Abstract
Recent studies have revealed that mesenchymal stem cells (MSCs) have a great potential in therapeutic applications. The low efficiency of MSC recruitment and homing to sites of diseased organ tissue, however, remains a major hurdle in their application for treatment of diseases. Stress is commonly associated with various diseases. At the present time, little information is available about the effect of stress on MSC function. Here, we employed a carbon tetrachloride (CCl4)-induced mouse liver fibrosis model to investigate whether constraint stress affects the migration of MSCs to fibrotic liver. MSC homing to the fibrotic liver was significantly inhibited in mice with restraint stress. Restraint stress induced an elevation of corticosterone level in the serum. Blocking glucocorticoid signaling with either corticosterone-synthesis inhibitor metyrapone (MET) or glucocorticoid receptor antagonist RU486 attenuated restraint stress-induced inhibition of MSCs migration. The serum concentration of stromal cell-derived factor-1 (SDF-1) increased in mice treated with CCl4. Restraint stress had no influence on expression of SDF-1 and hepatocyte growth factor (HGF) in the fibrotic liver. Culture with the serum of CCl4-treated mice or SDF-1 promoted MSC migration, which was suppressed by corticosterone. Exposure of MSCs to corticosterone decreased their expression of C-X-C chemokine receptor type 4 (CXCR4) and C-X-C chemokine receptor type 7 (CXCR7). These results demonstrate that the inhibitory effect of corticosterone on MSC migration might be mediated via decreasing the expression of CXCR4 and CXCR7 in MSCs. Interventions targeting the interaction between corticosterone and its receptor improve migration and homing of MSCs in hosts receiving transplantation of these cells.
Introduction
Mesenchymal stem cells (MSCs) were first identified in the bone marrow (BM). Together with hematopoietic stem cells, they represent the two stem cell populations in BM [1]. These primitive precursors have extensive capacities of self-renewal and differentiation into multiple lineages of cells, including osteoblasts, chondrocytes, and adipocytes. MSCs can be identified by their expression of CD73, CD90, and CD105 surface markers without expressing markers for hematopoietic cells, such as CD34 and CD45 [2]. Studies have shown that MSCs can be recruited from the BM to tissue sites of injury, inflammation, and malignant lesion to exert effects on injury repair and immune regulation. Therefore, MSCs appear to possess a great potential of therapeutic application for treatment of various diseases, including degenerative abnormalities and immune disorders [3]. Transplantation of MSCs has also emerged as a promising therapy for liver fibrosis [4]. It is easy to obtain a large number of MSCs due to its convenient isolation and in vitro expansion, since they also lack significant immunogenicity, MSCs have also been used as the vehicle for genetic modification to treat cancer. At the present time, the efficiency of MSCs migration and homing in vivo is relatively low, which influences the therapeutic effect of MSC therapy. Various factors might undermine MSCs migration and homing. Thus, it is of significance to explore the potential factors, and dealing with them can help to achieve an optimized result of MSC therapy [5].
In modern society, people frequently face various challenges from both career development and family life, which may render them to endure high levels of psychosocial stress. For patients who suffer from pain and anxiety associated with diseases and the related medical treatments, stress is often unavoidable. Stress, defined as allostasis, is the adaptive process of preserving stability in response to harmful conditions [6]. Selye defines that when exposed to prolonged stressors, host will develop general adaptation syndrome, which is considered as the stress response and includes the following three phases: the alarm reaction, stage of resistance, and stage of exhaustion [7]. One of the most important neuroendocrine pathways involved in the regulation of stress response is hypothalamus-pituitary-adrenal (HPA) axis, which leads to the secretion of glucocorticoids from the adrenal glands (corticosterone in rodents and cortisol in humans) [8,9]. Stress not only leads to psychological disorders such as depression [10], but also impairs normal functions of various organ systems, including the auditory and olfactory sensory systems [6]. Furthermore, recent evidence has revealed the potentially causative link of chronic stress with a variety of diseases, including cardiovascular diseases, type II diabetes, viral hepatitis, cirrhosis, and liver carcinoma [11,12]. Chronic stress can exert substantial influences on the immune system. It has long been known that glucocorticoids are potent mediators that regulate immune effector cell function by binding to glucocorticoid receptors [13]. Currently, information about the effects of stress on MSC functional activities remains scant.
In this study, we employed a murine model of liver fibrosis induced by carbon tetrachloride (CCl4) to investigate the effects of constraint stress on MSC recruitment to the fibrotic liver. The correlation between stress and alteration of corticosterone production, and the molecular mechanism underlying corticosterone-induced inhibition of MSC migration and homing to the diseased organ were exploited.
Materials and Methods
Animals
C57BL/6 mice 6–8 weeks old were purchased from the Shanghai Experimental Animal Center at the Chinese Academy of Sciences in Shanghai, China. Animals were housed under pathogen-free conditions. All experimental procedures were performed in accordance with the institutional animal welfare guidelines of the Second Military Medical University.
Isolation and culture of mouse BM MSCs
MSCs were isolated from C57BL/6 mouse BM by flushing the tibias and femurs with normal saline. Enhanced green fluorescence protein (EGFP)-MSCs were isolated from the BM of EGFP-expressing C57BL/6 mouse. Unfractionated BM cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (low glucose) (GIBCO, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; GIBCO, Invitrogen), 1× glutamax, and 1× penicillin–streptomycin at 37°C in an atmosphere of 5% CO2. Three days later, the culture medium was replaced to remove non-adherent cells. At the fifth day of culture, attached MSCs were removed by trypsin-EDTA. The MSCs were then resuspended in fresh medium. Purification of MSCs was carried out by culturing them for three passages. In the subsequent culture of MSCs, fibroblast growth factor basic (bFGF; GIBCO, Invitrogen) was added into culture medium at the concentration of 0.5 ng/mL.
Animal models
Liver fibrosis model: Liver injury and fibrosis were induced in mice by intragastrical administration (i.g.) of 5 μL CCl4/olive oil mixture (1:5 v/v) per gram body weight twice a week for 7 weeks before sacrifice. For the groups of control and stress mice that were not subject to CCl4 administration, olive oil was intragastrically administered.
Stress model
Constraint stress was induced in mice by holding them in 50 mL conical centrifuge tubes with holes for ventilation. Unrestrained control mice were allowed free movement in their cages, but deprived of food and water accesses during the stress period for stress mice. As shown in Figure 1A, mice were subjected to a 12 h cycle of constraint in the tubes from 8:00 pm to 8:00 am for 3 days from day 1 to 3 in the seventh week of CCl4 administration. On the fourth day, 1×106 EGFP-MSCs were administered to mice via tail vein injection for observation of MSC migration to the injured liver. The stress duration was then changed to 4 h from 6 pm to 10 pm during the next 3 days. On the seventh day in the seventh week, mice were sacrificed to collect liver, serum, and BM samples.
FIG. 1.
Restraint stress suppresses MSCs migration to CCl4-induced fibrotic liver in female mice. (A) Schematic diagram for mouse model subjected to restraint stress and CCl4-induced liver fibrosis. (B) Three days after 1×106 EGFP-MSCs were injected into the female mice via tail vein, mice were sacrificed and frozen section of the liver tissue was made, which were then observed under fluorescence microscope. (C) The quantification of fluorescent MSCs in the liver section of mice from indicated groups. **P<0.01 versus the group of CCl4; (D) The concentration of corticosterone in the serum of female mice from indicated groups was measured by ELISA. **P<0.01 versus the group of control and CCl4 respectively (×100 magnification; each group has six mice). CCl4, carbon tetrachloride; EGFP, enhanced green fluorescence protein; ELISA, enzyme-linked immunosorbent assay; MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/scd
In the first set of experiments as shown in Figure 1, mice were divided into four groups: (i) control, (ii) stress, (iii) CCl4, and (iv) stress+CCl4. Each group contained six mice.
To inhibit the stress-induced increase in blood corticosterone level, metyrapone (MET; Sigma-Aldrich, Saint Louis, MO) dissolved in Tween 80 (5% in saline) was administered to mice at 100 mg/kg via intraperitoneal injection 10 min before initiation of the constraint stress. Glucocorticoid receptor antagonist RU486 (Mifipristone; Sigma-Aldrich) dissolved in 0.9% saline containing 5% DMSO (Sigma-Aldrich) and 3 drops/mL of Tween 20 (Merck Schuchardt, Hohenbrunn, Germany) was administered to mice at 20 mg/kg via intraperitoneal injection at 9 am on every scheduled stress day.
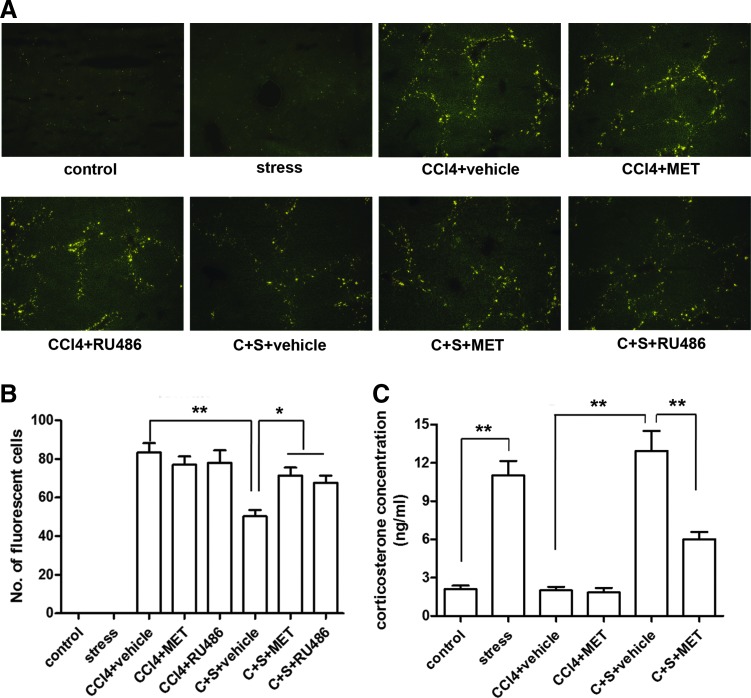
In the second set of experiments as illustrated in Figure 2, mice were divided into eight groups: (i) control, (ii) stress, (iii) CCl4+vehicle, (iv) CCl4+MET, (v) CCl4+RU486, (vi) CCl4 (C)+stress (S)+vehicle, (vii) C+S+MET, and (viii) C+S+RU486. About 0.9% saline containing 5% DMSO and 3 drops/mL of Tween 20, the solution of RU486 was taken as vehicle, which was also administered via intraperitoneal injection.
FIG. 2.
Interfering corticosterone signaling with corticosterone-synthesis inhibitor MET or CR antagonist RU486 attenuates the suppression of MSCs migration by restraint stress. (A) A representative image of frozen liver section of eight groups of mice with indicated treatment under fluorescence microscope. The solution of RU486 was taken as vehicle control. (B) The quantification of fluorescent MSCs in the liver section of mice from the above eight groups. (C) The concentration of corticosterone in the serum of mice from eight groups was measured by ELISA. (*P<0.05, **P<0.01 between the indicated groups; ×100 magnification). C, CCl4; CR, corticosterone receptor; MET, metyrapone; S, stress. Color images available online at www.liebertpub.com/scd
Enzyme-linked immunosorbent assay
Serum levels of corticosterone and stromal cell-derived factor-1 (SDF-1) were determined using Corticosterone EIA kits (Enzo Life Science, Lausen, Switzerland) and SDF-1 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN), respectively, using procedures provided by the manufacturers. All serum samples were 1:10 diluted before assessment.
Real-time reverse transcription-polymerase chain reaction
Total mRNA was extracted from cells using TRIzol reagent (Invitrogen). Message RNA expression by cells was determined by real-time reverse transcription-polymerase chain reaction (qRT-PCR) using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in an Mx4000 system (Stratagene, La Jolla, CA). Expression of each specific gene mRNA by cells was normalized with the endogenous GADPH mRNA. Thermal cycling conditions included an initial hold period at 95°C for 4 min, which was followed by a three-step PCR program of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s for a total of 40 cycles. Primers used in this study included SDF-1: 5′-ACAGACAAG-TGTGCATTGACCCGA-3′ (forward) and 5′-ATCGGCAGGAAGCGGGGAACT-3′ (reverse); hepatocyte growth factor (HGF): 5′-GGGCTGAAAAGATTGGATCA-3′ and 5′-TCGAACAAAAATACCAGGACG-3′; Nr3c1: 5′-ATGGGAGAGACCGAACAAA-3′ and 5′-TCCAGAAGCCGAAAGTCTGT-3′; C-X-C chemokine receptor type 4 (CXCR4): 5′-GGCTGTAGAGCGAGTGTTGC-3′ and 5′-GTAGAGGTTGACAGTGTAGAT-3′; C-X-C chemokine receptor type 7 (CXCR7): 5′-TCACCTACTTCACCGGCACC-3′ and 5′-ACATGGCTCTAGCGAGCAGG-3′; GAPDH: 5′-GGTGGTCTCCTCTGACTTCAACA-3′ and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on paraffin-embedded 4-μm sections of mouse liver tissue. The section was first deparaffinized and rehydrated, and then incubated in 3% hydrogen peroxide in methanol for 20 min to inactivate the endogenous peroxidase. Antigen retrieval was achieved by boiling the sections in buffer solution with pressure cooker for 2 min. Then, to block nonspecific binding, the sections were incubated with 1% bovine serum albumin for 30 min at room temperature. After incubation with rabbit polyclonal antibody against SDF-1/HGF (Boster, Wuhan, China) overnight at 4°C, the sections were incubated with horseradish peroxidase (HRP)-labeled anti-rabbit secondary antibody for 30 min at 37°C. A buffered diaminobenzidine (DAB) solution was used for visualization. At last, the sections were counterstained with hematoxylin and mounted with neutral gum. The results were expressed as the percentage of staining-positive cells, which were scored on a scale of 0 to 4 (0: no staining; 1:≤10%; 2: 11% to 30%; 3: 31% to 50%; 4:≥50%).
Transwell migration assay
MSCs were cultured in DMEM-low glucose medium containing 10% mouse serum, 10−7 M corticosterone (Sigma-Aldrich), 10−7 M RU486 (Sigma-Aldrich) with or without pretreatment with 100 ng/mL SDF-1 (Peprotech, Rocky Hill, NJ). Cells were cultured for 48 h and then were collected by trypsin treatment. Collected MSCs (1×105 in 200 μL culture medium) were added to the upper chamber. The lower chamber was added with 600 μL of culture medium. The cells were cultured at 37°C in an atmosphere of 5% CO2. After cells adhered to the membrane of transwell insert with 8-mm pores (BD Falcon, Franklin Lakes, NJ), the medium in the upper chamber was replaced by DMEM-low glucose medium without FBS, and the medium in the lower chamber was replaced by DMEM-low glucose medium containing 10% FBS. The cells were cultured for 48 h. Cells in the upper chamber were then removed with a cotton swab. The cells that migrated through the membrane and adhered to the lower surface of the membrane were fixed with methanol for 10 min. The fixed cells were stained with crystal violet solution (0.1%). Quantification of migrated cells was conducted by counting them in five randomized fields under a microscope at ×200 magnification.
Flow cytometry analysis
CXCR4 and CXCR7 expressions on MSCs were determined using direct immunofluorescent staining analyzed on a flow cytometry (Becton Dickinson, San Jose, CA). MSCs with or without pretreatment with 100 nM of corticosterone were trypsinized into single cell suspension. After incubation with 10% goat serum at 4°C for 10 min to block nonspecific binding, cells were washed with phosphate-buffered saline (PBS) twice. P-phycoerythrin (PE)-conjugated antibodies against CXCR4 (eBioscience, San Diego, CA) and CXCR7 (Biolegend, San Diego, CA) were added into 100 μL of cell suspension (1×106 cells/mL) and incubated for 20 min at 4°C. Then, the stained cells were washed twice with PBS, which was followed by filtering through nylon mesh to obtain single cell suspension. Finally, the stained cells were subjected to flow cytometry analysis.
Statistical analysis
All of in vitro experiments were repeated at least three times. The data were expressed as mean±standard deviation. Statistical analysis of the data was performed using GraphPad Prism 4 software. Student's t-test was used for comparison of difference between groups. Statistical significance was assumed at P<0.05.
Results
Restraint stress suppressed MSCs migration to CCl4-induced fibrotic liver in mice
To investigate the impact of constraint stress on MSCs migration in vivo, we employed a female mouse model of combined CCl4-induced hepatic fibrosis and constraint stress. To examine MSC migration in vivo, MSCs derived from mice expressing EGFP were administered to wild-type mice via tail vein injection (1×106 MSCs/mouse). The schematic diagram was shown in Figure 1A. By observing the frozen sections of the liver with a fluorescence microscope, we did not find EGFP-MSCs in the livers of mice in the control and stress group. In mice treated with CCl4, a large amount of EGFP-MSCs could be detected in the liver. This increase in the number of fluorescent cells was reduced ∼50% in mice subjected to constraint stress (P<0.01) (Fig. 1B, C). Measurement of hydroxyproline content in the liver with Sirius red staining showed that stress did not affect the development of liver fibrosis (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Increased production of corticosterone is an important response to stress. In this study, the concentration of corticosterone in the serum was significantly increased in stress-treated mice (P<0.01) (Fig. 1D).
To determine whether sex affects mouse response to stress, we carried out the same experiment in male mice. As shown in Supplementary Figure S2A, compared with male mice in CCl4 group, mice in CCl4 plus stress group showed a significant reduction of EGFP-MSCs in frozen liver sections (P<0.05). The extent of decrease in the number of migrated EGFP-MSCs in the liver in male mice was less than that in female mice. In addition, the baseline serum corticosterone level was lower in male mice than that in female mice. The serum corticosterone concentration of the female mice with stress treatment was more than five times as much as that of the control mice, while for the male mice the fold change was four times as indicated in Figure 2C and Supplementary Figure S2C, which suggests that constraint stress-induced elevation of corticosterone level was more in female mice than that in male mice. These data demonstrate that constraint stress significantly inhibits the migration and homing of MSCs to the injured liver. Alteration of corticosterone production likely plays a role in this process.
Corticosterone mediated the inhibitory effect of constraint stress on MSCs migration in vivo
To further verify whether corticosterone plays a role in the inhibition of MSC migration caused by restraint stress, we examined whether blocking glucocorticoid signaling with either corticosterone-synthesis inhibitor MET or glucocorticoid receptor antagonist RU486 would attenuate the suppression of MSCs migration in mice with restraint stress. The results showed that MET and RU486 administration had no effect on MSCs migration into the liver in mice without restraint stress. However, both MET and RU489 could significantly attenuate stress-induced inhibition of MSC migration (P<0.05) (Fig. 2A, B). Administration of MET inhibited corticosterone production during restraint stress treatment (P<0.01) (Fig. 2C). These results suggest that corticosterone mediates the stress-associated inhibition of MSC migration.
Restraint stress did not affect chemokine production in fibrotic liver
It is well known that MSCs have a tendency to home to injured tissues with inflammation. A potential signal for MSC migration and homing is locally produced chemokines in damaged tissues [3,14]. Therefore, we examined SDF-1 and HGF (two key chemokines reported to initiate MSC migration and homing) [15,16] levels in fibrotic liver tissues. qRT-PCR results demonstrated that administration of CCl4 significantly increased SDF-1 and HGF expression in the injured liver (P<0.01). However, this increase in SDF-1 and HGF expression was not affected by constraint stress or MET administration (Fig. 3A, C). The consistent results were obtained via IHC (Fig. 3B, D). These data suggest that corticosterone has no effect on SDF-1 and HGF expression in the fibrotic liver of mice treated with CCl4.
FIG. 3.
Restraint stress does not affect SDF-1 and HGF production in fibrotic liver. (A) qRT-PCR was performed to detect the mRNA expression level of SDF-1 in the mice livers from indicated groups. (B) IHC was performed to determine the expression of SDF-1 in the mice livers from indicated groups. (C) qRT-PCR was performed to detect the mRNA expression level of HGF in the mice livers from indicated groups. (D) IHC was performed to determine the expression of HGF in the mice livers from indicated groups (*P<0.05, **P<0.01 between the indicated groups and the control group; ×200 magnification). HGF, hepatocyte growth factor; IHC, immunohistochemistry; qRT-PCR, real-time reverse transcription–polymerase chain reaction; SDF-1, stromal cell-derived factor-1. Color images available online at www.liebertpub.com/scd
Corticosterone inhibited MSCs migration in vitro
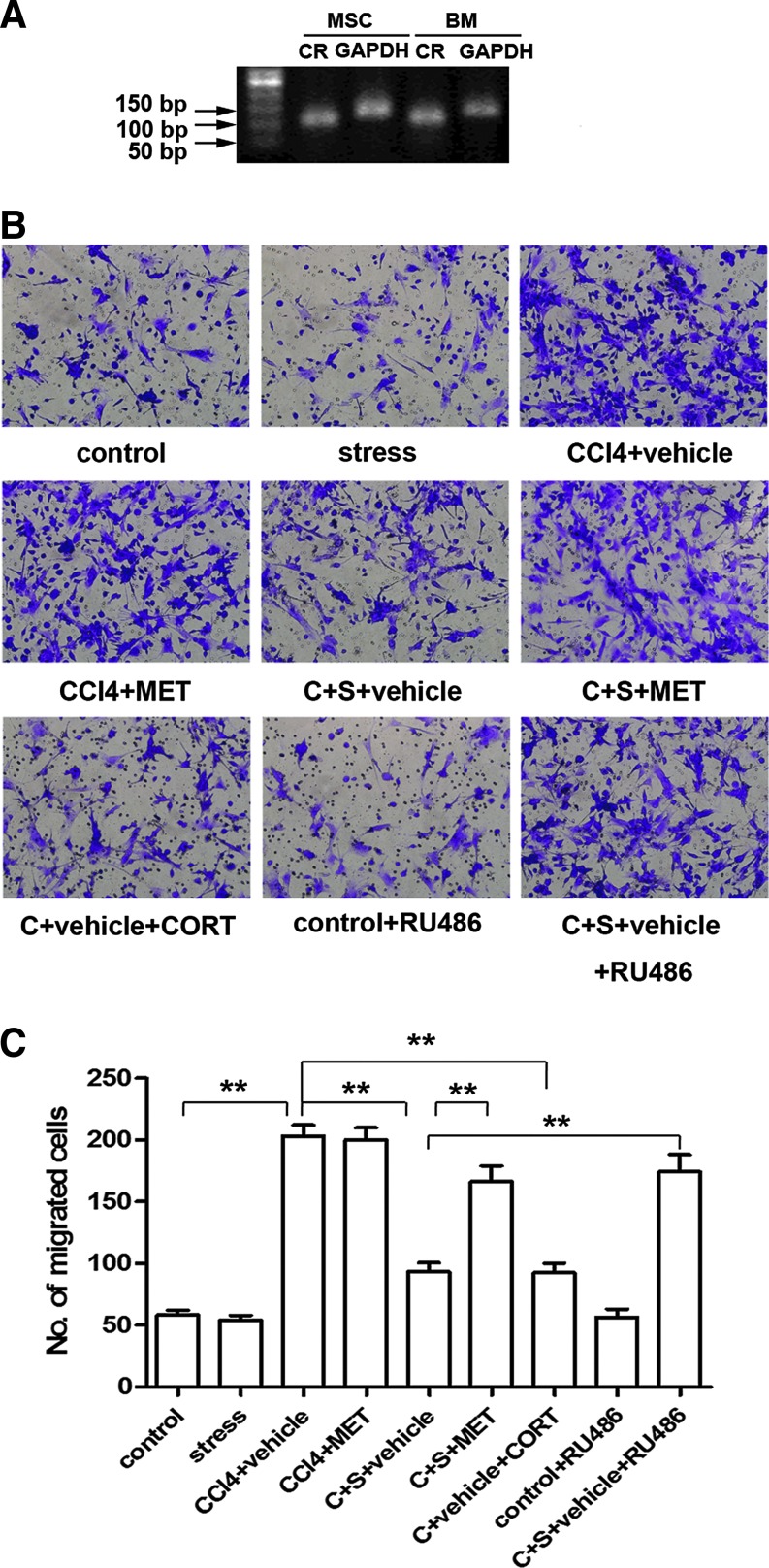
Cultured mouse MSCs and BM cells expressed corticosterone receptor (CR) (Fig. 4A), suggesting that these cells might respond to corticosterone. As shown in Figure 4B and C, there was no difference in migratory activity of MSCs cultured in medium containing serum either from control mice or mice with constraint stress. Culture of cells in the medium containing serum from mice treated with CCl4 showed a significantly enhancement of MSCs migration (P<0.01). This enhancement of MSC migratory activity was attenuated when cells were cultured in the medium containing serum from mice received CCl4 plus constraint stress (P<0.01). The attenuation of MSC migratory activity was not seen in the culture system containing serum from mice of C+S+MET group. These results support the role of corticosterone in mediating the inhibition of MSCs migration.
FIG. 4.
Corticosterone inhibits MSCs migration in vitro. (A) RT-PCR was performed to detect the expression of CR mRNA in cultured MSCs and BM derived from mice, and the amplified products were visualized in the agarose gel incorporated with 0.5 g/mL of ethidium bromide. (B) MSCs were cultured in DMEM-low glucose with 10% mice serum of indicated groups with or without 1×10−7 M corticosterone or 1×10−7 M RU486 for 48 h, and then they were subjected to transwell migration assay. Representative photographs of the migrated MSCs are shown. (C) Quantification of three independent experiments of transwell migration assay is shown. This is the significance of the quantification of violet cells. (**P<0.01 between the indicated groups; ×200 magnification). BM, bone marrow; CORT, corticosterone; DMEM, Dulbecco's modified Eagle's medium. Color images available online at www.liebertpub.com/scd
After addition of corticosterone (100 nM) into the culture medium containing serum from mice of the CCl4+vehicle group the number of migratory cells was markedly reduced (P<0.01). Blocking CR in MSCs with RU486 (100 nM) attenuated inhibition of MSC migration induced by C+S+vehicle serum (P<0.01). Consistently, blocking CR by siRNA also abolished the inhibitory effects of corticosterone on MSC migration as indicated in Supplementary Figure S3. These data demonstrate that corticosterone exerts a negative effect on MSC migration in response to the inflammatory stimulation.
Corticosterone inhibited SDF-1-enhanced MSCs migration and suppressed CXCR4/7 expression by MSCs
SDF-1 has been reported to be a crucial chemokine regulating MSCs migration. We examined the concentration of SDF-1 in mouse sera with ELISA. Consistent with our qRT-PCR and IHC results shown in Figure 3, ELISA determination revealed that the concentration of SDF-1 was markedly increased in serum samples of CCl4-treated mice in comparison with that in controls (P<0.05) (Fig. 5A). MSCs pretreated with 100 ng/mL SDF-1 showed substantially enhanced migratory capacity as indicated by transwell migration assay (P<0.05). This SDF-1-induced enhancement of MSC migration was significantly attenuated by corticosterone (P<0.05) (Fig. 5B, C). CXCR4 and CXCR7 are receptors for SDF-1 in MSCs. Cultured MSCs decreased their gene expression of both receptors following exposure to corticosterone (Fig. 5D). Flow cytometric analysis confirmed that corticosterone downregulated MSC expression of CXCR4 and CXCR7 receptors (Fig. 5E, F). In addition, we observed that expression of CXCR4 and CXCR7 by BM cells of mice with constraint stress was reduced ∼50% from the control level. CCl4 administration significantly enhanced their expression (P<0.05), which was significantly attenuated in mice with stress (P<0.05). In mice of C+S+MET group, the expression of these two receptors by BM cells was restored (Fig. 5G, H). These data suggest that the inhibitory effect of corticosterone on MSC migration might be mediated via decreasing the expression of SDF-1 receptors in MSCs.
FIG. 5.
Corticosterone inhibits SDF-1-enhanced MSCs migration and suppresses CXCR4/7 expression on MSCs. (A) The concentration of SDF-1 in the serum of mice from indicated groups was measured by ELISA. (B) MSCs were pretreated with 100 ng/mL SDF-1 with or without 1×10−7 M corticosterone for 48 h, and then they were subjected to transwell migration assay. Representative photographs of the migrated MSCs are shown. (C) Quantification of three independent experiments of transwell migration assay is shown. (D) qRT-PCR was performed to detect the mRNA expression levels of CXCR4 and CXCR7 in MSCs with or without corticosterone treatment for 48 h. (E, F) The content of CXCR4+ or CXCR7+ cells in MSCs with or without 1×10−7 M corticosterone treatment for 48 h was detected via flow cytometry analysis. (G, H) qRT-PCR was performed to detect the mRNA expression level of CXCR4 and CXCR7 in BM of mice from indicated groups. (*P<0.05, **P<0.01; ×200 magnification). CXCR4, C-X-C chemokine receptor type 4; CXCR7, C-X-C chemokine receptor type 7. Color images available online at www.liebertpub.com/scd
Discussion
Recent studies have revealed the promising potential of MSC-based therapy in the treatment of diverse diseases. Factors influencing MSC function following transplantation of these precursor cells have drawn a wide attention among investigators studying the MSC therapy [17–21]. In addition to understanding the regulation of functional activities involving MSC self-renewal/proliferation, differentiation, immunosuppression, senescence, and apoptosis, knowledge about mechanisms governing migration and homing of MSCs to target organ tissue sites is crucial for achieving an optimized result of MSC therapy. Rombouts and Ploemacher have reported that primary MSCs derived from mouse BM have a stronger capability of migration toward BM and spleen in vivo than those generated from the cultural expansion [22]. Migration of MSCs can be inhibited by culturing them in high confluence. This inhibition is mediated through the increase in production of tissue inhibitor of metalloproteinase (TIMP-3), a matrix metalloproteinase (MMP) inhibitor [23]. Culture conditions have also been shown to influence the ability of MSC migration. Annabi et al. have reported that hypoxic culture condition significantly enhances MSC migration in vitro [24]. Freyman et al. have reported that different methods of MSC delivery, such as intravenous (IV), intracoronary (IC), or endocardial (EC) delivery, result in different MSC engraftment rates in a porcine myocardial infarction model [25]. To the best of our knowledge, our current study is the first to investigate whether stress in the recipient of MSC therapy influences MSC migration and homing, thus affecting the therapeutic outcomes. We found that constraint stress significantly inhibited the engraftment of EGFP-MSCs in the injured liver in our established mouse liver fibrosis model of CCl4 administration. The level of serum corticosterone was increased in mice after constraint stress. Interfering glucocorticoid signaling with either corticosterone-synthesis inhibitor MET or glucocorticoid receptor antagonist RU486 would attenuate the suppression of MSC migration caused by constraint stress. These observations identify that corticosterone plays a crucial role in the suppressive effect of restraint stress on MSC migration.
Female and male subjects have differential vulnerability to the stress challenge both physically and psychologically [26,27]. It is well known that glucocorticoid plays an important role in the process of host response to stress. One of the reasons for the sex-related differential vulnerability to stress injury might be the difference of glucocorticoid metabolism in males versus females. Studies on rodent models have shown that higher baseline (unstressed) plasma corticosterone levels are generally observed in females. In response to stress stimuli, females usually show a more dramatic increase in corticosterone level in the circulation than do males. In addition, the stress-induced increase in corticosterone production is more persistent in females [28,29]. To define the effect of sex on stress-induced inhibition of MSC migration, we examined MSC homing to CCl4-induced fibrotic liver of both male and female mice. Our results showed that the baseline concentration of corticosterone in the serum of female mice was about two times as that in male mice. In response to constraint stress, female mice demonstrated a greater extent of increase in serum corticosterone level than that in male mice. In agreement with this difference of corticosterone metabolism between male and female animals, the number of EGFP-MSCs in fibrotic liver was decreased more dramatically in females than in males following treatment with constraint stress. These data suggest that corticosterone may be involved in mediating the negative effect of stress on MSC migration to the injured liver.
Interaction of chemokines with their corresponding receptors on the cell surface guides cell migration. The SDF-1/CXCR4 system has been reported to play a key role in mediating MSC migration [30]. Since the expression of CXCR4 was relatively low on MSCs, CXCR4 has been transduced into MSCs to enhance their migration and engraftment to target organs to improve the therapeutic efficacy of using these cells in vivo [31,32]. This study examined whether restraint stress could alter the level of SDF-1 expression in injured liver tissue. The results showed that there was no difference in the expression and production of SDF-1 in the liver tissue between C+vehicle group and C+S+vehicle group, indicating that restraint stress had no effect in SDF-1 production. These observations led us to shift our exploration toward the possible effect of restraint stress on the expression of SDF-1 receptors by MSCs. Our in vitro migration assay using serum from mice subjected to different treatments, synthetic corticosterone, and RU486 provided strong evidence that corticosterone could inhibit the enhancement of MSC migration induced by SDF-1 in the serum of mice treated with CCl4. qRT-PCR determination in our experiment indicated that stress-induced increase in production of corticosterone inhibited CXCR4/7 expression on BM cells in vivo. Observations in cultured MSCs confirmed that corticosterone could suppress the expression of CXCR4/7 on these precursor cells. Constraint stress might exert profound influence physically via different neuroendocrine pathways including adrenergic system and HPA axis [33,34]. However, there is also evidence indicating the crosstalk between the two pathways that adrenergic receptors can play a role in HPA activation upon stress stimuli [35,36]. Although the role of adrenergic receptors in MSCs migration was not detected in our study, it is possible that adrenergic activation might also involve in this process by modulating HPA axis and enhancing corticosterone production.
Although our study innovatively found that restraint stress negatively regulates MSCs migration, modulation of the migration of immune cells by constraint stress has long been reported, which serves as one of the important mechanisms underlying stress-induced immunosuppressive function. Mizobe et al. employed a mouse model of local intraperitoneal inflammation by injection of proteose peptone. They found that acute restraint stress (24 h restraint) could significantly suppress the migration of macrophages and granulocytes into the inflammatory site, which was mediated by stress-induced elevation of corticosterone production. Further evidence demonstrated that reduced migration of these immune cells was attributable to the downregulated expression of chemotactic factor MCP-1/JE caused by corticosterone [37]. Flint et al. found that stress hormones including corticosterone could inhibit the migration of T cells. Proteomic profiling of T cells isolated from control and stress mice and in vitro cultured T cells with or without stress hormones treatment revealed that stress-induced hormones downregulated the expression of crucial cytoskeletal proteins. This study provided evidence demonstrating that stress hormones especially corticosterone induced by acute stress inhibited T-cell migration via regulating actin cytoskeleton [38]. Above all, although having differences in the aspects of cell type, experimental model and underlying mechanism, consistently, our study also demonstrated the inhibitory effect of stress on cell migration caused by stress-induced hormones. Stress could exert a great variety of responses in organism, among which, influence on cell migration might be an important factor that we should pay attention to. Previous studies have repeatedly reported that glucocorticoids effect MSC differentiation. Low dose of dexamethasone (Dex), one steroid drug of the glucocorticoid class, enhances MSC proliferation, while high dose of Dex inhibits the proliferation of MSCs. Furthermore, high dose of Dex has been shown to exacerbate serum deprivation-induced apoptosis of MSCs, to impair MSC immunosuppressive capacity, and to inhibit VEGF and HGF expression by cells [21]. Blockage of endogenous glucocorticoids using RU486 significantly increases MSC proliferation and enhances the expression of FGF-2 (a stemness gene) and Sox-11 (a gene negatively associated with MSCs senescence) [39]. Consistent observations have been obtained by using small interfering RNA targeting glucocorticoid receptor gene expression in MSCs [40]. All these investigations share the same purpose of optimizing the culture system of MSCs for improvement of their clinical treatment applications. This study demonstrates that restraint stress increases corticosterone production in mice, which downregulates MSC expression of CXCR4/7. This reduction of MSC chemokine receptor expression in turn impairs the migratory activity of MSCs in response to appropriate chemokine stimuli. Correction of corticosterone-induced impairment of MSC migration and homing to sites of the target organ tissue in host with stress will contribute to improving the therapeutic efficiency of these precursor cells.
Conclusions
Our study demonstrates that corticosterone induced by restraint stress significantly inhibits MSC migration via downregulating CXCR4/7 expression. Interventions targeting the interaction between corticosterone and its receptor improve migration and homing of MSCs in hosts receiving transplantation of these cells.
Supplementary Material
Acknowledgments
Key Basic Research Project of China (grant no. 2012CBA01303, 2011CB966200); Key Project of National Natural Science Foundation of China (grant no. 81030041); National Natural Science Foundation of China (grant no. 31171321, 81101622, 81372330, 81101830); Special Funds for National Key Sci-Tech Special Project of China (grant no. 2012ZX10002-016, 2012ZX10002011-011); Shanghai Science and Technology Committee (grant no. 12ZR1454200, 12ZR1439800, 12431900802); Shanghai Municipal Health Bureau (grant no. 2011044) and Science Fund for Creative Research Groups, NSFC, China (grant no. 81221061); Youth Initial Funds of Second Military Medical University (grant no. 2013QN08).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Keating A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709–716 [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, Sun K, Jiang GC, Zhao X, et al. (2011). Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem 286:25007–25015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y. and Shi Y. (2012). Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med 1:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasir GA, Mohsin S, Khan M, Shams S, Ali G, Khan SN. and Riazuddin S. (2013). Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med 11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karp JM. and Leng Teo GS. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216 [DOI] [PubMed] [Google Scholar]
- 6.Dagnino-Subiabre A. (2013). Effects of chronic stress on the auditory system and fear learning: an evolutionary approach. Rev Neurosci 24:227–237 [DOI] [PubMed] [Google Scholar]
- 7.Selye H. (1976). Forty years of stress research: principal remaining problems and misconceptions. Can Med Assoc J 115:53–56 [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS. (2012). Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A 109 (Suppl 2):17180–17185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockrem JF. (2013). Individual variation in glucocorticoid stress responses in animals. Gen Comp Endocrinol 181:45–58 [DOI] [PubMed] [Google Scholar]
- 10.van Praag HM. (2005). Can stress cause depression?. World J Biol Psychiatry 6 (Suppl 2):5–22 [DOI] [PubMed] [Google Scholar]
- 11.Vere CC, Streba CT, Streba LM, Ionescu AG. and Sima F. (2009). Psychosocial stress and liver disease status. World J Gastroenterol 15:2980–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser R. and Kiecolt-Glaser JK. (2005). Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251 [DOI] [PubMed] [Google Scholar]
- 13.Webster JI, Tonelli L. and Sternberg EM. (2002). Neuroendocrine regulation of immunity. Annu Rev Immunol 20:125–163 [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain G, Fox J, Ashton B. and Middleton J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Ishikawa H, Kawai K, Tabata Y. and Suzuki S. (2013). Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials 34:9393–9400 [DOI] [PubMed] [Google Scholar]
- 16.van de Kamp J, Jahnen-Dechent W, Rath B, Knuechel R. and Neuss S. (2013). Hepatocyte growth factor-loaded biomaterials for mesenchymal stem cell recruitment. Stem Cells Int 2013:892065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque N, Rahman MT, Abu Kasim NH. and Alabsi AM. (2013). Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal 2013:632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das R, Jahr H, van Osch GJ. and Farrell E. (2010). The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 16:159–168 [DOI] [PubMed] [Google Scholar]
- 19.Razzouk S. and Schoor R. (2012). Mesenchymal stem cells and their challenges for bone regeneration and osseointegration. J Periodontol 83:547–550 [DOI] [PubMed] [Google Scholar]
- 20.Julavijitphong S, Wichitwiengrat S, Tirawanchai N, Ruangvutilert P, Vantanasiri C. and Phermthai T. (2014). A xeno-free culture method that enhances Wharton's jelly mesenchymal stromal cell culture efficiency over traditional animal serum-supplemented cultures. Cytotherapy 16:683–691 [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Pang B, Li Y, Zhu D, Pang T. and Liu Y. (2012). Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy 14:423–430 [DOI] [PubMed] [Google Scholar]
- 22.Rombouts WJ. and Ploemacher RE. (2003). Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17:160–170 [DOI] [PubMed] [Google Scholar]
- 23.De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M. and Van Riet I. (2007). Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 92:440–449 [DOI] [PubMed] [Google Scholar]
- 24.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J. and Beliveau R. (2003). Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 21:337–347 [DOI] [PubMed] [Google Scholar]
- 25.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M. and Wilensky RL. (2006). A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27:1114–1122 [DOI] [PubMed] [Google Scholar]
- 26.Bangasser DA. and Valentino RJ. (2012). Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol 32:709–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Guasti A, Fiedler JL, Herrera L. and Handa RJ. (2012). Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res 44:607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen H. and Yehuda R. (2011). Gender differences in animal models of posttraumatic stress disorder. Dis Markers 30:141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N. and Shibasaki T. (2009). Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 34:226–237 [DOI] [PubMed] [Google Scholar]
- 30.Yuan L, Sakamoto N, Song G. and Sato M. (2013). Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways. Stem Cells Dev 22:2384–2393 [DOI] [PubMed] [Google Scholar]
- 31.Marquez-Curtis LA, Gul-Uludag H, Xu P, Chen J. and Janowska-Wieczorek A. (2013). CXCR4 transfection of cord blood mesenchymal stromal cells with the use of cationic liposome enhances their migration toward stromal cell-derived factor-1. Cytotherapy 15:840–849 [DOI] [PubMed] [Google Scholar]
- 32.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG. and Kong D. (2008). Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 16:571–579 [DOI] [PubMed] [Google Scholar]
- 33.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, Spannuth WA, Deavers MT, et al. (2010). Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120:1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, Zhao Y, Wang X, Levine AJ. and Hu W. (2012). Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A 109:7013–7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvage D. (2012). Roles of the locus coeruleus and adrenergic receptors in brain-mediated hypothalamic-pituitary-adrenal axis responses to intracerebroventricular alcohol. Alcohol Clin Exp Res 36:1084–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin RJ, Hill MN. and Gorzalka BB. (2009). Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol 624:71–76 [DOI] [PubMed] [Google Scholar]
- 37.Mizobe K, Kishihara K, Ezz-Din El-Naggar R, Madkour GA, Kubo C. and Nomoto K. (1997). Restraint stress-induced elevation of endogenous glucocorticoid suppresses migration of granulocytes and macrophages to an inflammatory locus. J Neuroimmunol 73:81–89 [DOI] [PubMed] [Google Scholar]
- 38.Flint MS, Budiu RA, Teng PN, Sun M, Stolz DB, Lang M, Hood BL, Vlad AM. and Conrads TP. (2011). Restraint stress and stress hormones significantly impact T lymphocyte migration and function through specific alterations of the actin cytoskeleton. Brain Behav Immun 25:1187–1196 [DOI] [PubMed] [Google Scholar]
- 39.Yu Y, Wei N, Stanford C, Schmidt T. and Hong L. (2012). In vitro effects of RU486 on proliferation and differentiation capabilities of human bone marrow mesenchymal stromal cells. Steroids 77:132–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong L, Wei N, Joshi V, Yu Y, Kim N, Krishnamachari Y, Zhang Q. and Salem AK. (2012). Effects of glucocorticoid receptor small interfering RNA delivered using poly lactic-co-glycolic acid microparticles on proliferation and differentiation capabilities of human mesenchymal stromal cells. Tissue Eng Part A 18:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.