Abstract
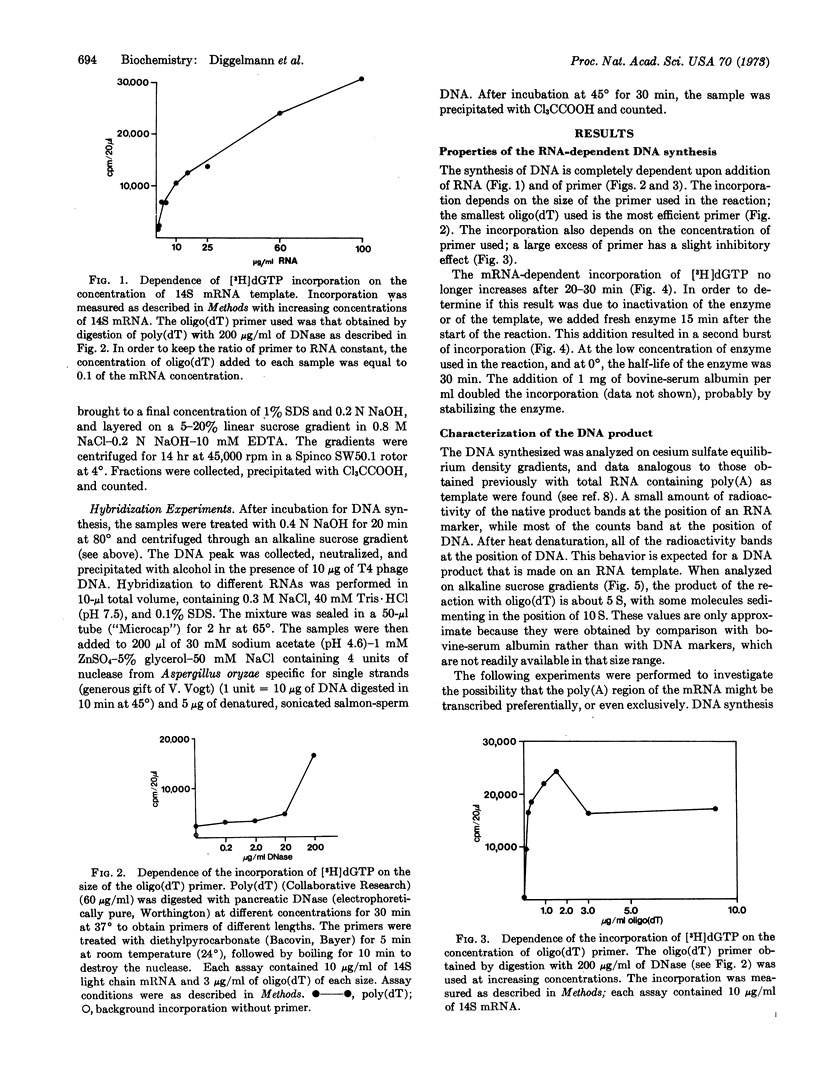
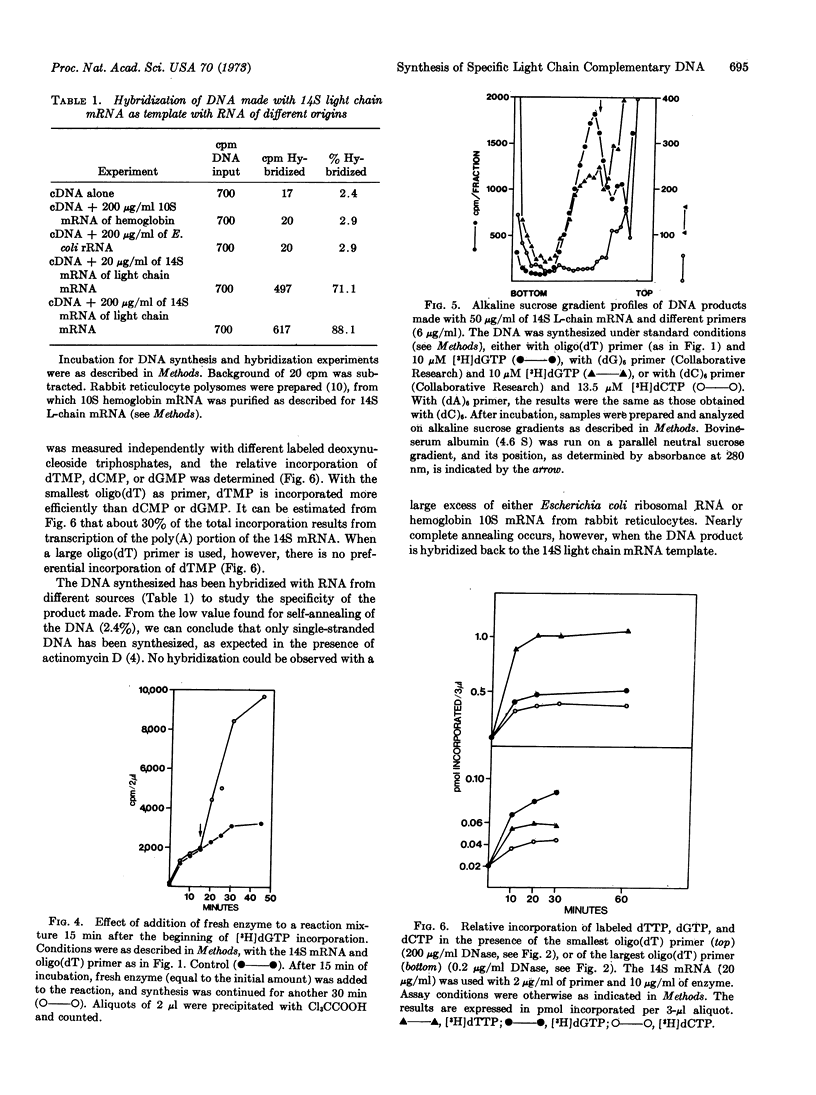
The 14S messenger RNA, which contains poly(adenylic acid), of MOPC 41 (mouse plasmocytoma) immunoglobulin light chain, purified to a single peak as shown by polyacrylamide gel electrophoresis, was used to synthesize complementary DNA with the RNA-dependent DNA polymerase of avian myeloblastosis virus. DNA synthesis is entirely dependent on added RNA template and oligo(dT) primer. Both the size and the concentration of the primer affect the reaction. The product behaves similarly to DNA during centrifugation in cesium sulfate density gradients. It is shown by hybridization that the DNA made is complementary to the purified template, light-chain mRNA. The high specific activity of the complementary DNA should make it suitable for gene-dosage experiments. According to alkaline sucrose gradient analyses, some complete complementary DNA transcripts of the 14S mRNA seem to be made. Oligo(dG) can also function as a primer for DNA synthesis, possibly by annealing to an internal cluster of cytidines in the mRNA, that correspond to the bases coding for amino-acids 119 and 120 of the MOPC 41 light chain.
Keywords: RNA-dependent DNA polymerase, hybridization, MOPC 41, mouse
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Zassenhaus P., Kates J. Synthesis of DNA from vaccinia messenger RNA templates. Nat New Biol. 1972 Aug 2;238(83):139–141. doi: 10.1038/newbio238139a0. [DOI] [PubMed] [Google Scholar]


