Abstract
Coexistence often involves niche differentiation either as the result of environmental divergence, or in response to competition. Disentangling the causes of such divergence requires that environmental variation across space is taken into account, which is rarely done in empirical studies. We address the role of environmental variation versus competition in coexistence between two rodent species: Rhabdomys bechuanae (bechuanae) and Rhabdomys dilectus dilectus (dilectus) comparing their habitat preference and home range (HR) size in areas with similar climates, where their distributions abut (allopatry) or overlap (sympatry). Using Outlying Mean Index analyses, we test whether habitat characteristics of the species deviate significantly from a random sample of available habitats. In allopatry, results suggest habitat selection: dilectus preferring grasslands with little bare soil while bechuanae occurring in open shrublands. In sympatry, shrubland type habitats dominate and differences are less marked, yet dilectus selects habitats with more cover than bechuanae. Interestingly, bechuanae shows larger HRs than dilectus, and both species display larger HRs in sympatry. Further, HR overlaps between species are lower than expected. We discuss our results in light of data on the phylogeography of the genus and propose that evolution in allopatry resulted in adaptation leading to different habitat preferences, even at their distribution margins, a divergence expected to facilitate coexistence. However, since sympatry occurs in sites where environmental characteristics do not allow complete species separation, competition may explain reduced inter-species overlap and character displacement in HR size. This study reveals that both environmental variation and competition may shape species coexistence.
Introduction
The concept of character displacement is the subject of regular debate in ecology [1–4]. Ecological character displacement is defined as a process where populations respond to competition by modifying their resource-use traits through phenotypic plasticity or genetic adaptation [5]. This response to competition plays an important role in generating and maintaining biodiversity as well as shaping the mechanisms of coexistence [1,6,7], particularly between species sharing similar niches [8]. However, solid empirical evidence demonstrating the process of character displacement is rare (shown in only 9 out of 144 studies reviewed in [4]), partly due to confusion between character variation and character displacement [4,9]. Character variation due to ecological heterogeneity could occur when species adapt to distinct environments in allopatry, and may not be interpreted as character displacement when the same species are found to be divergent in sympatry [9]. Moreover, when species occur along a gradient of environmental conditions, their traits may converge in sympatry despite competition [10]. In such conditions, ecological heterogeneity across space has been argued to be a more convincing cause of character variation than competition [4].
Our study aims to test the role of adaptation to distinct environments versus competition leading to character displacement in shaping coexistence between two sister species of the African four striped mouse: Rhabdomys bechuanae (sensu [11], hereafter bechuanae) and Rhabdomys dilectus dilectus (sensu [12], hereafter dilectus). We focus here on space use, an important dimension of the niche [13] because it determines access to resources, and hence could directly influence reproductive success and survival [14]. Further, the evolution of this complex trait could be shaped both by environmental conditions [15,16] and competitive interference in areas of coexistence [17,18].
Space use, or the spatial dimension of a species niche, can be described at beta and alpha scales [19]. The beta scale considers the climate and environmental conditions over the entire range of the species defining its environmental niche. The alpha scale considers niche variation between individuals and populations (i.e. “the niche variation hypothesis”, [20]) and allows for a more detailed assessment of niche characteristics.
We studied the spatial niche of the two striped mouse species at an alpha scale by analyzing their habitat use and home range (HR) characteristics. The striped mouse shows marked differentiation across climate and vegetation along an east-to-west gradient in southern Africa [11,21]. Large scale studies, modelling the two species’ niches over South Africa and Namibia suggested environmental divergence, dilectus being found in the wetter areas of the north-eastern parts of South Africa where grassland vegetation dominates, while bechuanae occupies warmer and drier regions and penetrates into the more mesic central part of South Africa within areas where open shrubland vegetation dominates [11,21]. Such a divergence could either be the result of adaptation in distinct environmental conditions, or reflect a large range of plastic responses to the environmental gradient occupied by the two species. Here, we test the role of adaptation versus plasticity in this divergence and disentangle the role of ecological heterogeneity versus competition in shaping species coexistence in the field.
The distribution of the two species abuts in areas with similar environmental conditions, where pockets of sympatric populations exist [22]. To distinguish habitat selection from character displacement, we compared habitat preference and HR characteristics of the two species at their distribution margins where allopatric and sympatric populations are found. We made the following predictions: first, if environmental niche divergence resulted from adaptation in allopatry, as suggested by the beta scale study, populations at the margins of the two species distribution, occurring in similar environments, would select different habitats. Second, if environmental heterogeneity in areas of sympatry is sufficient to allow species segregation, we would expect little competition, if any, on the spatial dimension of the species niche. Alternatively, if environmental heterogeneity does not allow complete habitat separation, competition is expected to induce character displacement, even if sympatry is only temporary, because the trait studied here, HR size, is expected to respond rapidly to interference competition [23].
Material and Methods
Ethics statement
Permits to work and handle animals in the field were obtained from the Free State and North West Province reserve ethics authorities (n°01/15700, 01/11262). Animals handling was performed under permissions from the French agriculture ministry to GG (C34–265), and Wits university ethics committee for CMSD (AESC n°: 2012/13/2A).
Study species
Rhabdomys sp. individuals forage alone during the day and rest at night in a nest either alone or in groups [24]. All Rhabdomys species are morphologically very similar, requiring genotyping for their identification. In our study, species identity was assessed by genotyping their Cytochrome Oxydase I mitochondrial gene (described in [22]).
Study area
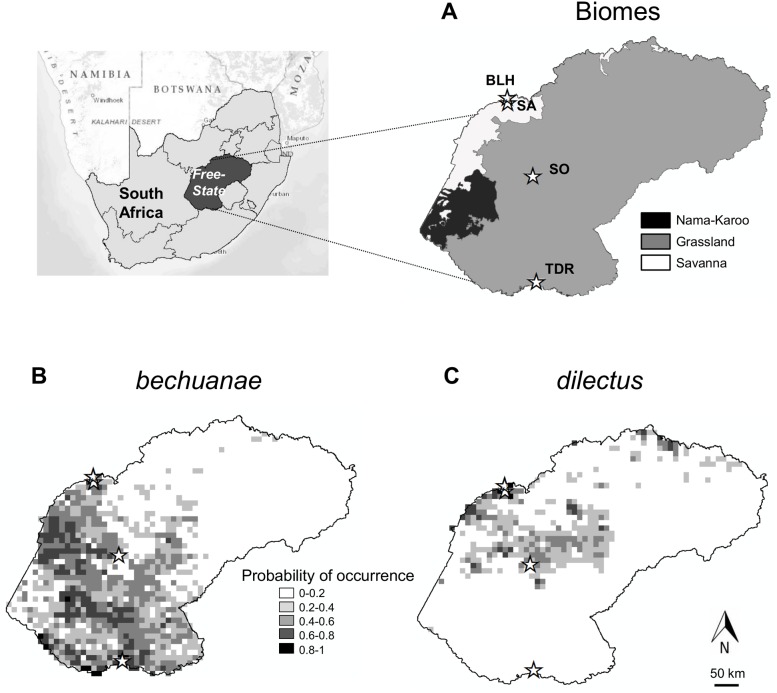
Mice were studied in four nature reserves located within the savanna and grassland biomes (sensu [25]) of central South Africa: three reserves in the Free State Province and one at its boundary with the North West Province (Fig. 1). The reserves occur along a north-south axis, from Bloemhof Dam (BLH; S27° 38’ E25° 40’) and Sandveld (SA; S27° 43’ E25° 45’), to Soetdoring (SO; S28° 50’ E26° 03’) and Tussen die Riviere (TDR; S30° 28’ E26° 09’). In these reserves, dilectus and bechuanae occur in different geographic settings, either as monospecific populations (hereafter: allpatric sites) or as regular but temporary mixed species populations (hereafter: sympatric sites, S1 Fig.). In sympatric sites mice of the two species could be trapped in the same traps (although not together).
Fig 1. Study area and species occurrence probability.
Details on biomes (A) and probabilities of occurrence of dilectus and bechuanae (B, C) (modified from Ganem et al. 2012). Star symbols indicate position of Bloemhof (BLH), Sandveld (SA), Soetdoring (SO) and Tussen die Riviere (TDR) Nature reserves.
We sampled a total of 22 sites across the four reserves, among which 11 were sampled at 2 to 3 occasions (Table 1). The HRs of the mice studied never overlapped between sites during the study period (which lasted roughly two weeks per site).
Table 1. Characteristics of studied sites across the four nature reserves.
| Reserve | Year | Season | Site | Geography | Radio-tracking | Total number of | Area (m2) | Percentage of area characterized with the 60x60 quadrats | Density index | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bechuanae | dilectus | |||||||||
| BLH | 2012 | spring | BLH1 | allopatry | yes | 0 | 8 | 30113 | 75 | 0.03 |
| BLH | 2012 | spring | BLH2 | allopatry | yes | 0 | 17 | 50319 | 31 | 0.04 |
| BLH | 2012 | spring | BLH3 | allopatry | yes | 0 | 13 | 85622 | 20 | 0.01 |
| SA | 2012 | spring | SA1 | allopatry | yes | 18 | 0 | 193027 | 49 | 0.01 |
| SA | 2011 | spring | SA1 | sympatry | yes | 96 | 43 | 193027 | 49 | 0.06 |
| SA | 2011 | spring | SA2 | sympatry | yes | 7 | 10 | 45531 | 19 | 0.05 |
| SA | 2012 | spring | SA3 | allopatry | yes | 4 | 0 | 44151 | 31 | 0.01 |
| SO | 2012 | spring | SO1 | allopatry | yes | 14 | 0 | 28150 | 52 | 0.02 |
| SO | 2012 | spring | SO2 | allopatry | yes | 4 | 0 | 17907 | 53 | 0.01 |
| SO | 2012 | spring | SO3 | allopatry | yes | 0 | 51 | 88514 | 64 | 0.05 |
| SO | 2012 | spring | SO4 | sympatry | yes | 1 | 10 | 107895 | 42 | 0.01 |
| TDR | 2012 | spring | TDR1 | allopatry | yes | 68 | 0 | 119527 | 48 | 0.07 |
| BLH | 2013 | autumn | BLH3 | allopatry | no | 0 | 3 | 85622 | 20 | |
| BLH | 2012 | spring | BLH4 | allopatry | no | 0 | 3 | 16763 | 52 | |
| SA | 2012 | autumn | SA1 | sympatry | no | 25 | 8 | 193027 | 49 | |
| SA | 2012 | autumn | SA3 | allopatry | no | 12 | 0 | 44151 | 31 | |
| SA | 2013 | autumn | SA3 | allopatry | no | 8 | 0 | 44151 | 31 | |
| SA | 2013 | autumn | SA4 | allopatry | no | 0 | 1 | 37110 | 48 | |
| SA | 2012 | autumn | SA4 | sympatry | no | 4 | 7 | 37110 | 48 | |
| SA | 2012 | spring | SA4 | sympatry | no | 1 | 2 | 37110 | 48 | |
| SA | 2012 | autumn | SA5 | allopatry | no | 17 | 0 | 19462 | 45 | |
| SA | 2012 | spring | SA5 | allopatry | no | 1 | 0 | 19462 | 45 | |
| SA | 2013 | autumn | SA5 | allopatry | no | 1 | 0 | 19462 | 45 | |
| SA | 2012 | autumn | SA6 | allopatry | no | 8 | 0 | 12751 | 71 | |
| SA | 2012 | autumn | SA7 | sympatry | no | 7 | 6 | 124315 | 29 | |
| SA | 2012 | spring | SA7 | sympatry | no | 5 | 1 | 124315 | 29 | |
| SA | 2012 | autumn | SA8 | allopatry | no | 4 | 0 | 7659 | 70 | |
| SA | 2012 | autumn | SA9 | allopatry | no | 18 | 0 | 43011 | 34 | |
| SA | 2013 | autumn | SA9 | allopatry | no | 5 | 0 | 43011 | 34 | |
| SO | 2012 | autumn | SO1 | allopatry | no | 4 | 0 | 28150 | 52 | |
| SO | 2012 | autumn | SO3 | allopatry | no | 0 | 52 | 88514 | 64 | |
| SO | 2012 | autumn | SO4 | allopatry | no | 0 | 11 | 107895 | 42 | |
| SO | 2012 | autumn | SO5 | allopatry | no | 7 | 0 | 18942 | 33 | |
| SO | 2012 | autumn | SO6 | allopatry | no | 0 | 1 | 6537 | 47 | |
| SO | 2012 | autumn | SO7 | sympatry | no | 1 | 1 | 9503 | 64 | |
| TDR | 2013 | autumn | TDR1 | allopatry | no | 4 | 0 | 119527 | 48 | |
| TDR | 2012 | spring | TDR2 | allopatry | no | 3 | 0 | 17701 | 57 | |
BLH: Bloemof, SA: Sandveld, SO: Soetdoring and TDR: Tussen Die Riviere nature reserves.
Although we did not monitor predation and competition with other species, we observed the presence of snakes, birds of prey and carnivore small mammals in all sites. Other rodent species were present in the trapping sites: the most frequent ones were Gerbiliscus sp. and Nannomys minutoides.
Trapping procedure
Trapping took place during the austral spring: October-November 2011 (SA), October-November 2012 (SA, SO, TDR, BLH), and autumn: April-May 2012 (SA, SO) and April-May 2013 (SA, TDR, BLH). Our trapping strategy aimed to sample most vegetation types available within the area. Small mammal live traps (Sherman and PVC traps of equivalent size) were baited with a mixture of oats, salt and peanut butter, and were provided with a piece of cotton wool as bait and to reduce thermal stress. The number of trap lines varied with the site size, and distance between traps was roughly 10m (10 to 30 traps/line). Traps were checked 2–3 times a day regularly between 7am and 7pm (mean±SD: 79.5±50.89 traps per day per site). Upon capture, each mouse was sexed, weighed, measured (body length) and individually marked with two ear tags (7mm, 0.17g; National Band and Tag Co., Newport, KY-USA). We also collected a piece of tail (≈1cm) for species identification. Overall, we trapped and genotyped 599 mice. Following [26], we estimated relative density within each site by computing the total number of striped mice captured during the first five trapping days divided by the total length of trap lines accounting for a 60 m buffer around the trap lines (roughly the average diameter of a mouse HR, Table 1).
Habitat characterization
Earlier studies addressing beta scale niche analysis [21,22] suggested that the two species could have different requirements in terms of vegetation cover and structure (i.e. grass versus woody vegetation). Here we aimed to test this hypothesis at an alpha scale, and characterized the vegetation structure of mouse habitats by measuring the percentage of trees, bushes, grass and bare soil over 60x60m quadrats, centred on a trap line (the first and last traps were at the centre of, respectively, the first and last quadrats on a given trap line). Furthermore, vegetation cover was determined within 1x1m metal square thrown to the right and left of a trap line at every second trap. Within these 1x1m quadrats, we evaluated the percentage of grass versus woody plants (small shrubs), the percentage of bare soil and an estimate of mouse visibility (an index ranging from 1, i.e. completely visible, to 5, i.e. completely hidden, a value determined by averaging the visibility of a dummy mouse that we placed in four different locations inside the metal square). Altogether we used 136 60x60m quadrats (5.9±6.31 per site) and 229 1x1m quadrats (10.4±15.37 per site).
Radiotracking
A total of 101 adult mice (body mass ≥ 23 grams), were equipped with VHF collars (MD 2C Holohil, Carp, Ontario, Canada) in October-November 2011 and 2012 in 11 distinct sites on the four reserves. Radiotracking was performed on foot with a wide-range receiver (AOR 8000) and a hand-held Telonics R4–14K antenna. Localization of a collared mouse followed the standard triangulation technique and its precise location was confirmed with the receiver cable used without the antenna. The receiver volume was set to 0 during the triangulation to reduce mice disturbance. The GPS coordinates of radio-collared individuals were recorded five times during the day (at about 7, 9, 11am and 2 and 4pm) and once at sunset (roughly 7pm).
Home range size and overlap estimations
HRs were defined as the areas encompassed within the 0.95 cumulative isopleth of the Utilization Distributions (UDs), estimated using the fixed kernel method with the reference smoothing parameter [27]. Our sampling regime was chosen after a calibration session where we followed 30 individuals for more than seven days (from 40 to 69 relocations). The estimated HR size of our controls stabilized after 27 relocations and a paired comparison of the HR size at 27 and 41 regular relocations did not show a significant difference (Wilcoxon test, V = 306, p = 0.14). Following [28], we chose a strategy maximizing the number of mice radiotracked with a sampling regime standardized to a minimum of 27 independent relocations, collected over five consecutive days.
We compared HR overlaps in a sympatric site between pairs of mice of the same species versus different species. We computed the overlap between each pair of HRs using their UD-based volume of intersection [29]. Because UDs are truncated at the 0.95 cumulative isopleth (excluding the poorly estimated UD tails), overlap values were normalized to 1 by dividing them by 0.95 (see [30] for details).
Statistical analyses
Statistical analysis was conducted with R-v2.15 [31]. Normality and heteroscedasticity of distributions were checked with a Shapiro test and visualized with the plot of the model’s residuals. When these conditions were not met even after data transformation, non-parametric tests were used. Significance level was set to 0.05, and adjusted for multiple comparisons with the sequential Bonferroni procedure when necessary. UD, overlap computations, and permutation tests (see below) were performed using home-made programs in Pascal.
Mice-habitat relationship assessed with trapping data
A total of 599 trapped mice were used in these analyses. A mouse was considered as potentially using a 60x60m quadrat when it was trapped within it, and a 1x1m quadrat when it was trapped less than 10m from it. Each quadrat was then assigned to one or the other species, to both species, or to none. A total of 89 60x60m quadrats and 227 1x1m quadrats were assigned to one or the two species.
We performed an Outlying Mean Index multivariate analysis to characterize the environmental niche of each species (i.e. OMI) [32]. Briefly, the OMI procedure generates ordination axes corresponding to the combination of environmental variables (here vegetation structure and cover) that are most relevant for the species under study, and provides a measure of the habitat conditions occupied by the species. Our two habitat parameters (vegetation structure and cover) were obtained at different sampling scales, hence, we carried out an OMI analysis at each scale (i.e. 60x60m and 1x1m). Each analysis produced a habitat niche position value (i.e. the mean habitat characteristics of species occurrence) and breadth (i.e. variance) for each of the four categories studied here, i.e. the two species in allopatric versus sympatric sites. We assessed marginality (i.e. deviation from a random sample of available conditions) of niche position and breadth of a given category on an OMI axis through comparisons with distributions obtained performing 1000 random permutations followed by bootstrap two-tailed tests.
The niche positions of the four population categories (two species in allopatry versus in sympatry) on the first OMI axis (OMI1) were compared with linear mixed ANOVAs (package nlme), with the category as a fixed effect and site as a random effect, followed by Tukey post-hoc tests when relevant (package mulcomp, glht function). The same procedure was applied on a subsample of the data comprised of only sympatric sites, to test whether excluding allopatric habitats from the analyses would detect species divergence in sympatry.
Mice-habitat relationships assessed with home range data
Habitat at the HR scale was characterized with the 60x60m quadrats that covered an area corresponding to at least 70% of the HR. Such a coverage was reached for 80 of the estimated HRs (S1 Table). The four vegetation structure variables measured within a quadrat were weighted by the relative proportions of the HR UD covered. These data were then analyzed following the same procedure as described above.
Determinants of home range size variation
For the purpose of this analysis, we reduced the four variables describing vegetation structure into one corresponding to the first axis of a Principal Component Analysis (PCA). This axis represented 76% of total variance. We tested the influence of body size, sex, population density, habitat (i.e. PCA1), geography (i.e. allopatry vs sympatry) and species on log-transformed HR size. Our data showed spatial autocorrelation (Moran test, p<0.001, library “spdep”), hence we applied the spatial simultaneous autoregressive error model estimation (sarlm model) in subsequent ANCOVA analyses. Our initial ANCOVA model comprised all factors as main effects and second and third order interactions with “species”, except for density which was included as a co-variable in the model. Preliminary analyses showed that density did not vary between species (KW, DF = 2, χ2 = 1.72, p = 0.42) or with the habitat parameter of the model (Spearman, ρ = 0.06, p = 0.58), and that body size did not differ between our sample of males and females (Anova, DF = 1,97, F = 0.61 p = 0.44). The most parsimonious model was obtained after sequential elimination of factors with non-significant effects (following [33]), and post-hoc checking that its AICc was significantly smaller to that of the initial model.
HR overlaps in a sympatric site
Because we did not know the species identity of mice during our field study, our selection of radiotracked mice could not be balanced. The between-species HR overlap analysis could eventually be performed for only one sympatric site (SA1), in which 6 dilectus (3 females and 3 males) and 14 bechuanae (9 females and 5 males) were radiotracked. Other sympatric sites contained too few radiotracked individuals of one species or the other to enable us to perform statistical tests. We computed three observed values: the mean overlap between any two dilectus HRs, the mean overlap between any two bechuanae HRs and the mean overlap between HRs of any two mice belonging to different species. To test the null hypothesis: “random overlap between species”, we determined all the possible partitions of 20 mice in a group of 6 (G6) and a group of 14 (G14). Because the degree of HR overlap between two mice could also depend on sex, we kept only the 12320 partitions (out of 38760) showing the observed sex ratios (i.e. 3 females and 3 males in G6 and 9 females and 5 males in G14). For each partition, we computed the mean overlap between two mice belonging to the same group (G6xG6 and G14xG14) or to different groups (G6xG14). Mean values are based on 15 (G6xG6), 91 (G14xG14) or 84 (G6xG14) observations. In this way, by considering the whole set of partitions, we built up three theoretical distributions expected under the null hypothesis, to which we compared the three observed values mentioned above. As in any permutation test, the probability to reject the null hypothesis, i.e. obtaining a value equal to or more extreme than the observed value in each of the three tests, was computed as P = 2(n e+1)/n (bilateral test), where n e is the number of these most extreme values and n is the total number of values (n = 12320).
Results
Variation of habitat preference within and between species
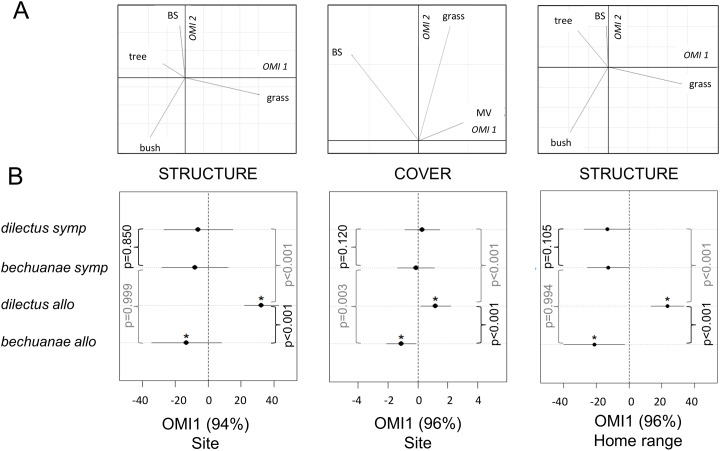
We compared the habitat characteristics of allopatric and sympatric mice of the two species at the population (site) and individual (HR) levels with three distinct OMI analyses. The first axes (i.e OMI1) always captured a significant proportion of the habitat variation (>94%, Fig. 2), and results were consistent across analyses. These three axes had positive values for presence of grass and mouse visibility, and negative values for the presence of woody type vegetation and bare soil (Fig. 2). The habitat niche positions of the two species in allopatric sites were significantly different from a random sample of available habitats (p<0.05), and from each other (respectively, at the site level, vegetation structure and cover, and at the HR level vegetation structure: z = -4.41, z = -7.69 and z = 5.28, p<0.001). The niche position of allopatric bechuanae showed significant negative values (i.e. habitat characterized by more woody vegetation and presence of bare soil than that in a random sample of available habitats), while those of dilectus were significantly positive (i.e. habitats characterized by the presence of more grass and cover than in a random sample). Unlike allopatric sites, the niche position of individuals of the two species in sympatry did not differ from random expectations (p>0.05). However, their positions differed from that of their allopatric counterparts. Indeed, in sympatry, bechuanae occurred in a habitat with higher mouse visibility and cover values (vegetation cover, z = 3.39, p = 0.003), but with similar values of vegetation structure (at the site and HR levels, Fig. 2) compared to its habitat in allopatry. In contrast, habitat characteristics of dilectus in allopatry and sympatry differed in the three analyses, as its habitat in sympatry was characterized by lower values of mouse visibility, cover and presence of grass than in allopatry (respectively, at the site level the vegetation structure and cover, at the HR level the vegetation structure: z = -3.88 z = -3.84 z = -4.29 p<0.001, Fig. 2).
Fig 2. Habitat niche divergence in sympatry and allopatry.
Habitat divergence between allopatric (allo) and sympatric (symp) populations of the two species as assessed with Outlying Mean Index (OMI) analyses. The upper row (A) shows the relative contribution of the different habitat variables: vegetation structure (tree, grass, bush and bare soil (BS)) and cover (bare soil (BS), grass and mouse visibility (MV)) to the first two OMI axes. The lower row (B) indicates the position (dot) and breadth (line) of each species niche along the first OMI axis (* when significantly different from random expectation). The p-values of Tukey tests are given for every pair comparison (black: inter-specific, grey: intra-specific).
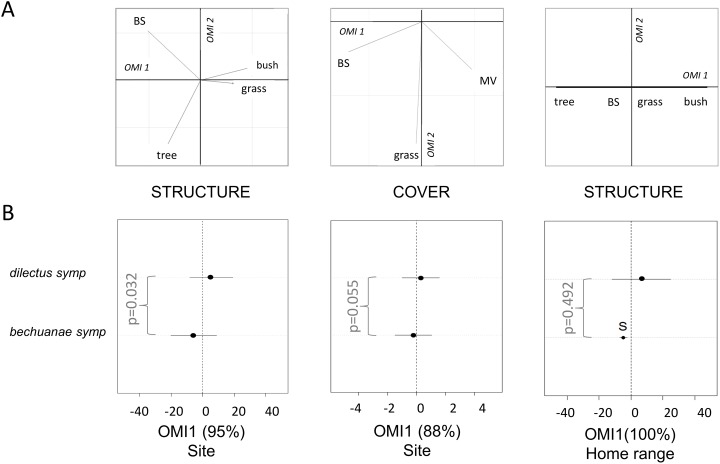
Considering only sympatric sites, the first OMI axes captured most of the data variation (>88%), and described a habitat gradient ranging from high values of bare soil and presence of trees (negative values of OMI1) to high values of grass, mouse visibility and presence of bushes (positive values of OMI1, Fig. 3). Despite the reduced power due to a smaller sample size (particularly for the HR level analysis), the results indicate that bechuanae occurs in micro-habitats characterized by more bare soil, woody vegetation and less mouse visibility than that of dilectus, although these differences (respectively at the site level, the vegetation structure and cover: p = 0.03 p = 0.05) were not strong (significance level adjusted for multiple testing α’ = 0.025; Fig. 3).
Fig 3. Details on habitat niche divergence in sympatry.
Habitat divergence of the two species in sympatric sites assessed with Outlying Mean Index (OMI) analyses. See legend in Fig. 2. The p-values follow Wilcoxon tests.
Variation of HR size within and between species
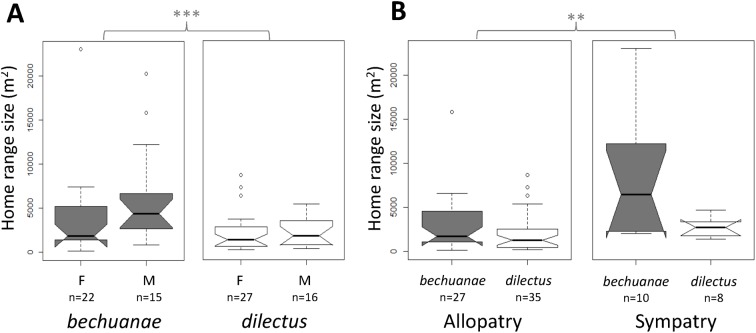
None of the first and second order interactions with species were significant predictors of HR size variation, indicating that the patterns described below were consistent across species. Males had larger HRs than females (z = 2.37, p = 0.02, Fig. 4, S2 Table) and population density and habitat did not significantly affect HR size (respectively, z = -0.89, p = 0.37 and z = 1.66, p = 0.10, S2 Table). Further, bechuanae had larger HRs than dilectus (z = 3.40, p<0.001) both in allopatry and sympatry, although the HRs of both species were smaller in allopatry than in sympatry (z = 2.82, p<0.01, Fig. 4, S2 Table).
Fig 4. Home range size estimates.
Home range size estimates (isopleth 0.95) across species (grey: bechuanae, white: dilectus), A: sex (F females and M males) and B: “geography” (** p<0.01, *** p<0.001 refers to Ancova results in S2 Table). Box-plots show the median (thick line), first and third quartiles. Non-overlapping notches are roughly equivalent to 95% confidence intervals.
Patterns of overlap in a sympatric site
Overlaps of HRs between the two species were significantly lower than random expectations (observed value: 0.011±0.048; permutation test p<0.01), while they were higher than expected within bechuanae (observed value: 0.057±1.265; p<0.01), and not different from random within dilectus (observed value: 0.023±0.052; p>0.5, S2 Fig.).
Discussion
Our study is among very few that attempted to disentangle the complex interaction between environment and competition in shaping character variation [23]. We focused on the spatial niche (i.e. habitat selection and HR size) of two sister species of striped mice whose distributions are mostly allopatric and characterized by distinct environmental conditions [11,12]. We assessed the influence of environmental variation and competition on habitat selection and HR size variation in an area where the distributions of the two species abut and where allopatric and sympatric populations can be compared under similar climatic conditions [22].
Earlier large scale investigations indicated that the environmental niche of the two studied species diverged: the habitats of bechuanae are dominated by warmer climates and drier open shrubland vegetation, while those of dilectus are characterized by wetter climates and grassland type vegetation, providing more cover [11,21,22]. Such beta scale studies are particularly relevant to address species niche characteristics over their entire range; however, they could suffer from confounding effects due to spatial autocorrelation of large scale environmental variables and are not expected to reveal micro-environmental heterogeneity [34], but see [35]. Nevertheless, using an alpha approach, the present study confirms habitat niche divergence at a fine scale and highlights a role for both environmental variation and competition in shaping the spatial niche of bechuanae and dilectus in sympatry.
Niche divergence
Habitat
Sister species are expected to have similar niches if they retain ancestral characteristics (niche conservatism, [36]), or when they evolve under similar conditions [37]. In all cases, contact or secondary sympatry between species sharing similar niches are expected to trigger character displacement [8,35]. Earlier studies of the striped mouse environmental niche indicated that evolution in allopatry took place under different environmental conditions [11,21], and that divergent adaptation may facilitate coexistence. The present study confirms divergence in habitat selection by the two species at the margins of their distributions but also indicates that the available habitat in sympatry is more similar to that of bechuanae in allopatry (i.e. less cover and more woody vegetation) than that of dilectus. Nevertheless, the latter still selects micro-habitats with more cover and less woody vegetation than bechuanae, confirming micro-habitat partitioning in sympatry as suggested by preliminary observations [22]. Such differences in habitat preference, consistent over the entire species range including its margins and sympatric zones, together with the largely allopatric distribution of the species, support evolution under different selective pressures in allopatry (i.e. adaptation), and a more recent secondary contact where these preferences are still expressed. Partition of the habitat niche could thus result in lower interspecific competition pressures facilitating co-existence [38,39]. Nevertheless our study indicates that habitat divergence in sympatry is tenuous compared to that in allopatry. Further, the habitat available in sympatry differed significantly from that in allopatry for dilectus, suggesting that the latter invaded the range of bechuanae and that coexistence occurs in areas to which dilectus might be less adapted.
Home Range
Lesser habitat partition in sympatry is expected to induce competition which we assessed comparing HR size variation, a trait known to be influenced by habitat features [16] and interspecific interference [40]. We found differences in HR size between the species: dilectus having smaller HRs than bechuanae. These differences exist despite sexual dimorphism in HR size (i.e larger in males than females), in both species, that could relate to behavioural [41,42] and physiological [16,43] sex differences.
Our study did not address the precise mechanisms of HR divergence; however, based on inference from the literature, we may expect that, like other species, striped mice adjust their HR size to available cover or shelter (e.g. [16] on the wood mouse) or to visibility of potential predators (e.g. [44] on roe deer). Such patterns are consistent with our observations that, the species showing preference for habitats with cover, dilectus, also has smaller HRs compared to bechuanae which selects habitats with more wood and less cover. Larger HRs may also provide access to patchily located shelters from predators [45], which may be the case for bechuanae which selects open shrubland type habitats. Differences in HR size may also indicate differences in food distribution, since smaller HRs were proposed to reflect more concentrated food distribution in other studies [44,46–49]. Surprisingly, we did not detect a significant influence of vegetation structure on HR size variation. Possible explanations might be that, either this variable only has an indirect effect on HR size (see above), or to lower resolution due to small sample size.
Character displacement
Differences in habitat preferences may facilitate coexistence between bechuanae and dilectus. However, as indicated above, sympatry occurs in habitats that are less favorable for dilectus and our study suggests that species segregation in sympatry may not be complete. Patterns of HR size variation in allopatry versus sympatry also suggest that competition may occur. Indeed, both species had a significant increase in their HR size in sympatry as compared to allopatry. Although we cannot exclude that such variation could be consistent with habitat variation as far as dilectus is concerned, this argument may not hold for bechuanae whose preferred habitat in allopatry and sympatry is similar.
A larger HR size in sympatry may reveal inter-species intolerance and competition [17,23,40]. In our study, larger HRs in sympatry compared to allopatry, at least in bechuanae, could be a response to competition and a strategy aimed at limiting costly interactions with the other species through character displacement [40]. Alternatively, it could be a strategy to occupy most of the available resources (e.g. nest sites). Patterns of HR overlap in our study suggest that our first hypothesis may be true, as bechuanae showed more HR overlaps than expected with members of the same species, while between species overlaps were lower than expected under random predictions.
Micro-habitat selection and space partition are expected to be adaptive responses to reduce competition [17,18,50]. Here, despite different habitat preferences, habitat segregation is tenuous in sympatry, resulting in bechuanae enlarging its HR possibly to avoid dilectus, making detours, or to control a larger number of shelters to outcompete dilectus.
Conclusion
It was argued that ecological complexity was not considered often enough in assessments of mechanisms of coexistence [9], and that evidence for character displacement resulting from species interference is rare [4]. Our study provides valuable field data in an interesting study model allowing to compare the spatial niche characteristics of two species in a relatively homogeneous sympatric and allopatric environment (a common garden setting). Furthermore, the alpha scale investigation, together with an earlier beta scale one [11,21,22], provides a comprehensive picture of how environmental heterogeneity and interference competition could shape the spatial niche of two sister species and influence patterns of coexistence. Future studies should include mechanistic experimental approach to address competition between the two species and determine the proximal mechanisms (e.g. the impact of competition on the species fitness) shaping the species range limits and patterns of co-existence.
Supporting Information
A: An example of distribution of allopatric and sympatric sites (SO1-SO6) within Soetdoring Nature Reserve. B: Distribution of the quadrats used for habitat assessment (vegetation structure and cover) around the trap lines.
(TIF)
From left to right: distributions of intra-species overlap values within dilectus (A) and bechuanae (B) and between the species (C). The red lines indicate position of observed mean values.
(TIF)
Number of HR estimates per species and sex in allopatric and sympatric sites, and size of the subsample for which the habitat was characterized.
(XLSX)
Results of the initial and minimal ANCOVA models. The models (sarlm residual, see text), testing factors that may influence HR size variation: habitat (PCA 1), sex, geography (allopatry vs sympatry), body size and population density. Bold p-values indicate significant effects.
(XLSX)
Acknowledgments
We are grateful for discussion with or help from: P. Caminade, M. Perriat-Sanguinet, the Succulent Karoo Research Station team, Y. Latour, O. Gimenez, L.-M. Chevin, S. Chamaille, J. Britton-Davidian, the reserve personnel, and FreePascal compiler (www.freepascal.org). This study was performed with permits from the Free State and North West Province reserve authorities (n°01/15700, 01/11262). ISEM 2014/216.
Data Availability
The data are available in FigShare (http://dx.doi.org/10.6084/m9.figshare.1281368).
Funding Statement
This work was supported by Free State DTEEA, SIBAGHE, the French CNRS/SA NRF agreements through PICS (n°4841 and n°81859) and GDRI (n°191) programs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dayan T, Simberloff D (2005) Ecological and community-wide character displacement: the next generation. Ecology Letters 8: 875–894. Available: http://doi.wiley.com/10.1111/j.1461-0248.2005.00791.x. Accessed 2013 August 6. [Google Scholar]
- 2. Brown W, Wilson E (1956) Character displacement. Systematic Zoology 5: 49–64. [Google Scholar]
- 3. Grant PR (1972) Convergent and divergent character displacement. Biological Journal of the Linnean Society 4: 39–68. [Google Scholar]
- 4. Stuart YE, Losos JB (2013) Ecological character displacement: glass half full or half empty? Trends in Ecology & Evolution 28: 402–408. Available: http://www.ncbi.nlm.nih.gov/pubmed/23537690. Accessed 7 August 2013. 10.1111/1365-2656.12336 [DOI] [PubMed] [Google Scholar]
- 5. Pfennig DW, Pfennig KS (2012) Evolution’s wedge competition and the origins of diversity Berley, California: 10.1016/S2214-109X(15)70024-0 [DOI] [Google Scholar]
- 6. Grant PR, Grant BR (2006) Evolution of character displacement in Darwin’s finches. Science 313: 224–226. Available: http://www.ncbi.nlm.nih.gov/pubmed/16840700. Accessed 2012 November 6. [DOI] [PubMed] [Google Scholar]
- 7. Chesson P (2000) Mechanisms of maintenance of species diversity. Annual review of Ecology and Systematics 31: 343–358. Available: http://www.jstor.org/stable/10.2307/221736. Accessed 2013 August 23. [Google Scholar]
- 8. Violle C, Nemergut DR, Pu Z, Jiang L (2011) Phylogenetic limiting similarity and competitive exclusion. Ecology Letters 14: 782–787. Available: http://www.ncbi.nlm.nih.gov/pubmed/21672121. Accessed 2013 December 14. 10.1111/j.1461-0248.2011.01644.x [DOI] [PubMed] [Google Scholar]
- 9. Goldberg EE, Lande R (2006) Ecological and reproductive character displacement on an environmental gradient. Evolution 60: 1344–1357. Available: http://www.ncbi.nlm.nih.gov/pubmed/16929652. [PubMed] [Google Scholar]
- 10. Case TJ, Taper ML (2000) Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. The American Naturalist 155: 583–605. [DOI] [PubMed] [Google Scholar]
- 11. Du Toit N, van Vuuren BJ, Matthee S, Matthee CA (2012) Biome specificity of distinct genetic lineages within the four-striped mouse Rhabdomys pumilio (Rodentia: Muridae) from southern Africa with implications for taxonomy. Molecular Phylogenetics and Evolution 65: 75–86. Available: http://www.ncbi.nlm.nih.gov/pubmed/22728170. Accessed 2012 November 7. 10.1016/j.ympev.2012.05.036 [DOI] [PubMed] [Google Scholar]
- 12. Rambau RV, Robinson TJ, Stanyon R (2003) Molecular genetics of Rhabdomys pumilio subspecies boundaries: mtDNA phylogeography and karyotypic analysis by fluorescence in situ hybridization. Molecular Phylogenetics and Evolution 28: 564–575. Available: http://linkinghub.elsevier.com/retrieve/pii/S1055790303000587. Accessed 2012 November 16. [DOI] [PubMed] [Google Scholar]
- 13. Lopez-Darias M, Schoener TW, Spiller DA, Losos JB (2012) Predators determine how weather affects the spatial niche of lizard prey: exploring niche dynamics at a fine scale. Ecology 93: 2512–2518. Available: http://www.ncbi.nlm.nih.gov/pubmed/23431582. [DOI] [PubMed] [Google Scholar]
- 14. Powell RA, Mitchell MS (2012) What is a home range? Journal of Mammalogy 93: 948–958. Available: http://www.bioone.org/doi/abs/10.1644/11-MAMM-S-177.1. Accessed 2012 November 4. [Google Scholar]
- 15. Schoener T (1974) Resource partitioning in ecological communities. Science 185: 27–39. [DOI] [PubMed] [Google Scholar]
- 16. Godsall B, Coulson T, Malo AF (2013) From physiology to space use: energy reserves and androgenization explain home-range size variation in a woodland rodent. Journal of Animal Ecology 83: 126–135. Available: http://www.ncbi.nlm.nih.gov/pubmed/23931095. Accessed 2013 December 16. 10.1111/1365-2656.12116 [DOI] [PubMed] [Google Scholar]
- 17. Arakaki S, Tokeshi M (2011) Analysis of spatial niche structure in coexisting tidepool fishes: null models based on multi-scale experiments. Journal of Animal Ecology 80: 137–147. 10.1111/j.1365-2656.2010.01749.x [DOI] [PubMed] [Google Scholar]
- 18. MacArthur R, Recher H, Cody M (1966) On the relation between habitat selection and species diversity. The American naturalist 100: 319–332. [Google Scholar]
- 19. Ackerly D, Schwilk D., Webb C. (2006) Niche evolution and adaptive radiation: testing the order of trait divergence. Ecology 87: 50–61. Available: http://www.ncbi.nlm.nih.gov/pubmed/16922302. [DOI] [PubMed] [Google Scholar]
- 20. Van Valen L (1965) Morphological variation and width of ecological niche. The American Naturalist 99: 377–390. [Google Scholar]
- 21. Meynard CN, Pillay N, Perrigault M, Caminade P, Ganem G (2012) Evidence of environmental niche differentiation in the striped mouse (Rhabdomys sp.): inference from its current distribution in southern Africa. Ecology and Evolution 2: 1008–1023. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3399166&tool=pmcentrez&rendertype=abstract. Accessed 2012 November 26. 10.1002/ece3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganem G, Meynard CN, Perrigault M, Lancaster J, Edwards S, et al. (2012) Environmental correlates and co-occurrence of three mitochondrial lineages of striped mice (Rhabdomys) in the Free State Province (South Africa). Acta Oecologica 42: 30–40. Available: http://linkinghub.elsevier.com/retrieve/pii/S1146609X12000045. Accessed 2012 December 19. [Google Scholar]
- 23. Mikami OK, Kawata M (2002) The effects of individual interactions and habitat preferences on spatial structure in a grassland bird community. Ecography 25: 200–214. Available: http://www.blackwell-synergy.com/links/doi/10.1034/j.1600-0587.2002.250208.x. [Google Scholar]
- 24. Schradin C (2004) Territorial defense in a group-living solitary forager: who, where, against whom? Behavioral Ecology and Sociobiology 55: 439–446. Available: http://www.springerlink.com/openurl.asp?genre=article&id=doi:10.1007/s00265-003-0733-x. Accessed 2012 November 2. [Google Scholar]
- 25. Mucina L, Rutherford M (2006) The Vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria, South Africa. [Google Scholar]
- 26. Stapp P, Van Horne B (1997) Response of Deer Mice (Peromyscus maniculatus) to shrubs in shortgrass prairie: linking small-scale movements and the spatial distribution of individuals. Functional Ecology 11: 644–651. [Google Scholar]
- 27. Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70: 164–168. [Google Scholar]
- 28. Börger L, Franconi N, De Michele G, Gantz A, Meschi F, et al. (2006) Effects of sampling regime on the mean and variance of home range size estimates. Journal of Animal Ecology 75: 1393–1405. Available: http://www.ncbi.nlm.nih.gov/pubmed/17032372. Accessed 2013 August 8. [DOI] [PubMed] [Google Scholar]
- 29. Seidel K (1992) Statistical properties and applications of a new measure of joint space use for wildlife. University of Washington, Seattle, USA. [Google Scholar]
- 30. Benhamou S, Valeix M, Chamaillé-Jammes S, Macdonald DW, Loveridge AJ (2014) Movement-based analysis of interactions in African lions. Animal Behaviour 90: 171–180. Available: http://linkinghub.elsevier.com/retrieve/pii/S0003347214000645. Accessed 2014 March 21. [Google Scholar]
- 31. R Development Core Team. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 10.1016/j.neuroimage.2011.01.013 [DOI] [Google Scholar]
- 32. Doledec S, Chessel D, Gimaret-Carpentier C (2000) Niche separation in community analysis: a new method. Ecology 81: 2914–2927. [Google Scholar]
- 33. Zar J (1999) Biostatistical Analysis. 4th ed Ryu T, Snavely S, editors Prentice-Hall; Upper Saddle River, USA. [Google Scholar]
- 34. Soberón J (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters 10: 1115–1123. Available: http://www.ncbi.nlm.nih.gov/pubmed/17850335. Accessed 2014 May 23. [DOI] [PubMed] [Google Scholar]
- 35. McCormack JE, Zellmer AJ, Knowles LL (2010) Does niche divergence accompany allopatric divergence in Aphelocoma jays as predicted under ecological speciation? Insights from tests with niche models. Evolution 64: 1231–1244. Available: http://www.ncbi.nlm.nih.gov/pubmed/19922442. Accessed 2014 May 23. 10.1111/j.1558-5646.2009.00900.x [DOI] [PubMed] [Google Scholar]
- 36. Peterson AT, Soberon J, Sanchez-Cordero V (1999) Conservatism of ecological niches in evolutionary time. Science 285: 1265–1267. Available: http://www.sciencemag.org/cgi/doi/10.1126/science.285.5431.1265. Accessed 2014 May 26. [DOI] [PubMed] [Google Scholar]
- 37. Losos JB (2011) Convergence, adaptation, and constraint. Evolution 65: 1827–1840. Available: http://www.ncbi.nlm.nih.gov/pubmed/21729041. Accessed 2014 June 3. 10.1111/j.1558-5646.2011.01289.x [DOI] [PubMed] [Google Scholar]
- 38. Schluter D, Mcphail JD (1992) Ecological character displacement and speciation in sticklebacks. The American naturalist 140: 85–108. 10.1086/285404 [DOI] [PubMed] [Google Scholar]
- 39. Lira A, Souza A, Silva Filho A, Albuquerque C (2013) Spatio-temporal microhabitat use by two co-occurring species of scorpions in Atlantic rainforest in Brazil. Zoology 116: 182–185. Available: http://www.ncbi.nlm.nih.gov/pubmed/23664851. Accessed 2013 November 1. 10.1016/j.zool.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 40. Trombulak SC (1985) The influence of interspecific competition on home range size in chipmunks (Eutamias). Journal of Mammalogy 66: 329–337. [Google Scholar]
- 41. Clutton-Brock TH (1989) Mammalian mating systems. Proceedings of the Royal Society of London 236: 339–372. Available: http://www.ncbi.nlm.nih.gov/pubmed/2567517. [DOI] [PubMed] [Google Scholar]
- 42. Schradin C (2006) Whole-day follows of striped mice (Rhabdomys pumilio), a diurnal murid rodent. Journal of Ethology 24: 37–43. Available: http://www.springerlink.com/index/10.1007/s10164-005-0158-2. Accessed 2012 December 19. [Google Scholar]
- 43. Schradin C, Scantlebury M, Pillay N, König B (2009) Testosterone levels in dominant sociable males are lower than in solitary roamers: physiological differences between three male reproductive tactics in a sociably flexible mammal. The American Naturalist 173: 376–388. Available: http://www.ncbi.nlm.nih.gov/pubmed/19199528. Accessed 2012 November 12. 10.1086/596535 [DOI] [PubMed] [Google Scholar]
- 44. Tufto J, Andersen R, Linnell J (1996) Habitat use and ecological correlates of home range size in a small cervid: the roe deer. Journal of Animal Ecology 65: 715–724. [Google Scholar]
- 45. Holmes W (1991) Predator risk affects foraging behaviour of pikas: observational and experimental evidence. Animal Behaviour 42: 111–119. [Google Scholar]
- 46. Schradin C, Pillay N (2005) Intraspecific variation in the spatial and social organization of the african striped mouse. Journal of Mammalogy 86: 99–107. [Google Scholar]
- 47. Schradin C, Schmohl G, Rödel HG, Schoepf I, Treffler SM, et al. (2010) Female home range size is regulated by resource distribution and intraspecific competition: a long-term field study. Animal Behaviour 79: 195–203. Available: http://linkinghub.elsevier.com/retrieve/pii/S0003347209004965. Accessed 2012 November 4. [Google Scholar]
- 48. Stradiotto A, Cagnacci F, Delahay R, Tioli S, Nieder L, et al. (2009) Spatial organization of the yellow-necked mouse: effects of density and resource availability. Journal of Mammalogy 90: 704–714. [Google Scholar]
- 49. Jonsson P, Hartikainen T, Koskela ESA (2002) Determinants of reproductive success in voles: space use in relation to food and litter size manipulation. Evolutionary Ecology 16: 455–467. [Google Scholar]
- 50. Hawes ML (1977) Home range, territoriality, and ecological separation in sympatric shrews, Sorex vagrans and Sorex obscurus. Journal of Mammalogy 58: 354–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: An example of distribution of allopatric and sympatric sites (SO1-SO6) within Soetdoring Nature Reserve. B: Distribution of the quadrats used for habitat assessment (vegetation structure and cover) around the trap lines.
(TIF)
From left to right: distributions of intra-species overlap values within dilectus (A) and bechuanae (B) and between the species (C). The red lines indicate position of observed mean values.
(TIF)
Number of HR estimates per species and sex in allopatric and sympatric sites, and size of the subsample for which the habitat was characterized.
(XLSX)
Results of the initial and minimal ANCOVA models. The models (sarlm residual, see text), testing factors that may influence HR size variation: habitat (PCA 1), sex, geography (allopatry vs sympatry), body size and population density. Bold p-values indicate significant effects.
(XLSX)
Data Availability Statement
The data are available in FigShare (http://dx.doi.org/10.6084/m9.figshare.1281368).