Abstract
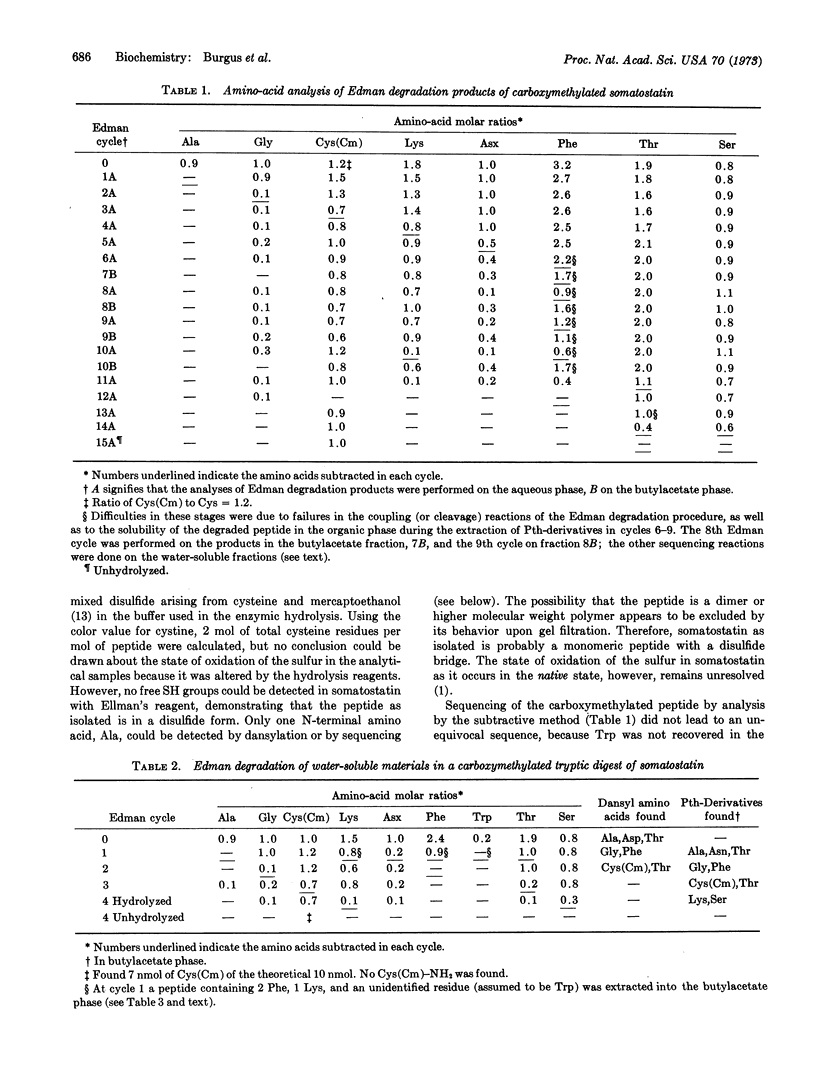
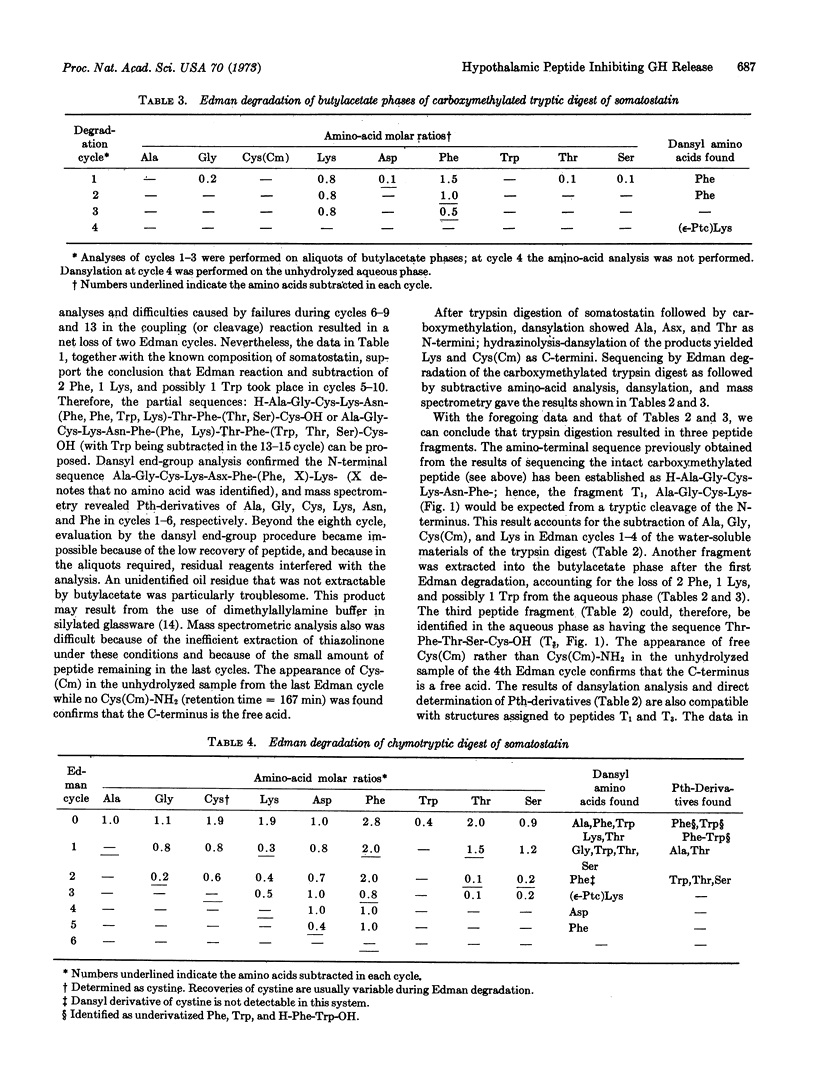
Somatostatin, a peptide isolated from ovine hypothalamic tissue that inhibits the release of radioimmunoassayable growth hormone in vitro from rat or human pituitary cells or in vivo in rats, has the primary structure [Formula: see text]. The structure was established by submitting the carboxymethylated peptide, the carboxymethylated tryptic digest, and the chymotryptic digest of the peptide to Edman degradation. Degradation products were analyzed by amino-acid analysis, as well as in some cases by determination of N-termini by dansylation or by determination of phenylthiohydantoins by mass spectrometry.
Keywords: somatotropin release, Edman degradation, dansylation, mass spectrometry
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E. Studies on the reduction and re-formation of protein disulfide bonds. J Biol Chem. 1961 May;236:1361–1363. [PubMed] [Google Scholar]
- Amoss M., Burgus R., Blackwell R., Vale W., Fellows R., Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971 Jul 2;44(1):205–210. doi: 10.1016/s0006-291x(71)80179-1. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Burgus R., Butcher M., Amoss M., Ling N., Monahan M., Rivier J., Fellows R., Blackwell R., Vale W., Guillemin R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fairwell T., Lovins R. E. Quantitative protein sequencing using mass spectrometry: mass spectral analysis of 2-anilino-5-thiazolinone derivatives of amino acids without prior conversion to the phenyl thiohydantoins. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1280–1289. doi: 10.1016/s0006-291x(71)80011-6. [DOI] [PubMed] [Google Scholar]
- HILL R. L., SCHMIDT W. R. The complete enzymic hydrolysis of proteins. J Biol Chem. 1962 Feb;237:389–396. [PubMed] [Google Scholar]
- Inglis A. S., Liu T. Y. The stability of cysteine and cystine during acid hydrolysis of proteins and peptides. J Biol Chem. 1970 Jan 10;245(1):112–116. [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Ling N., Burgus R., Rivier J., Vale W., Brazeau P. The use of mass spectrometry in deducing the sequence of somatostatin--a hypothalamic polypeptide that inhibits the secretion of growth hormone. Biochem Biophys Res Commun. 1973 Jan 4;50(1):127–133. doi: 10.1016/0006-291x(73)91073-5. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- YAMASHIRO D. PARTITION CHROMATOGRAPHY OF OXYTOCIN ON 'SEPHADEX'. Nature. 1964 Jan 4;201:76–77. doi: 10.1038/201076a0. [DOI] [PubMed] [Google Scholar]



