Abstract
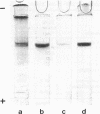
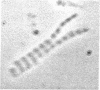
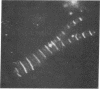
Published information on the properties of two proteins from chicken muscle, creatine kinase (MM-creatine kinase) and an M-line protein, suggested that they might be identical molecules. Different published procedures were used to purify the two proteins to homogeneity, and the properties of the two preparations were compared. Creatine kinase specific activity increased during purification of M-line protein, reaching a value comparable to that of purified MM-creatine kinase. The two proteins migrated identically in two electrophoretic systems and, after electrophoresis, both could be stained for creatine kinase activity. Double immunodiffusion tests with antibody prepared against MM-creatine kinase established the serological identity of the two protein preparations. Immunofluorescent studies showed that antiserum against MM-creatine kinase was bound in a regular pattern at the centers of the A-band regions of isolated myofibrils. These data show conclusively that the M-line protein and MM-creatine kinase are identical.
Keywords: myofibril, M-line protein, localization, isoenzymes
Full text
PDF
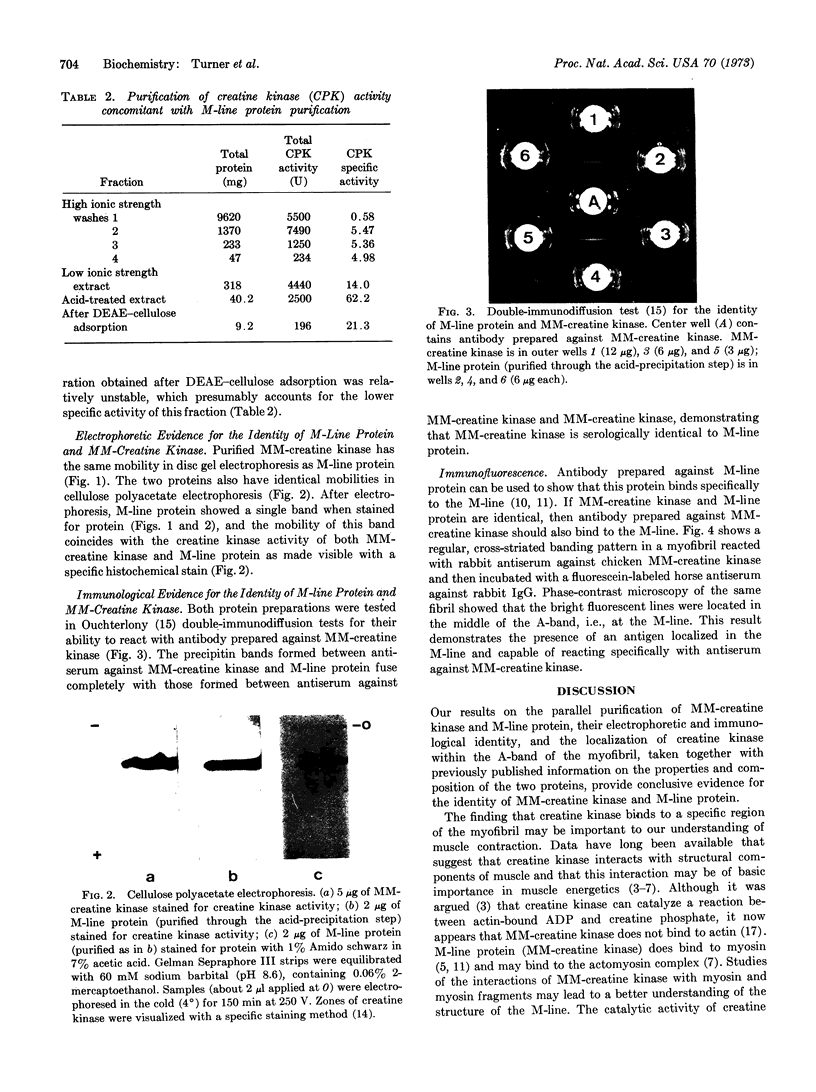

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H., Pette D. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968 Nov;6(2):163–171. doi: 10.1111/j.1432-1033.1968.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Dawson D. M., Eppenberger H. M., Kaplan N. O. Creatine kinase: evidence for a dimeric structure. Biochem Biophys Res Commun. 1965 Nov 22;21(4):346–353. doi: 10.1016/0006-291x(65)90200-7. [DOI] [PubMed] [Google Scholar]
- Dawson D. M., Eppenberger H. M., Kaplan N. O. The comparative enzymology of creatine kinases. II. Physical and chemical properties. J Biol Chem. 1967 Jan 25;242(2):210–217. [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Kodama A., Ebashi F. Troponin. I. Preparation and physiological function. J Biochem. 1968 Oct;64(4):465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Hooton B. T., Watts D. C. Adenosine 5 -triphosphate--creatine phosphotransferase from dystrophic mouse skeletal muscle. A genetic lesion associated with the catalytic-site thiol group. Biochem J. 1966 Sep;100(3):637–646. doi: 10.1042/bj1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Holt P. G., Knight J. O., Kakulas B. A. Incubation film technique for the histochemical localization of creatine kinase. Histochemie. 1971;26(2):120–125. doi: 10.1007/BF00293502. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morimoto K., Harrington W. F. Isolation and physical chemical properties of an M-line protein from skeletal muscle. J Biol Chem. 1972 May 25;247(10):3052–3061. [PubMed] [Google Scholar]
- Ottaway J. H. Evidence for binding of cytoplasmic creatine kinase to structural elements in heart muscle. Nature. 1967 Jul 29;215(5100):521–522. doi: 10.1038/215521b0. [DOI] [PubMed] [Google Scholar]
- Roy B. P., Laws J. F., Thomson A. R. Preparation and properties of creatine kinase from the breast muscle of normal and dystrophic chicken (Gallus domesticus). Biochem J. 1970 Nov;120(1):177–185. doi: 10.1042/bj1200177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROHMAN R. C. Studies on the enzymic interactions of the bound nucleotide of the bound nucleotide of the muscle protein actin. Biochim Biophys Acta. 1959 Apr;32:436–449. doi: 10.1016/0006-3002(59)90617-1. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V., Häcker W. The regulatory proteins of the myofibril. Characterization and biological activity of the calcium-sensitizing factor (troponin A). Biochem J. 1972 Jan;126(1):237–249. doi: 10.1042/bj1260237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin A. L., Karpati G., Bulcke J. A. Immunohistochemical localization of creatine phosphokinase in skeletal muscle. Proc Natl Acad Sci U S A. 1969 Sep;64(1):171–175. doi: 10.1073/pnas.64.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUNIK B., HOLTZER H. The distribution of muscle antigens in contracted myofibrils determined by fluorescein-labeled antibodies. J Biophys Biochem Cytol. 1961 Oct;11:67–75. doi: 10.1083/jcb.11.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI K., MASE R. Coupled reaction of creatine kinase and myosin A-adenosine triphosphatase. J Biol Chem. 1962 Feb;237:397–403. [PubMed] [Google Scholar]
- YAGI K., NODA L. Phosphate transfer to myofibrils by ATP-creatine transphosphorylase. Biochim Biophys Acta. 1960 Sep 23;43:249–259. doi: 10.1016/0006-3002(60)90435-2. [DOI] [PubMed] [Google Scholar]