Abstract
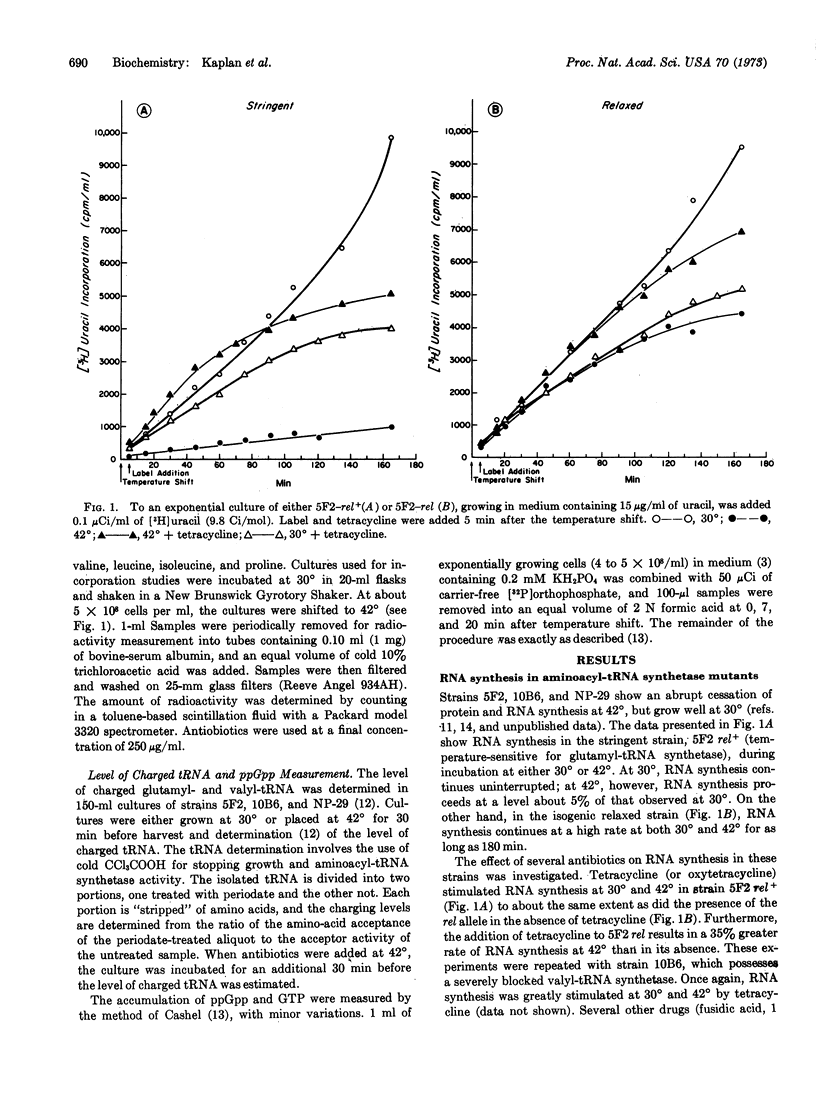
It has been possible to demonstrate the complete absence of either charged tRNAGlu or charged tRNAVal at 42° by the use of two stringent strains of E. coli, one temperature-sensitive for glutamyl-tRNA synthetase and the other temperature-sensitive for valyl-tRNA synthetase. In both strains, stable RNA synthesis ceases, and guanosine tetraphosphate accumulates upon incubation at the nonpermissive temperature. Unique among a series of antibiotics tested, only tetracycline was able to stimulate stable RNA synthesis and to cause disappearance of the guanosine nucleotide. In this regard tetracycline and the “relaxed” gene product appear to be analogous.
Keywords: RNA synthesis, guanosine tetraphosphate, tetracycline
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G., Suchanek M. C. Characterization of mutants of Escherichia coli temperature-sensitive for ribonucleic acid regulation: an unusual phenotype associated with a phenylalanyl transfer ribonucleic acid synthetase mutant. J Bacteriol. 1971 Nov;108(2):627–638. doi: 10.1128/jb.108.2.627-638.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Control of RNA synthesis in Escherichia coli. I. Amino acid dependence of the synthesis of the substrates of RNA polymerase. J Mol Biol. 1968 Jul 14;34(2):317–330. doi: 10.1016/0022-2836(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel D. H., Elkins B. N. The stimulation of ribonucleic acid synthesis by ribosome inhibitors in amino acid-starved Escherichia coli. Biochim Biophys Acta. 1968 Sep 24;166(2):466–474. doi: 10.1016/0005-2787(68)90234-7. [DOI] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. Formation of ppGpp in a relaxed and stringent strain of Escherichia coli during diauxie lag. Biochemistry. 1971 Oct 12;10(21):3980–3982. doi: 10.1021/bi00797a027. [DOI] [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. Levallorphan-induced accumulation of ppGpp in Escherichia coli. Biochemistry. 1972 Apr 11;11(8):1363–1366. doi: 10.1021/bi00758a006. [DOI] [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. MSI accumulation induced by sodium chloride. Biochemistry. 1972 Feb 15;11(4):615–618. doi: 10.1021/bi00754a023. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Anderson D. Selection of temperature-sensitive activating enzyme mutants in Escherichia coli. J Bacteriol. 1968 Mar;95(3):991–997. doi: 10.1128/jb.95.3.991-997.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. Correlation between the rate of ribonucleic acid synthesis and the level of valyl transfer ribonucleic acid in mutants of Escherichia coli. J Bacteriol. 1969 May;98(2):579–586. doi: 10.1128/jb.98.2.579-586.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Raué H. A., Ab G., Gruber M. Role of the ribosome in stringent control of bacterial RNA synthesis. Biochim Biophys Acta. 1971 Aug 12;246(1):157–160. doi: 10.1016/0005-2787(71)90081-5. [DOI] [PubMed] [Google Scholar]



