Excel (Microsoft® Office software by Microsoft Corporation). Numbers of immunostained cells in parthenogenetic embryos were compared with the corresponding developmental stages of in vivo-fertilized control embryos.
Results
Perturbed H3K9 acetylation and normal H3K14 acetylationin parthenogenetic embryos
Immunostaining analyses of control blastocysts revealed a higher level of acetylated histone H3 lysine 9 (H3K9Ac) in the ICM as compared with the TE (Fig. 1B), as previously described [50]. On the other hand, asymmetrical H3K9 acetylation patterns were not observed among blastomeres or between Cdx2+ (green) and Cdx2− blastomeres in control morulae (Fig. 1A). At the morula stage, the pattern and level of H3K9 acetylation in parthenotes were similar to those in controls (Fig. 1A). However, the distribution of H3K9 acetylation in parthenogenetic blastocysts was the opposite of that in controls, with a higher level in the TE as compared with the ICM (indicated by arrows in Fig. 1B). Therefore, acetylation of H3K9 in parthenotes was increased in the TE but decreased in the ICM, as compared with controls. Abnormal distribution of H3K9 acetylation between the ICM and TE in mouse parthenogenetic blastocysts (Fig. 1B) is reminiscent of the loss of asymmetrical H3K9 acetylation in somatic cell nuclear transfer (SCNT)-cloned bovine blastocysts [50].
FIG. 1.
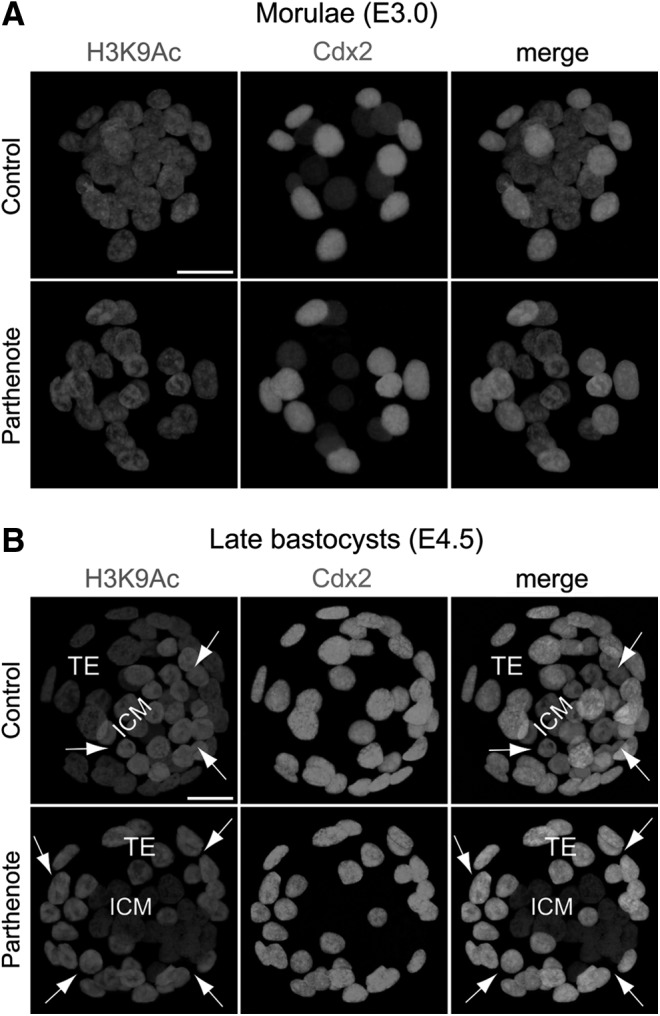
Reversed asymmetrical distribution of acetylated histone H3 lysine 9 (H3K9Ac) in parthenogenetic blastocysts. Double immunostaining of acetylated histone H3K9 and Cdx2 revealed relatively homogeneous staining among all nuclei of both control (normal fertilized) and parthenogenetic morulae (E3.0) (A) and asymmetrical staining between the inner cell mass (ICM) and trophectoderm (TE) of control and parthenogenetic blastocysts (E4.5) (B). Cdx2 was detected in the outer nuclei of morulae and the TE of blastocysts. Note that the asymmetrical distribution of H3K9Ac between the ICM and the TE was reversed in parthenogenetic blastocysts as compared with control blastocysts (B); that is, the H3K9Ac level was apparently higher in the ICM than TE in controls, but was significantly lower in the ICM than TE in parthenotes. Arrows indicate cells with a relatively high H3K9Ac level. Scale bars: 20 μm.
Since a previous study demonstrated that H3K9 and H3K14 acetylation co-occurred at several gene loci in mouse ES cells [51], we also examined H3K14 acetylation in parthenogenetic embryos. Unlike H3K9 acetylation, H3K14 acetylation was homogeneous among all blastomeres at both the morula and blastocyst stages, and its level and pattern were indistinguishable between controls and parthenotes (Fig. 2).
FIG. 2.
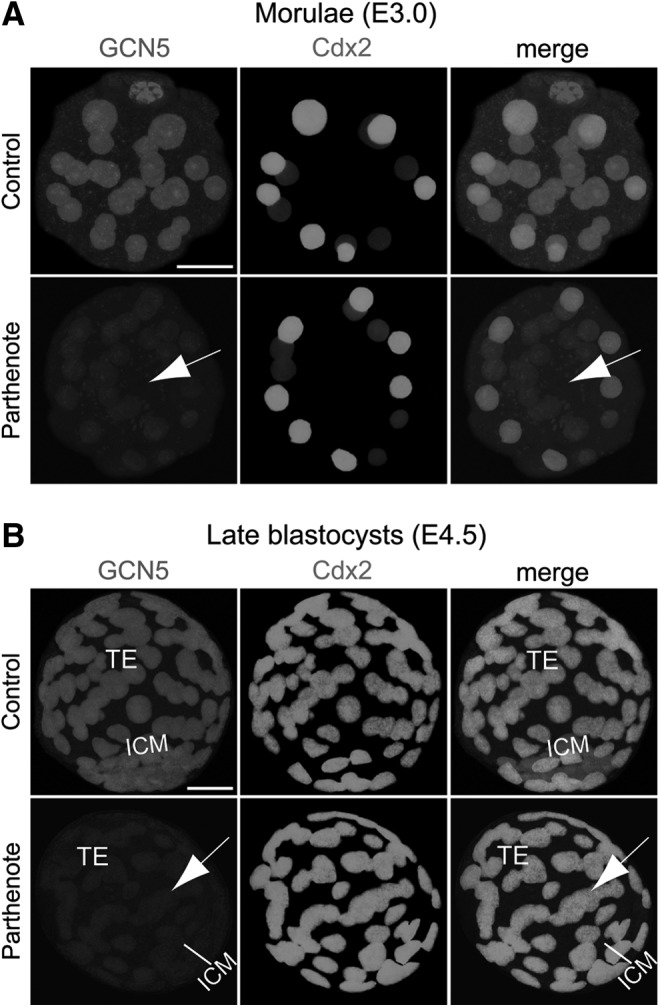
Decreased expression of GCN5 acetyltransferase in parthenogenetic morulae and blastocysts. Double immunostaining against GCN5 and Cdx2 revealed significantly decreased GCN5 expression (indicated by arrows) throughout the whole parthenogenetic embryos (including both the outer Cdx2+ and inner Cdx2- blastomeres) compared with control embryos at both the morula (A) and blastocyst (B) stages. Scale bars: 20 μm.
Normal expression of p300 and decreased expression of GCN5, HDAC1, and Tip60 in parthenogenetic embryos
It has been reported that both H3K9 and H3K14 acetylation are regulated by the acetyltransferases GCN5/PCAF, while H3K9Ac is also regulated by Tip60 acetyltransferase, and H3K14 acetylation is also regulated by the acetyltransferases p300/CBP and/or Myst3 [51–54]. Interestingly, a recent study demonstrated that the expression of GCN5 and HDAC1 was decreased in mouse parthenogenetic embryos at the four-cell stage, predominantly in or around the nuclei [55].
Consistent with the previous finding, we observed decreased GCN5 and HDAC1 expression in parthenotes compared with controls at both the morula and blastocyst stages (Figs. 2 and 3). While GCN5 expression showed a global reduction throughout the whole embryos (Fig. 2), HDAC1 expression was predominantly decreased in the Cdx2+ outer blastomeres and TE (indicated by arrows in Fig. 3), as HDAC1 was expressed at a much lower level in the Cdx2− inner blastomeres and ICM of both controls and parthenotes (Fig. 3). In contrast to the predominant reduction of HDAC1 expression in the outer blastomeres and TE of parthenotes, Tip60 showed a predominant decrease of expression in the inner blastomeres and ICM of parthenotes (indicated by arrows in Fig. 4). Similar to GCN5, Tip60 displayed a homogeneous expression pattern throughout the whole control embryos at both the morula and blastocyst stages (Fig. 4). It is noteworthy that the level of Tip60 expression in the Cdx2+ outer blastomeres and TE of parthenotes was comparable with the expression level in controls (Fig. 4). The reduction of HDAC1 and Tip60 expression in the outer and inner blastomeres of parthenotes, respectively, may impose an additive effect on H3K9 acetylation, leading to an increased and decreased level of H3K9Ac in the TE and ICM of parthenogenetic blastocysts (Fig. 1), respectively.
FIG. 3.
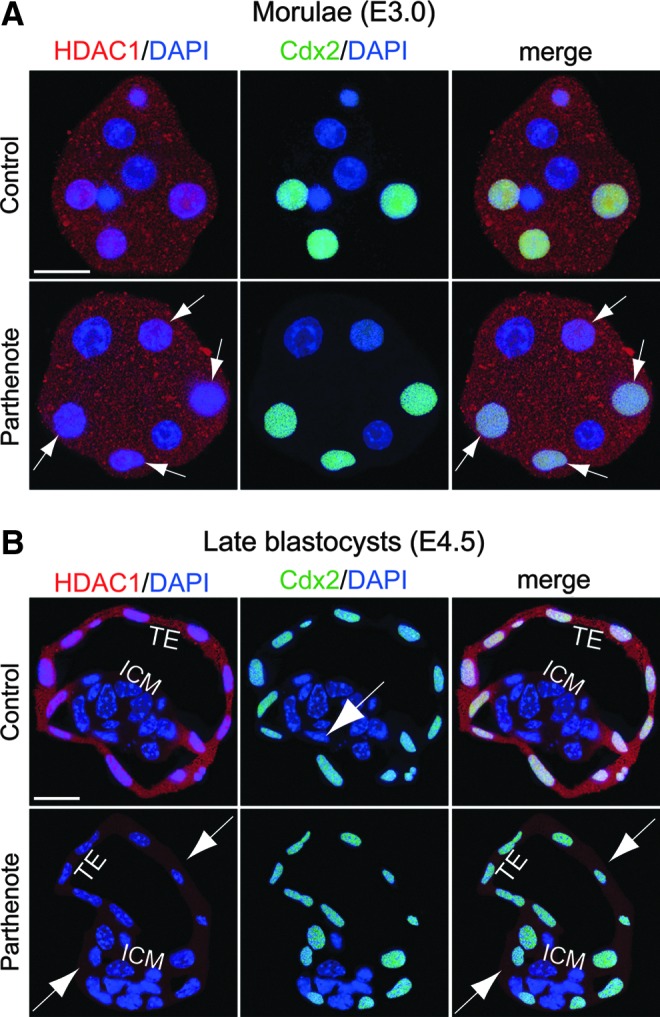
Decreased expression of histone deacetylase 1 (HDAC1) deacetylase in the outer blastomeres and TE of parthenogenetic embryos. Double immunostaining for HDAC1 (red) and Cdx2 (green) indicated that HDAC1 was expressed at a significantly higher level in the Cdx2+ blastomeres (ie, the outer blastomeres of morulae and TE of blastocysts) (A) than in the Cdx2- blastomeres (ie, the inner blastomeres of morulae and ICM of blastocysts) (B) in control embryos. At the morula stage, HDAC1 expression was detectable in both the nuclei and cytoplasm of parthenogenetic outer blastomeres, although at a reduced level compared with control blastomeres [indicated by arrows in (A)]. At the blastocyst stage, the reduction of HDAC1 expression in parthenotes was magnified so that both the TE and ICM displayed only a background staining level of HDAC1 in the cytoplasm [indicated by arrows in (B)]. Scale bars: 20 μm.
FIG. 4.
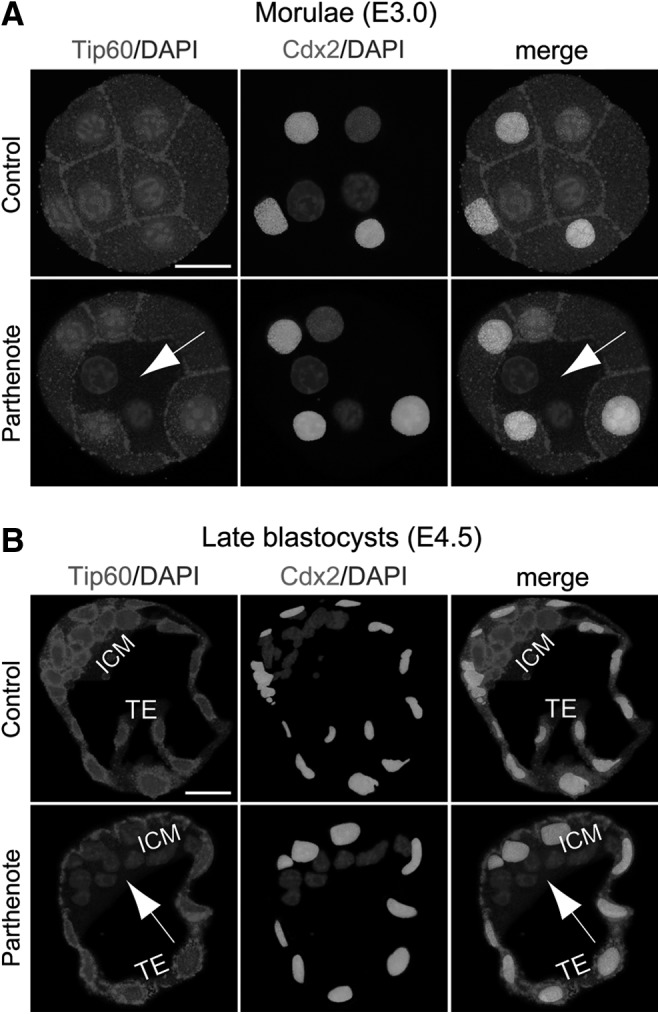
Decreased expression of TAT-interactive protein 60 (Tip60) acetyltransferase in the inner blastomeres and ICM of parthenogenetic embryos. Double immunostaining against Tip60 and Cdx2 indicated that, in parthenogenetic embryos, Tip60 expression was dramatically reduced in the Cdx2− blastomeres (indicated by arrows), including the inner blastomeres of morulae (A) and ICM of blastocysts (B), compared with control embryos, which exhibited strong and homogeneous Tip60 expression throughout the whole embryos. Scale bars: 20 μm.
H3K27 trimethylation is decreased in parthenogenetic TE
Since parthenogenetic embryos have been previously demonstrated to exhibit incomplete X inactivation and overexpression of X-linked genes [56–60] and levels of trimethylation of histone H3 lysine 27 (H3K27Me3) have been reported to be associated with the inactivated paternal X chromosome [61–64], we hypothesized that patterns of this epigenetic mark may be altered in parthenotes. H3K27 trimethylation is detectable in preimplantation embryos from the 16-cell morula stage onward [61–66], and we observed multiple H3K27Me3 punctate signals in all nuclei of both control and parthenogenetic morulae (Fig. 5A). At the blastocyst stage (Fig. 5B), a single strong H3K27Me3 punctate signal was detected in all nuclei of the control TE, but no specific punctate signals above the background level were detected in nuclei of the parthenote TE, consistent with loss of X inactivation in the parthenote TE [56,57,60]. On the other hand, both control and parthenogenetic blastocysts displayed multiple, strong punctate signals of H3K27 trimethylation in the ICM (Fig. 5B). Therefore, trimethylation of H3K27 was normal in the parthenogenetic morula and ICM, but decreased in the parthenogenetic TE.
FIG. 5.
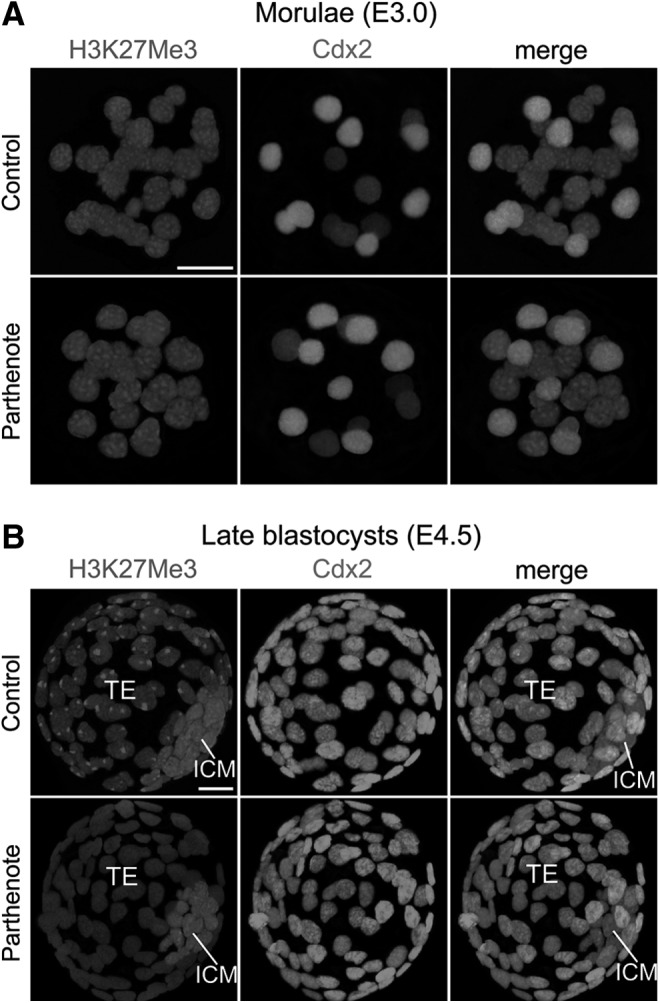
Absence of punctate staining against trimethylated histone H3 lysine 27 (H3K27Me3) in nuclei of the parthenogenetic TE. Immunostaining against dimethylated histone H3K27 revealed multiple punctate signals in all nuclei of both control (normally fertilized) and parthenogenetic morulae (E3.0) (A). At the blastocyst stage, however, the single punctate staining pattern of H3K27Me3 observed in all nuclei of the control TE (expressing Cdx2 with fluorescence) was absent in the parthenogenetic TE (B). Note the higher staining level in the ICM as compared with the TE in both control and parthenogenetic blastocysts (B). Scale bars: 20 μm.
Ezh2 phosphorylation is increased, while H2A mono-ubiquitination is decreased in parthenogenetic blastocysts
Previous studies have demonstrated that decreased H3K27 methylation results from increased phosphorylation of serine 21 of the Polycomb group methyltransferase Ezh2 [43,67]; thus, we examined the levels of total and phosphorylated Ezh2 proteins in parthenogenetic blastocysts. We found that the amounts of total Ezh2 in controls and parthenotes were comparable (Fig. 6A), whereas the level of Ser21-phosphorylated Ezh2 was considerably higher in the parthenote TE than in the control TE (Fig. 6B). On the other hand, the level of Ezh2 phosphorylation was very low in the ICM of both controls and parthenotes (Fig. 6B). Hence, the amount of phosphorylated Ezh2 was greatly increased in the parthenogenetic TE, while total Ezh2 was unaffected.
FIG. 6.
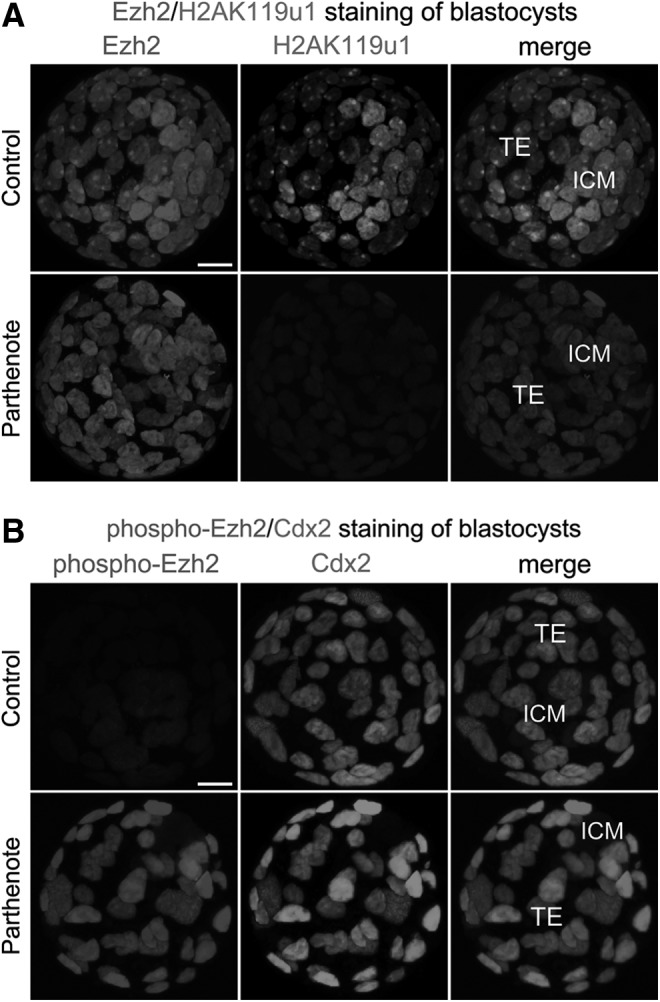
Dramatically decreased mono-ubiquitination of histone H2A lysine 119 (H2AK119u1) and phosphorylation of enhancer of zeste homolog 2 (Ezh2) methyltransferase in parthenogenetic blastocysts. Immunostaining was used to reveal that levels of total Ezh2 methyltransferase were similar between the nuclei of the TE of control and parthenogenetic blastocysts (A). The level of ubiquitinated histone H2AK119 was significantly decreased in both the TE and ICM of parthenogenetic blastocysts (A), while the level of serine 21-phosphorylated Ezh2 was dramatically higher in the parthenogenetic TE than in the control TE (B). Note that the ICM in both control and parthenogenetic blastocysts exhibited only a background level of Ezh2 phosphorylation (B). Scale bars: 20 μm.
H3K27 methylation recruits the Polycomb repressive complex 1 (PRC1) to histone H2A, resulting in mono-ubiquitination of histone H2A at lysine 119 (H2AK119u1) [68,69]; we, therefore, hypothesized that the level of H2AK119u1 would be reduced in parthenotes. As shown in Fig. 6A, we observed only background levels of H2AK119u1 immunostaining in both the TE and ICM of parthenogenetic blastocysts. In contrast, control blastocysts contained a specific H2AK119u1 punctate signal (green) in the nucleus of each TE cell, which co-localized with the punctate signal of Ezh2 (red); furthermore, multiple, strong punctate signals were observed in the nucleus of each ICM cell (Fig. 6A). Our results indicated that increased phosphorylation of Ezh2 in the parthenogenetic TE is concomitant with a significant decrease of both trimethylation of H3K27 and mono-ubiquitination of H2AK119.
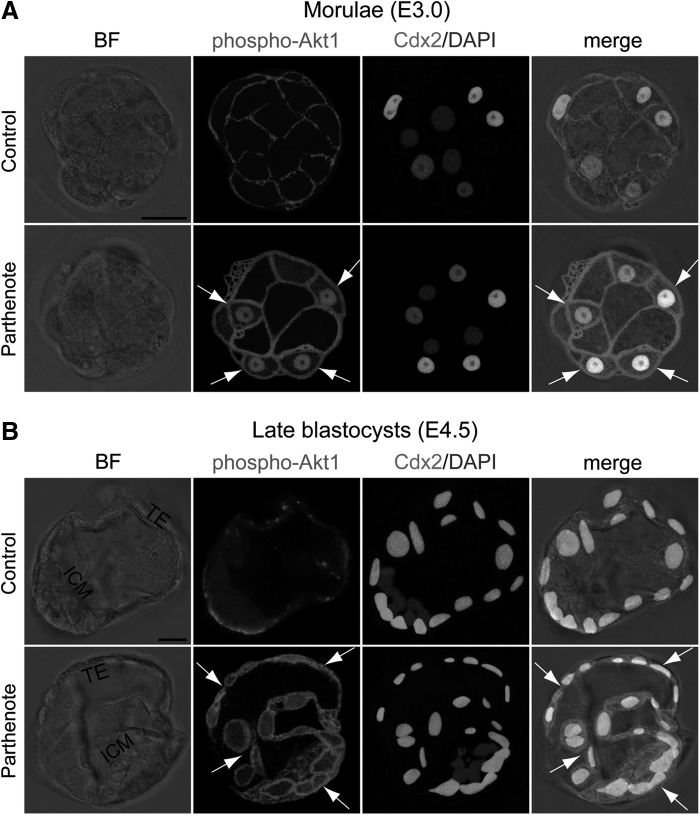
Akt1 phosphorylation is increased in parthenogenetic embryos
The serine/threonine protein kinase Akt1 (also known as protein kinase B α) has been shown to suppress H3K27 trimethylation via phosphorylation of Ezh2 [43]. As such, we hypothesized that over-activation of Akt1 may accompany the increase in phosphorylated Ezh2 in parthenogenetic blastocysts. Previous studies have reported that Akt1 is maximally activated when its serine at position 473 is phosphorylated [70,71], and the level of Ser473-phosphorylated Akt1 is positively correlated with the level of Ser21-phosphorylated Ezh2, but inversely correlated with the level of trimethylated histone H3K27 [43]; we, therefore, investigated the level of Ser473-phosphorylated Akt1 in parthenotes. We found that Akt1 phosphorylation was greatly increased in parthenotes as compared with controls, at both the morula and blastocyst stages (Fig. 7). Ser473-phosphorylated Akt1 was increased primarily at the plasma membrane of parthenotes, but an increase was also observed in the cytoplasm at both the morula and blastocyst stages (indicated by arrows in Fig. 7), as well as in the nuclei of most outer blastomeres at the morula stage (Fig. 7A). Interestingly, by the blastocyst stage, phosphorylated Akt1 was no longer detected in the nuclei of parthenotes (Fig. 7B). Moreover, the level of Akt1 phosphorylation in controls was higher at the morula than at the blastocyst stage (compare Fig. 7A, B), and Ser473-phosphorylated Akt1 displayed an apical staining pattern on the TE at the blastocyst stage (Fig. 7B), consistent with the results of an earlier study [72].
FIG. 7.
Increased phosphorylation of Akt1 in parthenogenetic morulae and blastocysts. Double immunostaining against serine 473-phosphorylated Akt1 and Cdx2 revealed that the phosphorylation level of Akt1 was greatly increased at the plasma membrane and in the cytoplasm of parthenogenetic Cdx2+ blastomeres, during both the morula (E3.0) (A) and blastocyst (E4.5) (B) stages (indicated by arrows). While Ser473-phospho-Akt1 staining was present in the nuclei of most outer blastomeres in parthenogenetic morulae [nuclei indicated by arrows in (A)], nuclear staining of phospho-Akt1 was absent from parthenogenetic blastocysts [arrows in (B)]. Scale bars: 20 μm.
Trimethylation of H3K4 and H3K9 is unaffected in parthenogenetic embryos
In addition to the histone modifications mentioned earlier, trimethylation of lysine 4 and lysine 9 of histone H3 has also been demonstrated to be required for maintaining pluripotency and regulating ICM/TE lineage specification. Trimethylation of histone H3 lysine 4 (H3K4Me3) is observed at actively transcribed gene loci [73–75], and is also associated with the promoters of poised developmental genes and active pluripotency genes, including Oct4, Nanog, and Sox2; of these genes, Nanog and Sox2 display aberrant expression in parthenogenetic morulae and blastocysts [19,76–78]. Furthermore, parthenogenetic embryos at the one- and two-cell stages lose the asymmetrical staining of trimethylated H3K4 between the maternal and paternal genomes [37]. However, our immunofluorescence analyses revealed comparable levels of H3K4 trimethylation between controls and parthenotes at both the morula and blastocyst stages, and staining against trimethylated H3K4 was homogeneous throughout all nuclei of control and parthenogenetic morulae and blastocysts (Supplementary Fig. S1).
Trimethylation of histone H3 lysine 9 (H3K9Me3) is also a key epigenetic mark in developing embryos, and is associated with DNA methylation, inactivated paternal X chromosomes in blastomeres, and repression of TE-specific genes in the ICM [64,79–82]. Immunostaining against H3K9Me3 was also homogeneous throughout the whole embryo at both the morula and blastocyst stages, with no difference between the ICM and TE [62,64,83,84]. Our results, thus, indicate that levels of H3K9 trimethylation are comparable between control and parthenogenetic embryos at both the morula and blastocyst stages (Supplementary Fig. S1).
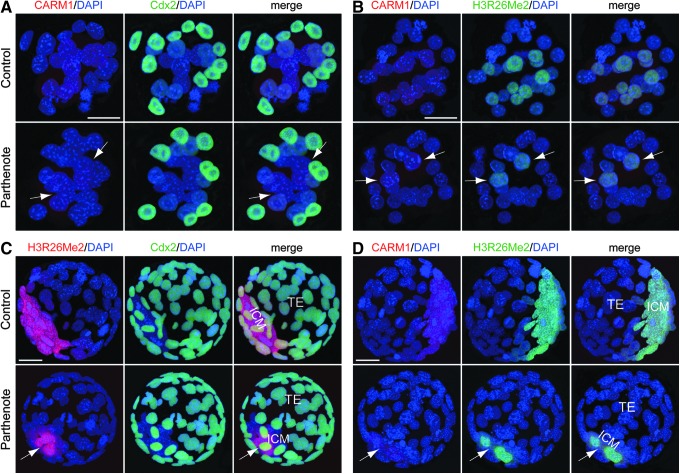
H3R26 dimethylation and CARM1 expression are reduced in parthenogenetic embryos
In a previous study, we observed decreased Nanog and Sox2 expression in parthenotes at both the morula and blastocyst stages [19]; given that the expression of Nanog and Sox2 is upregulated by CARM1-mediated methylation of histone H3 arginine methylation [85,86], we hypothesized that histone H3 arginine methylation may be disrupted in parthenotes. Immunostaining was used to reveal that both CARM1 expression and dimethylation of histone H3 arginine 26 (H3R26Me2) were significantly decreased in parthenotes, with fewer positive nuclei and lower levels in each nucleus as compared with controls, at both the morula (Fig. 8B) and blastocyst (Fig. 8C, D) stages (indicated by arrows). We observed weak immunostaining signals against CARM1 at both the morula (Fig. 8A, B) and blastocyst (Fig. 8D) stages, in agreement with previous reports that CARM1 mRNA is downregulated by the two-cell stage [85] and protein is decreased by the morula stage [87]. At the morula stage, both CARM1 expression (Fig. 8A, B) and H3R26 dimethylation (Fig. 8B) were predominantly detected in the inner blastomeres, whereas a low level of H3R26Me2 was also observed in the outer blastomeres (especially in control morulae; Fig. 8B). The percentages of CARM1-positive blastomeres in control and parthenogenetic morulae were 36.5±2.8% and 10.4±2.9%, respectively, while the percentages of H3R26Me2-positive blastomeres in control and parthenogenetic morulae were 96.6±2.4% and 54.2±3.1%, respectively (n=12, p<0.01, Student's t test). At the blastocyst stage, H3R26 dimethylation colocalized with CARM1 expression (Fig. 8D); both signals were detected in both the ICM (higher level) and TE (lower level) of controls but detected only in the ICM of parthenotes (Fig. 8C, D). The percentages of CARM1-positive/H3R26-positive blastomeres in control and parthenogenetic blastocysts were 51.6±4.6% and 14.2±4.4%, respectively (n=12, p<0.01, Student's t test). Taken together, CARM1 expression and H3R26 dimethylation exhibited an ∼2–3.5-fold reduction in parthenotes as compared with controls during preimplantation development.
FIG. 8.
Decreased coactivator-associated arginine methyltransferase 1 (CARM1) expression and dimethylation of histone H3 arginine 26 (H3R26Me2) in parthenogenetic embryos. (A, B) Immunostaining against CARM1 (red) revealed greatly decreased numbers of positive cells in parthenogenetic morulae [indicated by arrows in (A) and (B)]. Immunostaining signals against dimethylated histone H3R26 [green in (B)] revealed co-localization with those against CARM1 [red in (B)], and a dramatic decrease in the number of positive cells in the parthenogenetic morula [indicated by arrows in (B)]. (C, D) Immunostaining against dimethylated histone H3R26 [red in (C) and green in (D)] revealed significantly lower signals in parthenogenetic blastocysts, primarily in the ICM (indicated by arrows). Immunostaining signals against CARM1 methyltransferase [red in (D)] were co-localized with those of H3R26Me2 [green in (B)], and were also significantly decreased in parthenogenetic blastocysts [this decrease was also primarily in the ICM, as indicated by the arrows in (D)]. Scale bars: 20 μm.
TCF7L2 is decreased, while miR-181c is increased in parthenogenetic embryos
Recent studies have reported increased expression of Wnt signaling components, including the cAMP-dependent protein kinase PKA and transcription factor TCF7L2, in parthenogenetic blastocysts [88], as well as inhibition of CARM1 expression by miR-181c microRNA, a downstream effector of Wnt signaling [40,42]. We, thus, proceeded to investigate whether the observed decrease in CARM1 expression in parthenogenetic morulae and blastocysts correlated with expression of TCF7L2 and miR-181c. In control embryos, both TCF7L2 (Fig. 9) and miR-181c (Fig. 10) were predominantly expressed in the Cdx2-positive outer blastomeres of morulae and TE, with very weak expression in the inner blastomeres of morulae and ICM. In contrast, parthenotes exhibited strong expression of both TCF7L2 and miR-181c in the inner blastomeres of morulae and ICM (indicated by arrows in Figs. 9 and 10). Increased TCF7L2 expression in parthenotes may be associated with increased miR-181c expression, which would result in inhibition of CARM1 expression.
FIG. 9.
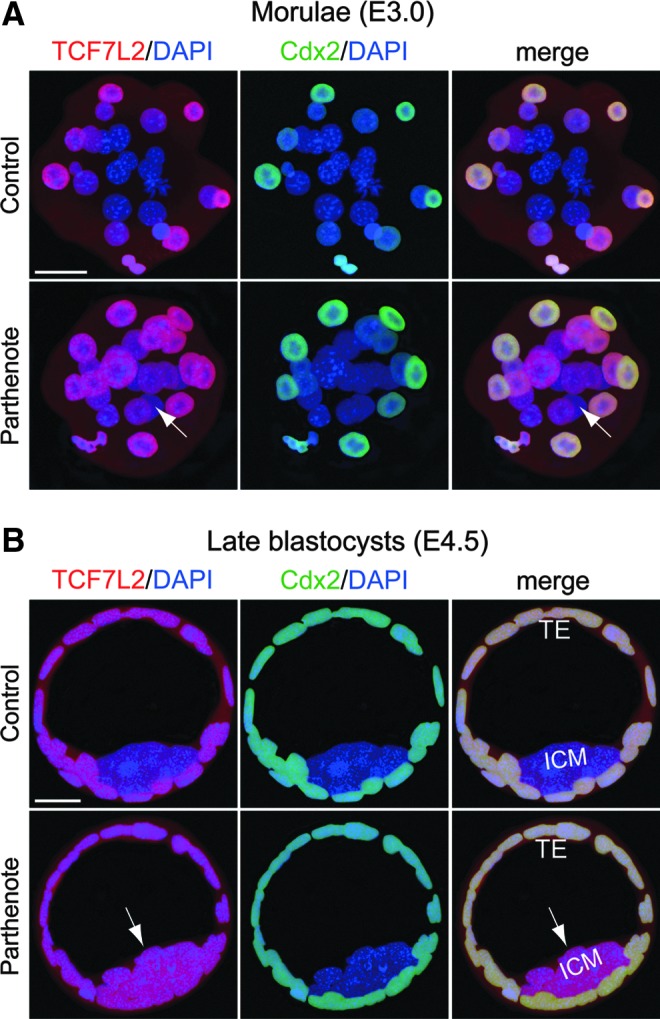
Increased expression of the Wnt effector transcription factor 7L2 (TCF7L2) in parthenogenetic morulae and blastocysts. Double immunostaining against TCF7L2 (red) and Cdx2 (green) revealed dramatically increased TCF7L2 expression (indicated by arrows) in the inner blastomeres of parthenogenetic morulae (A) and parthenote ICM (B) as compared with control embryos. On the other hand, TCF7L2 expression in the outer blastomeres of morulae and TE was comparable between control and parthenogenetic embryos (A, B). Scale bars: 20 μm.
FIG. 10.
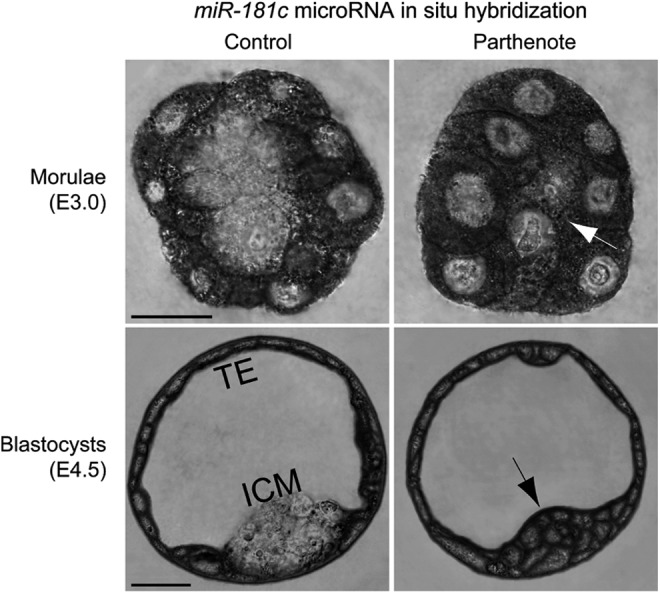
Increased expression of the CARM1 inhibitor miR-181c microRNA in parthenogenetic morulae and blastocysts. RNA in situ hybridization was performed using an LNA™-modified DNA oligonucleotide probe against miR-181c microRNA. Expression of miR-181c was dramatically increased in the inner blastomeres of parthenogenetic morulae and the ICM of parthenogenetic blastocysts (indicated by arrows), as compared with control embryos. On the other hand, miR-181c expression in the outer blastomeres of morulae and TE was comparable between control and parthenogenetic embryos. Scale bars: 20 μm.
Discussion
The presence of both paternal and maternal genomes is required to establish epigenetic asymmetry in the zygote, which is essential for correct nuclear reprogramming and restoration of cell totipotency during early preimplantation development [34,36]. The absence of one parental genome can contribute to aberrant expression of imprinted, development-related, and pluripotency genes, as alteration of epigenetic asymmetry has been suggested to change the developmental potency of cells [8,34,36,37,89]. However, aberrant nuclear reprogramming and loss of epigenetic asymmetry was also observed between zygote pronuclei of mammalian embryos cloned by SCNT, despite the nuclei carrying both paternal and maternal genomes [34,36,37,90]. This suggests that perturbed epigenetic asymmetry does not necessarily result from loss of a parental genome. It is likely that the in vitro activation and culture of oocytes per se is an important contributor to the aberrant nuclear reprogramming during early preimplantation development. In addition, superovulation per se has been demonstrated to perturb DNA methylation and expression of Line-1 retrotransposon and several imprinted genes, including PEG1/MEST and H19 [91–94]. However, it remains to be unraveled whether superovulation affects histone modifications as well. In our study, the patterns and levels of H3K9Ac and H3K14Ac; methylation of H3 lysine 4, lysine 9, lysine 27, and arginine 26, as well as ubiquitylation of H2A lysine 119, are consistent with those shown in previous studies [50,62,64–68,85,95,96]. Hence, the putative effect of superovulation on histone signatures may be negligible in this study.
Multiple lines of evidence have demonstrated that epigenetic asymmetry between blastomeres imposes heritable instructions for lineage-specific differentiation, and may, thus, direct lineage allocation between the morula and blastocyst stages [34,35,97]. Our findings of perturbed histone-modifying signaling proteins and aberrant histone signatures in parthenogenetic morulae and blastocysts are summarized in Fig. 11. Of the epigenetic modifications analyzed, asymmetrical DNA methylation between the ICM and TE lineages and differential methylation of histone H3 arginine among blastomeres since the four-cell stage were demonstrated to be the most important for lineage allocation and cell fate determination [85,97]. We observed a significant reduction in H3 arginine methylation and CARM1 expression in parthenogenetic embryos at both the morula and blastocyst stages; these findings, combined with our previous report that expression of Nanog and Sox2 is markedly decreased in such embryos [19], suggest a possible mechanism for impaired lineage allocation and cell fate determination in parthenotes. It was recently reported that CARM1 translation is downregulated by the miR-181 family of microRNAs [42], which are, in turn, upregulated by the Wnt/Tcf signaling pathway [40]. Interestingly, Wnt signaling was increased in parthenogenetic blastocysts, with increased expression of the TCF7L2 transcription factor and the cAMP-dependent protein kinase PKA [88]. We report here that the expression of both TCF7L2 and miR-181c is dramatically increased in the inner blastomeres of morulae and ICM in parthenotes, perhaps accounting for the decrease in CARM1 expression.
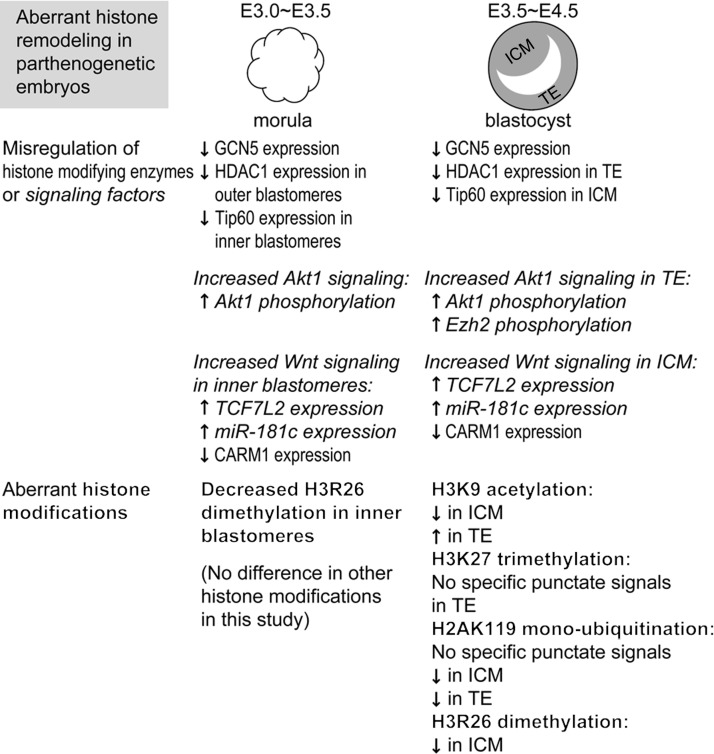
FIG. 11.
Summary of aberrant histone modifications and misregulation of histone-modifying enzymes and associated signaling factors in parthenogenetic morulae and blastocysts. Of the histone modifications analyzed in this study, only the dimethylation level of histone H3 arginine 26 (H3R26) was different between control and parthenogenetic embryos at the morula stage. At the blastocyst stage, however, levels of the following histone modifications were altered in parthenotes: H3R26 dimethylation, histone H3 lysine 9 (H3K9) acetylation, histone H3 lysine 27 (H3K27) trimethylation, and histone H2A lysine 119 (H2AK119) mono-ubiquitination. Decreased dimethylation of H3R26 in parthenotes may be due to decreased expression of CARM1 methyltransferase, which may result from the observed increase in TCF7L2 (a component of Wnt signaling) and expression of miR-181c microRNA at both the morula and blastocyst stages. Decreased trimethylation of H3K27 in parthenote TE, on the other hand, is associated with decreased mono-ubiquitination of H2AK119 and increased phosphorylation of Ezh2 methyltransferase, which may result from the observed increase in phosphorylation of Akt1 in parthenogenetic blastocysts. In addition, we found that H3K9 acetylation is decreased in parthenote ICM and increased in parthenote TE as compared with control blastocysts, whereas the underlying molecular mechanism remains to be elucidated.
Other asymmetric histone modifications in mammalian blastocysts include H3K9Ac and di-/tri-methylation of histone H3 lysine 27 (H3K27Me2/Me3) [34,35,50,62,64]. Previous studies reported that H3K9 acetylation was associated with active gene transcription [98–101], and the expression of both Nanog and Sox2 was upregulated by H3K9 acetylation [73,102,103]. Our finding that levels of acetylated H3K9 are decreased in the parthenogenetic ICM (Fig. 1B) may be associated with reduced expression of Nanog and Sox2 [19]. The decrease of H3K9Ac in the parthenogenetic ICM may be associated with reduced expression of Tip60 acetyltransferase in the Cdx2− inner blastomeres of parthenotes (Fig. 4), whereas the increase of H3K9Ac in the parthenogenetic TE may be attributable to reduced expression of HDAC1 deacetylase in the Cdx2+ outer blastomeres of parthenotes (Fig. 3). Another histone modification, H3 lysine 14 acetylation (H3K14Ac), which has been shown to co-occur with H3K9Ac at multiple gene regulatory elements [51], did not show a significant change of pattern or level in parthenotes (Supplementary Fig. S2). Interestingly, H3K14Ac has been reported to be required for not only Nanog expression but also Oct4 expression in mouse preimplantation embryos or ES cells [95,104]. Unaffected acetylation of H3K14 in parthenotes, which may be attributable to unchanged expression of p300 [51,52] (Supplementary Fig. S3), may be associated with normal Oct4 expression in parthenotes as observed [19].
On the other hand, H3K27 methylation has been shown to be associated with the inactivation of the paternal X chromosome, as well as with promoters of pluripotency genes and poised developmental genes in TE cells of normal blastocysts [61,63,64,76–78,105]. We found loss of specific punctate signals of H3K27 dimethylation in the TE, but not ICM, of parthenogenetic blastocysts (Fig. 5B), suggesting impaired X inactivation or aberrant expression of poised developmental genes in the parthenogenetic TE, for example, ectopic expression of Gata4 in the parthenote TE [19]. Interestingly, it was recently reported that H3K27Me3-positive signals were present in <10% of Gata4-positive hypoblast cells in in vitro-cultured human embryos [106]. In contrast to perturbed H3K9 acetylation and H3K27 trimethylation in parthenogenetic blastocysts, expression of both H3K9Ac and H3K27Me3 was normal in parthenogenetic morulae (Figs. 1A and 2A), despite the aberrant expression of lineage-specific genes Sox2, Nanog, and Gata4 [19] as well as acetylation-regulatory enzymes GCN5, HDAC1, and Tip60 in parthenotes at the morula stage. This suggests that restricted expression of lineage-specific genes may regulate the expression pattern of histone-modifying enzymes and, subsequently, contribute to the establishment of asymmetric patterns of H3K9Ac and H3K27Me3 between embryonic and extraembryonic lineages.
It has been reported that activated Akt1 phosphorylates Ezh2 methyltransferase and inhibits H3K27 trimethylation and H2AK119 ubiquitylation [43,67–69]. We observed a dramatic increase of Ser21-phosphorylated Ezh2 and Ser473-phosphorylated Akt1 in parthenogenetic blastocysts, in association with decreased H3K27 trimethylation and H2AK119 mono-ubiquitination. It is noteworthy that increased phosphorylation of Akt1 was observed in parthenotes at the morula stage, preceding the occurrence of perturbedH3K27 trimethylation and H2AK119 mono-ubiquitination at the blastocyst stage (Fig. 5A and data not shown). In addition to Ezh2, both H3K27Me3 and H2AK119u1 are subject to regulation by the binding partner of Ezh2, the Polycomb protein embryonic ectoderm development (EED), which inhibits expression of TE lineage-specific genes, including Cdx2 and Gata3 [66]. Since Cdx2 expression is unchanged in parthenotes ([19] and this study), it is less likely that EED contributes to the perturbed pattern of H3K27Me3 in parthenogenetic TE. Another candidate of H3K27Me3-regulatory protein is KDM6B/JDJM3 (lysine-specific demethylase 6B, also known as Jumonji domain-containing protein 3) [66,107]. However, previous studies have reported that the histone demethylases JDJM3 and JDJM2C were expressed normally in parthenogenetic embryos [107,108], and depletion of these histone demethylases caused a developmental arrest before the blastocyst stage [107,108], unlike parthenotes, which could develop till the hatched blastocyst stage in vitro [19]. Therefore, KDM6B/JDJM3 demethylase may be ruled out as a misregulated candidate in parthenotes.
It is noteworthy that, although the levels of both H3K27 trimethylation and H2AK119 mono-ubiquitination significantly decreased in the parthenogenetic TE (Figs. 5B and 6A), H3K27Me3 was still present in the parthenogenetic ICM (Fig. 5B), where no specific signal of H2AK119u1 was detected (Fig. 6A). Since H3K27Me3 is directly recruited by the PRC2 and H2AK119u1 is directly recruited by PRC1, which is downstream of PRC2 [67–69], it is plausible that perturbed expression or activity of the components of PRC1 would affect the level of H2AK119u1 but not H3K27Me3. Interestingly, recent studies have demonstrated that the histone H3 lysine 36-specific demethylase Fbxl10/Kdm2b is a pivotal interacting partner of Ring1b, a core component of PRC1, and is required for H2AK119 ubiquitylation and suppression of extra-embryonic endoderm-specific genes, including Gata4 and Gata6, in order to maintain pluripotency of ES cells [109,110]. Therefore, loss of H2AK119u1 in the parthenogenetic ICM may be associated with dramatically increased Gata4 expression as observed [19]. Future studies will be required to further elucidate whether the expression of Fbxl10/Kdm2b or Ring1b, or the interaction between these two proteins is perturbed in parthenogenetic embryos.
Supplementary Material
Acknowledgments
The authors thank Chien-Hong Chen for providing the parthenogenetic activation protocol and Li-Wen Lo of the Core Facility of the Genomics Research Center for expert assistance with confocal microscopy. They also thank Drs. Hung-Chih Kuo and Cheng-Fu Kao in the Institute of Organismic and Cellular Biology for their critical comments on this project and article. This work was supported by grants from 100-2321-B-001-036, 101-2321-B-182A-004, and 102-2321-B-182A-004 from National Science Council to J.Y.
Prior conference presentation of the submitted material: it was presented in the annual meetings of Taiwan Society for Stem Cell Research in 2008 and 2009.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cassar G, John TM. and Etches RJ. (1998). Observations on ploidy of cells and on reproductive performance in parthenogenetic turkeys. Poult Sci 77:1457–1462 [DOI] [PubMed] [Google Scholar]
- 2.Fujita MK. and Moritz C. (2009). Origin and evolution of parthenogenetic genomes in lizards: current state and future directions. Cytogenet Genome Res 127:261–272 [DOI] [PubMed] [Google Scholar]
- 3.Parker HM, Kiess AS, Wells JB, Young KM, Rowe D. and McDaniel CD. (2010). Genetic selection increases parthenogenesis in Chinese painted quail (Coturnix chinensis). Poult Sci 89:1468–1472 [DOI] [PubMed] [Google Scholar]
- 4.Sinclair EA, Pramuk JB, Bezy RL, Crandall KA. and Sites JW, Jr., (2010). DNA evidence for nonhybrid origins of parthenogenesis in natural populations of vertebrates. Evolution 64:1346–1357 [DOI] [PubMed] [Google Scholar]
- 5.Cheng L. (2008). More new lines of human parthenogenetic embryonic stem cells. Cell Res 18:215–217 [DOI] [PubMed] [Google Scholar]
- 6.Revazova ES, Turovets NA, Kochetkova OD, Agapova LS, Sebastian JL, Pryzhkova MV, Smolnikova VI, Kuzmichev LN. and Janus JD. (2008). HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells 10:11–24 [DOI] [PubMed] [Google Scholar]
- 7.Allen ND, Barton SC, Hilton K, Norris ML. and Surani MA. (1994). A functional analysis of imprinting in parthenogenetic embryonic stem cells. Development 120:1473–1482 [DOI] [PubMed] [Google Scholar]
- 8.Barton SC, Arney KL, Shi W, Niveleau A, Fundele R, Surani MA. and Haaf T. (2001). Genome-wide methylation patterns in normal and uniparental early mouse embryos. Hum Mol Genet 10:2983–2987 [DOI] [PubMed] [Google Scholar]
- 9.Bonk AJ, Li R, Lai L, Hao Y, Liu Z, Samuel M, Fergason EA, Whitworth KM, Murphy CN, Antoniou E. and Prather RS. (2007). Aberrant DNA methylation in porcine in vitro-, parthenogenetic-, and somatic cell nuclear transfer-produced blastocysts. Mol Reprod Dev 75:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean W, Bowden L, Aitchison A, Klose J, Moore T, Meneses JJ, Reik W. and Feil R. (1998). Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development 125:2273–2282 [DOI] [PubMed] [Google Scholar]
- 11.Horii T, Kimura M, Morita S, Nagao Y. and Hatada I. (2008). Loss of genomic imprinting in mouse parthenogenetic embryonic stem cells. Stem Cells 26:79–88 [DOI] [PubMed] [Google Scholar]
- 12.Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM, Biniszkiewicz D, Yanagimachi R. and Jaenisch R. (2001). Epigenetic instability in ES cells and cloned mice. Science 293:95–97 [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Sun B, Wang W, Zhang Z, Gao F, Shi G, Cui B, Kong X, He Z, et al. (2007). Activation of paternally expressed imprinted genes in newly derived germline-competent mouse parthenogenetic embryonic stem cell lines. Cell Res 17:792–803 [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Lerou P, Yabuuchi A, Lengerke C, Ng K, West J, Kirby A, Daly MJ. and Daley GQ. (2007). Histocompatible embryonic stem cells by parthenogenesis. Science 315:482–486 [DOI] [PubMed] [Google Scholar]
- 15.Mitalipov S, Clepper L, Sritanaudomchai H, Fujimoto A. and Wolf D. (2007). Methylation status of imprinting centers for H19/IGF2 and SNURF/SNRPN in primate embryonic stem cells. Stem Cells 25:581–588 [DOI] [PubMed] [Google Scholar]
- 16.Rugg-Gunn PJ, Ferguson-Smith AC. and Pedersen RA. (2007). Status of genomic imprinting in human embryonic stem cells as revealed by a large cohort of independently derived and maintained lines. Hum Mol Genet 16:R243–R251 [DOI] [PubMed] [Google Scholar]
- 17.Szabó P. and Mann JR. (1994). Expression and methylation of imprinted genes during in vitro differentiation of mouse parthenogenetic and androgenetic embryonic stem cell lines. Development 120:1651–1660 [DOI] [PubMed] [Google Scholar]
- 18.Zvetkova I, Apedaile A, Ramsahoye B, Mermoud JE, Crompton LA, John R, Feil R. and Brockdorff N. (2005). Global hypomethylation of the genome in XX embryonic stem cells. Nat Genet 37:1274–1279 [DOI] [PubMed] [Google Scholar]
- 19.Chen YH. and Yu J. (2012). Ectopic expression of Fgf3 leads to aberrant lineage segregation in the mouse parthenote preimplantation embryos. Dev Dyn 241:1651–1664 [DOI] [PubMed] [Google Scholar]
- 20.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS. and Schultz RM. (2000). Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 62:1526–1535 [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Gonzalez R, Ramirez MA, Bilbao A, De Fonseca FR. and Gutiérrez-Adán A. (2007). Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects. Mol Reprod Dev 74:1149–1156 [DOI] [PubMed] [Google Scholar]
- 22.Kafer GR, Kaye PL, Pantaleon M, Moser RJ. and Lehnert SA. (2011). In vitro manipulation of mammalian preimplantation embryos can alter transcript abundance of histone variants and associated factors. Cell Reprogram 13:391–401 [DOI] [PubMed] [Google Scholar]
- 23.Li T, Vu TH, Ulaner GA, Littman E, Ling JQ, Chen HL, Hu JF, Behr B, Giudice L. and Hoffman AR. (2005). IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. Mol Hum Reprod 11:631–640 [DOI] [PubMed] [Google Scholar]
- 24.Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM. and Bartolomei MS. (2004). Selective loss of imprinting in the placenta following preimplantation development in culture. Development 131:3727–3735 [DOI] [PubMed] [Google Scholar]
- 25.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM. and Bartolomei MS. (2008). Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet 17:1–14 [DOI] [PubMed] [Google Scholar]
- 26.Young LE. and Fairburn HR. (2000). Improving the safety of embryo technologies: possible role of genomic imprinting. Theriogenology 53:627–648 [DOI] [PubMed] [Google Scholar]
- 27.Kawahara M, Wu Q, Yaguchi Y, Ferguson-Smith AC. and Kono T. (2006). Complementary roles of genes regulated by two paternally methylated imprinted regions on chromosomes 7 and 12 in mouse placentation. Hum Mol Genet 15:2869–2879 [DOI] [PubMed] [Google Scholar]
- 28.Obata Y, Kaneko-Ishino T, Koide T, Takai Y, Ueda T, Domeki I, Shiroishi T, Ishino F. and Kono T. (1998). Disruption of primary imprinting during oocyte growth leads to the modified expression of imprinted genes during embryogenesis. Development 125:1553–1560 [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Kumagai T, Kawahara M, Ogawa H, Hiura H, Obata Y, Takano R. and Kono T. (2006). Regulated expression of two sets of paternally imprinted genes is necessary for mouse parthenogenetic development to term. Reproduction 131:481–488 [DOI] [PubMed] [Google Scholar]
- 30.Bui HT, Wakayama S, Mizutani E, Park KK, Kim JH, Van Thuan N. and Wakayama T. (2011). Essential role of paternal chromatin in the regulation of transcriptional activity during mouse preimplantation development. Reproduction 141:67–77 [DOI] [PubMed] [Google Scholar]
- 31.Barton SC, Adams CA, Norris ML. and Surani MA. (1985). Development of gynogenetic and parthenogenetic inner cell mass and trophectoderm tissues in reconstituted blastocysts in the mouse. J Embryol Exp Morphol 90:267–285 [PubMed] [Google Scholar]
- 32.Mognetti B. and Sakkas D. (1996). Defects in the allocation of cells to the inner cell mass and trophectoderm of parthenogenetic mouse blastocysts. Reprod Fertil Dev 8:1193–1197 [DOI] [PubMed] [Google Scholar]
- 33.Sturm KS, Flannery ML. and Pedersen RA. (1994). Abnormal development of embryonic and extraembryonic cell lineages in parthenogenetic mouse embryos. Dev Dyn 201:11–28 [DOI] [PubMed] [Google Scholar]
- 34.Morgan HD, Santos F, Green K, Dean W. and Reik W. (2005). Epigenetic reprogramming in mammals. Hum Mol Genet 14:R47–R58 [DOI] [PubMed] [Google Scholar]
- 35.Reik W, Santos F, Mitsuya K, Morgan H. and Dean W. (2003). Epigenetic asymmetry in the mammalian zygote and early embryo: relationship to lineage commitment?. Phil Trans R Soc Lond B Biol Sci 358:1403–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos F. and Dean W. (2004). Epigenetic reprogramming during early development in mammals. Reproduction 127:643–651 [DOI] [PubMed] [Google Scholar]
- 37.Merico V, Barbieri J, Zuccotti M, Joffe B, Cremer T, Redi CA, Solovei I. and Garagna S. (2007). Epigenomic differentiation in mouse preimplantation nuclei of biparental, parthenote and cloned embryos. Chromosome Res 15:341–360 [DOI] [PubMed] [Google Scholar]
- 38.Maalouf WE, Alberio R. and Campbell KHS. (2008). Differential acetylation of histone H4 lysine during development of in vitro fertilized, cloned and parthenogenetically activated bovine embryos. Epigenetics 3:199–209 [DOI] [PubMed] [Google Scholar]
- 39.Nowak-Imialek M, Wrenzycki C, Herrmann D, Lucas-Hahn A, Lagutina I, Lemme E, Lazzari G, Galli C. and Niemann H. (2008). Messenger RNA expression patterns of histone-associated genes in bovine preimplantation embryos derived from different origins. Mol Reprod Dev 75:731–743 [DOI] [PubMed] [Google Scholar]
- 40.Ji J, Yamashita T. and Wang XW. (2011). Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, Li A, Xie Y, Li J, et al. (2010). A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics 11:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Jiang J, Xu C, Wang Y, Sun L, Guo X. and Liu H. (2013). MicroRNA-181 regulates CARM1 and histone arginine methylation to promote differentiation of human embryonic stem cells. PLoS One 8:e53146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha T-L, Zhou BP, Xia W, Wu Y, Yang C-C, Chen C-T, Ping B, Otte AP. and Hung M-C. (2005). Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 310:306–310 [DOI] [PubMed] [Google Scholar]
- 44.Carvacho I, Lee HC, Fissore RA. and Clapham DE. (2013). TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep 5:1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang D, Pan L, Yang LH, He XK, Huang XY. and Sun FZ. (2005). Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Human Reprod 20:3053–3061 [DOI] [PubMed] [Google Scholar]
- 46.Imahie H, Takahashi M, Toyoda Y. and Sato E. (2002). Differential effects of cytochalasin B on cytokinesis in parthenogenetically activated mouse oocytes. J Reprod Dev 48:31–40 [Google Scholar]
- 47.Gao S. (2006). Protocols for nuclear transfer in mice. Methods Mol Biol 325:25–33 [DOI] [PubMed] [Google Scholar]
- 48.Abramczuk J. and Sawicki W. (1975). Pronuclear synthesis of DNA in fertilized and parthenogenetically activated mouse eggs. Exp Cell Res 92:361–372 [DOI] [PubMed] [Google Scholar]
- 49.Han BS. and Gao JL. (2013). Effects of chemical combinations on the parthenogenetic activation of mouse oocytes. Exp Therapeut Med 5:1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos F, Zakhartchenko V, Stojkovic M, Peters A, Jenuwein T, Wolf E, Reik W. and Dean W. (2003). Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr Biol 13:1116–1121 [DOI] [PubMed] [Google Scholar]
- 51.Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H. and Tora L. (2012). H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics 13:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, Wang C, Brindle PK, Dent SY. and Ge K. (2011). Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J 30:249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagy Z. and Tora L. (2007). Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26:5341–5357 [DOI] [PubMed] [Google Scholar]
- 54.Lee KK. and Workman JL. (2007). Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol 8:284–295 [DOI] [PubMed] [Google Scholar]
- 55.Peng M, Li Y, Huang H. and Jin F. (2014). The expression of GCN5, HDAC1 and DNMT1 in parthenogenetically activated mouse embryos. J Obstet Gynaecol 1–5 [DOI] [PubMed] [Google Scholar]
- 56.Brown AL. and Kay GF. (1999). Bex1, a gene with increased expression in parthenogenetic embryos, is a member of a novel gene family on the mouse X chromosome. Hum Mol Genet 8:611–619 [DOI] [PubMed] [Google Scholar]
- 57.Latham KE. and Rambhatla L. (1995). Expression of X-linked genes in androgenetic, gynogenetic, and normal mouse preimplantation embryos. Dev Genet 17:212–222 [DOI] [PubMed] [Google Scholar]
- 58.Nesterova TB, Barton SC, Surani MA. and Brockdorff N. (2001). Loss of Xist imprinting in diploid parthenogenetic preimplantation embryos. Dev Biol 235:343–350 [DOI] [PubMed] [Google Scholar]
- 59.Sturm K, Lafferty M. and Tam PPL. (1999). Pgk1 and Hprt gene activity in the peri-implantation mouse embryo is influenced by the parental origin of the X-chromosome. Int J Dev Biol 43:69–73 [PubMed] [Google Scholar]
- 60.Tada T. and Takagi N. (1992). Early development and X-chromosome inactivation in mouse parthenogenetic embryos. Mol Reprod Dev 31:20–27 [DOI] [PubMed] [Google Scholar]
- 61.Cheng MK. and Disteche CM. (2004). Silence of the fathers: early X inactivation. Bioessays 26:821–824 [DOI] [PubMed] [Google Scholar]
- 62.Erhardt S, Su IH, Schneider R, Barton S, Bannister AJ, Perez-Burgos L, Jenuwein T, Kouzarides T, Tarakhovsky A. and Surani MA. (2003). Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development 130:4235–4248 [DOI] [PubMed] [Google Scholar]
- 63.Heard E. (2004). Recent advances in X-chromosome inactivation. Curr Opin Cell Biol 16:247–255 [DOI] [PubMed] [Google Scholar]
- 64.Okamoto I, Otte AP, Allis CD, Reinberg D. and Heard E. (2004). Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303:644–649 [DOI] [PubMed] [Google Scholar]
- 65.Rugg-Gunn PJ, Cox BJ, Ralston A. and Rossant J. (2010). Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc Natl Acad Sci U S A 107:10783–10790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saha B, Home P, Ray S, Larson M, Paul A, Rajendran G, Behr B. and Paul S. (2013). EED and KDM6B coordinate the first mammalian cell lineage commitment to ensure embryo implantation. Mol Cell Biol 33:2691–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sparmann A. and van Lohuizen M. (2006). Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6:846–856 [DOI] [PubMed] [Google Scholar]
- 68.Lee MG, Villa R, Trojer P, Norman J, Yan K-P, Reinberg D, Croce LD. and Shiekhattar R. (2007). Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 318:447–450 [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Gong Y, Yue J, Qiang B, Yuan J. and Peng X. (2008). Cooperation between EZH2, NSPc1-mediated histone H2A ubiquitination and Dnmt1 in HOX gene silencing. Nucleic Acids Res 36:3590–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P. and Hemmings BA. (1996). Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 71.Wang R. and Brattain MG. (2006). AKT can be activated in the nucleus. Cell Signal 18:1722–1731 [DOI] [PubMed] [Google Scholar]
- 72.Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Ratajczak CK. and Moley KH. (2005). The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Dev Biol 284:377–386 [DOI] [PubMed] [Google Scholar]
- 73.Hattori N, Imao Y, Nishino K, Hattori N, Ohgane J, Yagi S, Tanaka S. and Shiota K. (2007). Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells 12:387–396 [DOI] [PubMed] [Google Scholar]
- 74.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J. and Kouzarides T. (2002). Active genes are tri-methylated at K4 of histone H3. Nature 419:407–411 [DOI] [PubMed] [Google Scholar]
- 75.Sims RJ, III, Millhouse S, Chen C-F, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL. and Reinberg D. (2007). Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription post-initiation factors and pre-mRNA splicing. Mol Cell 28:665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adamo A, Sesé B, Boue S, Castaño J, Paramonov I, Barrero MJ. and Belmonte JCI. (2011). LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol 13:652–659 [DOI] [PubMed] [Google Scholar]
- 77.Dahl JA, Reiner AH, Klungland A, Wakayama T. and Collas P. (2010). Histone H3 lysine 27 methylation asymmetry on developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation embryos. PLoS One 5:e9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang H, Shukla A, Wang X, Chen W- Y, Bernstein BE. and Roeder RG. (2011). Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 144:513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson JP, Lindroth AM, Cao X. and Jacobsen SE. (2002). Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416:556–560 [DOI] [PubMed] [Google Scholar]
- 80.Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S, Chen T, Li E, Jenuwein T. and Peters AH. (2003). Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol 13:1192–1200 [DOI] [PubMed] [Google Scholar]
- 81.Lohmann F, Loureiro J, Su H, Fang Q, Lei H, Lewis T, Yang Y, Labow M, Li E, Chen T. and Kadam S. (2010). KMT1E mediated H3K9 methylation is required for the maintenance of embryonic stem cells by repressing trophectoderm differentiation. Stem Cells 28:201–212 [DOI] [PubMed] [Google Scholar]
- 82.Tamaru H. and Selker EU. (2001). A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414:277–283 [DOI] [PubMed] [Google Scholar]
- 83.Huang J-C, Lei Z-L, Shi L-H, Miao Y-L, Yang J-W, Ouyang Y-C, Sun Q-Y. and Chen D-Y. (2007). Comparison of histone modifications in in vivo and in vitro fertilization mouse embryos. Biochem Biophys Res Commun 354:77–83 [DOI] [PubMed] [Google Scholar]
- 84.Yeo S, Lee KK, Han YM. and Kang YK. (2005). Methylation changes of lysine 9 of histone H3 during preimplantation mouse development. Mol Cells 20:423–428 [PubMed] [Google Scholar]
- 85.Torres-Padilla ME, Parfitt DE, Kouzarides T. and Zernicka-Goetz M. (2007). Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Q, Bruce AW, Jedrusik A, Ellis PD, Andrews RM, Langford CF, Glover DM. and Zernicka-Goetz M. (2009). CARM1 is required in embryonic stem cells to maintain pluripotency and resist differentiation. Stem Cells 27:2637–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parfitt DE. and Zernicka-Goetz M. (2010). Epigenetic modification affecting expression of cell polarity and cell fate genes to regulate lineage specification in the early mouse embryo. Mol Biol Cell 21:2649–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu N, Enkemann SA, Liang P, Hersmus R, Zanazzi C, Huang J, Wu C, Chen Z, Looijenga LH, Keefe DL. and Liu L. (2010). Genome-wide gene expression profiling reveals aberrant MAPK and Wnt signaling pathways associated with early parthenogenesis. J Mol Cell Biol 2:333–344 [DOI] [PubMed] [Google Scholar]
- 89.Liu H, Kim JM. and Aoki F. (2004). Regulation of histone H3 lysine 9 methylation in oocytes and early pre-implantation embryos. Development 131:2269–2280 [DOI] [PubMed] [Google Scholar]
- 90.Wang F, Kou Z, Zhang Y. and Gao S. (2007). Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol Reprod 77:1007–1016 [DOI] [PubMed] [Google Scholar]
- 91.Fauque P, Jouannet P, Lesaffre C, Ripoche MA, Dandolo L, Vaiman D. and Jammes H. (2007). Assisted reproductive technology affects developmental kinetics, H19 imprinting control region methylation and H19 gene expression in individual mouse embryos. BMC Dev Biol 7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sato A, Otsu E, Negishi H, Utsunomiya T. and Arima T. (2007). Aberrant DNA methylation of imprinted loci in superovulated oocytes. Human Reprod 22:26–35 [DOI] [PubMed] [Google Scholar]
- 93.Fortier AL, Lopes FL, Darricarrere N, Martel J. and Trasler JM. (2008). Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Human Mol Genet 17:1653–1665 [DOI] [PubMed] [Google Scholar]
- 94.Liang XW, Cui XS, Sun SC, Jin YX, Heo YT, Namgoong S. and Kim NH. (2013). Superovulation induces defective methylation in line-1 retrotransposon elements in blastocyst. Reprod Biol Endocrinol 11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo L, Qi ST, Miao DQ, Liang XW, Li H, Ou XH, Huang X, Yang CR, Ouyang YC, et al. (2012). The roles of parathyroid hormone-like hormone during mouse preimplantation embryonic development. PLoS One 7:e40528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu FR, Liu Y, Shang MB, Yang XX, Ding B, Gao JG, Wang R. and Li WY. (2012). Differences in H3K4 trimethylation in in vivo and in vitro fertilization mouse preimplantation embryos. Genet Mol Res 11:1099–1108 [DOI] [PubMed] [Google Scholar]
- 97.Hemberger M. and Dean W. (2007). Epigenetic arbitration of cell fate decisions: tipping the bias. Dev Cell 12:176–178 [DOI] [PubMed] [Google Scholar]
- 98.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, et al. (2007). Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318 [DOI] [PubMed] [Google Scholar]
- 99.Nishida H, Suzuki T, Kondo S, Miura H, Fujimura Y. and Hayashizaki Y. (2006). Histone H3 acetylated at lysine 9 in promoter is associated with low nucleosome density in the vicinity of transcription start site in human cell. Chromosome Res 14:203–211 [DOI] [PubMed] [Google Scholar]
- 100.Roh TY, Cuddapah S. and Zhao K. (2005). Active chromatin domains are defined by acetylation islands revealed by genome-wide mapping. Genes Dev 19:542–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki T, Kondo S, Wakayama T, Cizdziel PE. and Hayashizaki Y. (2008). Genome-wide analysis of abnormal H3K9 acetylation in cloned mice. PLoS ONE 3:e1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li X, Kato Y, Tsuji Y. and Tsunoda Y. (2008). The effects of trichostatin A on mRNA expression of chromatin structure-, DNA methylation-, and development-related genes in cloned mouse blastocysts. Cloning Stem Cells 10:133–142 [DOI] [PubMed] [Google Scholar]
- 103.Sikorska M, Sandhu JK, Deb-Rinker P, Jezierski A, Leblanc J, Charlebois C, Ribecco-Lutkiewicz M, Bani-Yaghoub M. and Walker PR. (2008). Epigenetic modifications of SOX2 enhancers, SRR1 and SRR2, correlate with in vitro neural differentiation. J Neurosci Res 86:1680–1693 [DOI] [PubMed] [Google Scholar]
- 104.Zhong X. and Jin Y. (2009). Critical roles of coactivator p300 in mouse embryonic stem cell differentiation and Nanog expression. J Biol Chem 284:9168–9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, et al. (2008). Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teklenburg G, Weimar CH, Fauser BC, Macklon N, Geijsen N, Heijnen CJ, Chuva de Sousa Lopes SM. and Kuijk EW. (2012). Cell lineage specific distribution of H3K27 trimethylation accumulation in an in vitro model for human implantation. PLoS ONE 7:e32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canovas S, Cibelli JB. and Ross PJ. (2012). Jumonji domain-containing protein 3 regulates histone 3 lysine 27 methylation during bovine preimplantation development. Proc Natl Acad Sci U S A 109:2400–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Zhang M, Zhang Y, Kou Z, Han Z, Chen DY, Sun QY. and Gao S. (2010). The histone demethylase JMJD2C is stage-specifically expressed in preimplantation mouse embryos and is required for embryonic development. Biol Reprod 82:105–111 [DOI] [PubMed] [Google Scholar]
- 109.He J, Shen L, Wan M, Taranova O, Wu H. and Zhang Y. (2013). Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nat Cell Biol 15:373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu X, Johansen JV. and Helin K. (2013). Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol Cell 49:1134–1146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.