Abstract
Background:
Kisspeptins (kp) activate a receptor coupled to a Gαq subunit (GPR54 or KiSS-1R) receptor to perform a variety of functions, including inhibition of cell motility, chemotaxis, and metastasis. In this study we have investigated whether kp-10, the most potent member of the kisspeptin family, can modulate CXCR4 (C-X-C chemokine receptor type 4) expression and mesenchymal stem cells (MSCs) migration that may influence the development of tumors.
Materials and Methods:
We compared the directional migration of MSCs treated with 10-100 or 500 nM kp-10 for 24 hours and no treated cells using an in vitro transmembrane migration assay. In addition, Chloromethylbenzamido Dialkylacarbocyanine (CM-Dil) labeled adipose-derived mesenchymal stem cells treated with 10-100 or 500 nM kp-10 and no treated cells were transfused via the tail vein to the melanoma tumor bearing C57BL/6 mice. After 24 hours, the mice were scarified, the tumors were dissected, and the tumor cell suspensions were analyzed by flow cytometry for detection of CM-Dil+ MSCs.
Results:
We have found that kp-10 increased the MSCs migration at 100 nM, while it decreased the MSCs migration at 500 nM, both in vitro and in vivo, with a significant increase of CXCR4 expression at 100 nM kp-10 compared to the no treated cells, but it had no significant difference between the various concentrations of kp-10.
Conclusion:
Thus, our data showed that kp-10 can differently affect MSCs migration in various concentrations, probably through different effects on CXCR4 expression in various concentrations.
Keywords: Kisspeptin-10, mesenchymal stem cell, CXCR4, migration
INTRODUCTION
Mesenchymal stem cells (MSCs) are multipotent cells that reside in several adult tissues such as bone marrow and adipose tissue. They are a relatively available pool of stem cells, able to differentiate into various cell types like osteoblasts, chondrocytes, fibroblasts, and adipocytes.[1] It has been shown that MSCs migrate to the sites of injury, ischemia, and tumor microenvironments. It has been recently identified that MSCs can modulate tumor progression. They exert their growth-promoting effects either via paracrine secretion of growth factors and anti-apoptotic factors or by differentiating into tumor-associated fibroblasts, which can enhance tumor growth, metastasis formation, and therapy resistance.[2,3,4]
The mechanisms by which MSCs migrate across the endothelium and home to the target tissues are not yet fully understood, however, extensive studies have shown that the migration of MSCs is dependent upon the different cytokine/receptor pairs SDF-1/CXCR4, SCF-c-Kit, HGF/c-Met, VEGF/VEGFR, PDGF/PDGFR, MCP-1/CCR2, and HMGB1/RAGE.[5]
A study on MSCs homing demonstrated that increased expression of CXCR4 (C-X-C chemokine receptor type 4) potentiates their movement into various tissues.[6] On the other hand SDF-1 (Stromal cell-drived factor-1), which is known as CXCL12 (Chemokine (C-X-C motif) ligand 12)[7] exerts pleiotropic effects, to regulate the essential processes of tumor metastasis, such as, motility of malignant cells, their chemoattraction and adhesion and also plays an important role in tumor vascularization.[8]
One of the metastasis suppressor genes that specially inhibits the spread of tumor cells to distant organs is the KISS1 gene, which encodes a hydrophobic protein of the 145 amino acid that can be broken into a 54 amino acid metastin (kp-54) and shorter peptides (kp-10, kp-13, kp-14), which are collectively called kisspeptins, in which, kp-10 is the shortest and the most active kisspeptin.[9,10,11] They activate their cognate receptor GPR54 in different target tissues to perform a variety of functions, including inhibition of metastasis and monitoring of reproductive function. Also, a couple of kisspeptin/GPR54 can influence cell signaling by interaction with other receptors such as CXCR4 and GNRH receptors.[12] Kiss-1 has been shown to suppress metastasis in melanoma, bladder cancer, gastric carcinoma, and thyroid, ovarian, endometrial, pancreatic, and esophageal cancers.[13,14,15,16,17,18,19,20]
As MSCs migration to the tumor microenvironment may potentially influence the development of a tumor, this study aims to investigate whether kp-10 can modulate MSCs migration and CXCR4 expression.
MATERIALS AND METHODS
Mesenchymal stem cell isolation and culture
The MSCs were isolated off the inguinal adipose tissues of a four- to five-week-old female C57BL/6 mice (n = 4) (Center for Laboratory Animals, Pasteur Institute, Iran). Under sterile conditions, the adipose tissue was cut with a scalpel into millimeter pieces. The tissues were washed using phosphate buffered saline (PBS; Invitrogen, USA) and were treated with 0.075% collagenase type I (Sigma-Aldrich, USA) for 45 minutes, at 37°C, by manual stirring every 10 minutes. The same volume of Dulbecco's Modified Eagle Media (DMEM) supplemented with 10% Fetal bovine serum (FBS) was added to stop the process of digestion. The cellular content was obtained after centrifugation at 1400 rpm for 10 minutes. Following this, the cells were washed and cultured in a culture medium supplemented with 0.50 mg/ml amphotericin B (Gibco, USA), at 37°C, in 5% CO2, and the adherent MSCs were grown in a culture medium supplemented with 1 ng/ml fibroblast growth factor (FGF-2) (Sigma-Aldrich, USA) and 10 ng/ml epidermal growth factor (EGF) (Sigma-Aldrich, USA) to maintain the cells in an undifferentiated state. The identification of mesenchymal stem cell markers was carried out using monoclonal antibodies against cells surface markers. Subsequently, after reaching 70-80% confluency, the cells were used for experiments.[21]
Melanoma cell culture
The mouse melanoma cell line B16F10 (cell bank of Pasteur Institute, Iran) was cultured in DMEM (Sigma-Aldrich, USA) supplemented with 10% FBS (Invitrogen, USA), 1 g/L glucose, 1% L-glutamine, and 1% penicillin-streptomycin (Invitrogen, USA), at 37°C, in a CO2 atmosphere.[22]
Mesenchymal stem cell migration assay in vitro
The in vitro migration assay was performed in the transwell inserts, with an 8-μm pore size, the membrane of which was uncoated (SPL, Germany). The cells were treated by PBS (Phosphate buffered saline) with 10-100 or 500 nM kp-10 (Anaspec, USA) for 24 hours and one other group of cells was not treated with kp-10. Then the cells were trypsinized and a number of 2 × 104 mesenchymal stem cells in 200 μl serum-free medium were seeded into the top chamber. Then, 30% of Fetal calf serum (FCS) or 4 × 104 melanoma cells in 500 μl medium were added to the lower chamber as a chemoattractant. After 24 hours of incubation at 37˚C, the non-migrated cells were removed from the top of the membrane, whereas, cells on the bottom of the membrane were trypsinized with 300 μl of trypsin and were suspended into the medium and counted in five minutes by flow cytometry (BD Calibur™ (Becton Dickinson)) after obtaining the appropriate gate. Results were obtained by three experiments.[23,24]
Mesenchymal stem cell migration assay in vivo
Female C57BL/6 mice, six to eight weeks of age were purchased from the Pasteur Institute of Iran. The animals were used according to the guidelines of the Institute of Health for the Care of Laboratory Animals and the Ethics Committee. The mice served as recipients for tumor inoculation of subcutaneous models of melanoma and injection of MSCs via the tail vein. The mice were divided into four groups and each one consisted of six mice. Melanoma cells numbering 106, in 200 μl volume of PBS were inoculated into the left back flank of the mice. To assess migration of the intravenously injected MSCs to melanoma tumors, CM-Dil-labeled MSCs were used on day 21. For each mouse about 5 × 105 MSCs were used, after 24 hours of treatment with 10-100 or 500 nM kp-10 (Anaspec, USA). The no-treated cells were stained with the CM-Dil (Molecular probes, USA) that was dissolved in dimethyl sulfoxide, and the cells were incubated at 37°C for five minutes, and subsequently at 4°C for 15 minutes (density, 1 μg CM-Dil per million cells), and then washed twice and re-suspended in PBS, after which the cells were injected into the tail vein of the mice. After 24 hours, the mice were sacrificed and the tumors were dissected and treated with 0.1% collagenase type I and then lysed in Red Blood Cell Lysis Solution (BD Biosciences). After washing twice with PBS, the cell suspensions were analyzed by flow cytometry for detection of CM-Dil+ MSCs.[25,26]
Flow cytometry analysis
Expression of the CXCR4 protein was determined in the MSCs of the fourth passage. A number of 5 × 104 mesenchymal stem cells, after 24 hours of treatment with 10-100 or 500 nM kp-10 (Anaspec, USA), and also no treated cells, were trypsinized. Then the cells were washed with PBS twice and incubated with 5 μl of anti-mouse CXCR4 PE (dilution 1:200, eBioscience, USA) or Rat IgG isotype control antibody for 45 minutes in the dark, under room temperature, and analyzed with flow cytometry (BD Calibur™ (Becton Dickinson)). The results were indicative of three experiments.[24,27]
Statistical analysis
Data were obtained from three experiments and were presented as mean ± SEM. Analysis of data was performed with SPSS 16.0 using the Kruskal-Wallis test followed by the Mann-Whitney test to determine a significant difference at P < 0.05 between the control and experimental groups.
RESULT
The effect of Kisspeptin-10 on MSCs migration
We have evaluated the effect of kp-10 on the migration of MSCs in vitro and in vivo. To assess migration in vitro, all the cells were treated with 10-100 or 500 nM kp-10 for 24 hours and the no-treated cells were seeded into the top chamber in a serum-free medium and allowed to migrate across an uncoated transwell membrane for 24 hours, toward 30% FCS or melanoma cells, and the number of migrated cells were counted by flow cytometry. To evaluate migration in vivo, 24 hours after the MSC injection, the number of CM-Dil+ MSCs in the cell suspension of the tumor was measured. There were statistically significant differences among the groups both in vitro and in vivo. The MSCs showed decreased migration ability toward 30% FCS at the highest concentration of kp-10 compared to all the groups and also toward the melanoma cells and the tumor compared to 10 and 100 nM kp-10, but migration at lower concentrations was increased, especially at 100 nM kp-10 compared to other groups, in all the experiments [Figures 1a–c] (P < 0.05).
Figure 1.
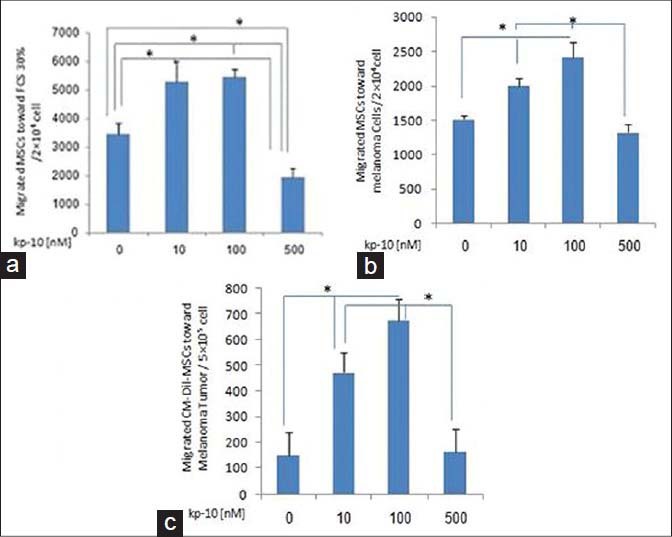
MSC Migration Assay. (A and B) Treated MSCs with 10-100 or 500 nM kp-10 for 24 hours and the no-treated cells were seeded into the top chamber of the Transwell (8 μm) in serum-free medium and 30% FCS or melanoma cells was added to the bottom camber. After incubation for 24 hours, at 37°C, the number of migrated cells across the membrane was counted by flow cytometry. All the results are indicative of three experiments. (c) Number of CM-Dil+ MSCs, 24 hours after injection, was measured in cell suspension of tumor by flow cytometry (n = 6). kp-10 significantly decreased migration of MSCs toward 30% FCS at the highest concentration compared to all groups and toward melanoma cells and tumor compared to 10 and 100 nM kp-10, but migration was increased at lower concentrations, especially at 100 nM kp-10, compared to other groups in all experiments. Results were compared between groups by the Kruskal-Wallis test (* P < 0.05)
Results were as follows in the in vitro and in vivo experiments, respectively: No treated cells; 3479 ± 378, 1515 ± 60, 152 ± 89, kp-10 (10 nM); 5315 ± 700, 2002 ± 112.1, 473 ± 79.7, kp-10 (100 nM); 5475 ± 270, 2408 ± 231.4, 676 ± 83.8, kp-10 (500 nM); 1950 ± 300, 1325 ± 125, 164 ± 90.5.
The effect of Kisspeptin-10 on the expression of CXCR4 in MSCs
The expression level of CXCR4 in treated cells with 10, 100, or 500 nM kp-10 for 24 hours and no-treated cells was determined after a 45-minute incubation with anti-mouse CXCR4 PE or Rat IgG isotype control antibody and was analyzed by flow cytometery. The expression of CXCR4 was significantly increased at 100 nM compared to the no-treated cells, but it had no significant difference between the various concentrations of kp-10 (P < 0.05) [Figure 2].
Figure 2.
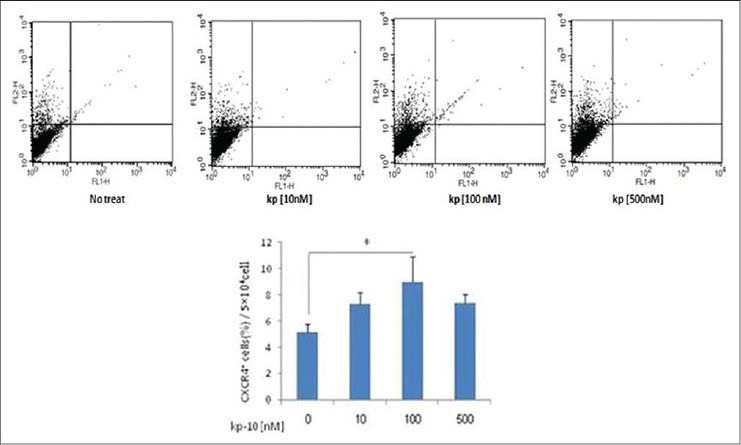
Expression of CXCR4 in MSCs. Dot Plot shows the distribution pattern of sample cells, FL2 shows CXCR4+ cells. For each samples, 5 × 104 cells were analyzed by flow cytometry. After 24 hours, the MSCs were treated with 10-100 or 500 nM kp-10 and the no-treated cells diluted in PBS and incubated with anti-mouse CXCR4 PE (dilution 1:200) or Rat IgG isotype control antibody for 45 minutes, in the dark, at room temperature. The fluorescence intensity was determined using flow cytometry. The expression of CXCR4 was increased at 100 nM compared to the no-treated cells, but it had no significant difference between various concentrations. The results are indicative of three experiments and are compared between groups with the Kruskal-Wallis test (*P < 0.05)
Results were as follows: No treated cells; 5.14 ± 0.66, kp-10 (10 nM); 7.3 ± 0.87, kp-10 (100 nM); 9 ± 1.9, kp-10 (500 nM); 7.3 ± 0.69.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the effects of kp-10 on MSCs migration and CXCR4 expression. Our results showed that kp-10 significantly increased MSCs migration at the 100 nM concentration, while it decreased MSCs migration at 500 nM and increased CXCR4 expression at 100 nM kp-10, compared to the no-treated cells, but it had no significant difference between the various concentrations of kp-10.
The KISS-1 gene is one of the 14 genes that has been shown to suppress metastasis of malignant cells and it only binds the GPCR.[27] KISS-1 can get involved with each step of the metastatic cascade including, invasion, angiogenesis, migration and homing, vessel infiltration, and proliferation. Kisspeptins activate their cognate receptor, GPR54, in diverse target tissues, to perform different functions, including, inhibition of tumor metastasis and control of reproductive function. However, there is reliable evidence to suggest that kisspeptin can activate many different signals through GPR54, which include activation of phospholipase c (plc) and accumulation of inositol triphosphate, mobility and mobilization of intracellular calcium, and activation of protein kinase c. Kisspeptin also has active pathways that are associated with MAPK, particularly ERK1/2, p38, and PI3K/Akt. In addition, couple kisspeptin/GPR54 can affect cell signaling by interaction with other receptors such as CXCR4 and GNRH receptors.[12,28]
The interesting finding of this study is that the highest concentration of kp-10 reduced MSC migration. It is shown that tumors produce a number of cytokines and chemokins, which act as ligands for receptors. Extracellular signals transmitted through intracellular signaling pathways include phosphoinositide 3-kinase (PI-3K)/Akt, MAPK/ERK1/2, and Rho GTP ase, to induce or modulate MSC migration.[29] The role of Rho A in the regulation of cell migration in cancer cells such as MDA-MB231 and MDA-MB435 is indicated.[9] Gu et al., 2003, have also shown that activation of the Rho GTPase status plays an important role in the proliferation and migration of the hematopoietic stem/progenitor cells.[6]
Cho et al., 2011, have demonstrated that Rho A is a downstream key protein of kiss1/kiss1R signaling for breast tumorogenecity and tumor progression and showed that 100 nmol/L concentration of kp-10 in the HEK293 cells increases the GTP-bound Rho A, thus it seems that kiss1/kiss1r signaling is required for cell growth and motility.[30] Furthermore, another study has reported that kp-10 stimulates invasion of MDA-MB231 and Hs578T breast cancer cells via an increase in MMP-9 secretion and activity, such that maximum invasion and migration was observed by 10 to 100 nM kp-10.[9] However, some other studies reported that kp-10 reduced cell migration in response to cellular stimuli in a dose-dependent manner; kp-10 at 1 and 10 μM concentrations inhibited migration and invasion of MDA-MB-231 and MCF-7 cells via activation of TNFα-RhoA-NF-kB.[31] In addition, 1 and 10 μM metastin induced ERK1 and P38 activation in PANC-1 cells and resulted in decreased cell migration.[19] In another study, it was reported that kp-10 at a dose window of 10-9 to 10-11 M reduced breast cancer cell migration and Akt activation.[32] So, it seems that there is a distinctive dose-dependent pattern effect of kisspeptin on cell migration and the underlying signal transduction pathways.[12] Furthermore, it seems that KISS1/KISS1R signaling could be cell-type-specific.[30]
Another finding was that kp-10 significantly increased CXCR4 expression at 100 nM kp-10 compared to the no-treated cells, but it had no significant difference between the various concentrations of kp-10.
Several studies have shown that mesenchymal stem cells have a very high tendency toward tumor cells and it appears that SDF-1 and CXCR4 receptor play a major role in modulating the mobility of MSCs.[6,8] It has been reported that kp-10 decreases the expression of CXCR4 in breast cancer cells at a dose of 10−8 to 10−7 M.
It increased CXCR4 expression at 10−12 M kp-10, but at the 10−11 and 10−9 M kp-10 concentration no change in CXCR4 expression was observed.[32] On the other hand, Navenot et al., 2005, have shown that kp-10 blocked chemotaxis of tumor cells expressing CXCR4 in response to SDF-1/CXCL12, but they demonstrated that pretreatment with 100 nM kp-10 did not induce the down-modulation of CXCR4 expression on the cell surface, reduce affinity for SDF-1/CXCL12 ligand or alter activation of the stimulated Gαi by this ligand.[27]
According to our knowledge this study is the first analysis of the effect of kp-10 on the levels of CXCR4 expression in MSCs. Similar to our results, Navenot and colleagues have shown that kp-10 does not induce decreased expression of CXCR4. Thus in the present study, we suggest that kp-10 can differently affect MSC migration in various concentrations, probably through different effects on CXCR4 expression in various concentrations, or maybe through distinct effects of different concentrations of kisspeptin on other signaling pathways such as MAPK/ERK1/2, Rho GTPase, Akt, and MMP-9 activity. To observe this process we require further studies.
CONCLUSION
Our data showed that kp-10 can differently affect MSCs migration in various concentrations, probably through different effects on CXCR4 expression in various concentrations.
ACKNOWLEDGMENTS
This study was funded by the Isfahan University of Medical Sciences (IUMS).
Footnotes
Source of Support: Isfahan University of Medical Sciences (IUMS)
Conflict of Interest: None declared.
REFERENCES
- 1.Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901–6. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–70. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 3.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–9. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson MR, Chae SS, Jain RK, Duda DG. Direct evidence for lineage-dependent effects of bone marrow stromal cells on tumor progression. Am J Cancer Res. 2011;1:144–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Deak E, Seifried E, Henschler R. Homing pathways of mesenchymal stromal cells (MSCs) and their role in clinical applications. Int Rev Immunol. 2010;29:514–29. doi: 10.3109/08830185.2010.498931. [DOI] [PubMed] [Google Scholar]
- 6.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–91. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 7.Burger JA, Kipps TJ. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 8.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–45. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 9.Zajac M, Law J, Cvetkovic DD, Pampillo M, McColl L, Pape C, et al. GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS One. 2011;6:e21599. doi: 10.1371/journal.pone.0021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30:4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 12.Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: Do multiple signals underlie function diversity? Peptides. 2009;30:10–5. doi: 10.1016/j.peptides.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–83. doi: 10.1158/1078-0432.ccr-1519-02. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer: Loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol. 2003;162:609–17. doi: 10.1016/S0002-9440(10)63854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–72. doi: 10.1002/ijc.20357. [DOI] [PubMed] [Google Scholar]
- 16.Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab. 2002;87:2399. doi: 10.1210/jcem.87.5.8626. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–76. doi: 10.1007/s10585-005-8186-4. [DOI] [PubMed] [Google Scholar]
- 18.Jiang T, Zhang SL, Lin B, Meng LR, Gao H. Expression and clinical significance of KISS-1 and GPR54 mRNA in endometrial carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:229–31. [PubMed] [Google Scholar]
- 19.Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, et al. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res. 2001;61:7422–5. [PubMed] [Google Scholar]
- 21.Skurk T, Ecklebe S, Hauner H. A novel technique to propagate primary human preadipocytes without loss of differentiation capacity. Obesity (Silver Spring) 2007;15:2925–31. doi: 10.1038/oby.2007.349. [DOI] [PubMed] [Google Scholar]
- 22.Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–8. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Yusenko MV, Kovacs G. Lack of KISS1R expression is associated with rapid progression of conventional renal cell carcinomas. J Pathol. 2011;223:46–53. doi: 10.1002/path.2764. [DOI] [PubMed] [Google Scholar]
- 24.Huang YL, Qiu RF, Mai WY, Kuang J, Cai XY, Dong YG, et al. Effects of insulin-like growth factor-1 on the properties of mesenchymal stem cells in vitro. J Zhejiang Univ Sci B. 2012;13:20–8. doi: 10.1631/jzus.B1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu KX, Wang MH, Fan C, Wang L, Guo M, Ai HS. CM-DiI labeled mesenchymal stem cells homed to thymus inducing immune recovery of mice after haploidentical bone marrow transplantation. Int Immunopharmacol. 2011;11:1265–70. doi: 10.1016/j.intimp.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navenot JM, Wang Z, Chopin M, Fujii N, Peiper SC. Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: A potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Res. 2005;65:10450–6. doi: 10.1158/0008-5472.CAN-05-1757. [DOI] [PubMed] [Google Scholar]
- 28.Navenot JM, Fujii N, Peiper SC. Activation of Rho and Rho-associated kinase by GPR54 and KiSS1 metastasis suppressor gene product induces changes of cell morphology and contributes to apoptosis. Mol Pharmacol. 2009;75:1300–6. doi: 10.1124/mol.109.055095. [DOI] [PubMed] [Google Scholar]
- 29.Dai LJ, Moniri MR, Zeng ZR, Zhou JX, Rayat J, Warnock GL. Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett. 2011;305:8–20. doi: 10.1016/j.canlet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Cho SG, Wang Y, Rodriguez M, Tan K, Zhang W, Luo J, et al. Haploinsufficiency in the prometastasis Kiss1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer Res. 2011;71:6535–46. doi: 10.1158/0008-5472.CAN-11-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SG, Li D, Stafford LJ, Luo J, Rodriguez-Villanueva M, Wang Y, et al. KiSS1 suppresses TNFalpha-induced breast cancer cell invasion via an inhibition of RhoA-mediated NF-kappaB activation. J Cell Biochem. 2009;107:1139–49. doi: 10.1002/jcb.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olbrich T, Ziegler E, Türk G, Schubert A, Emons G, Gründker C. Kisspeptin-10 inhibits bone-directed migration of GPR54-positive breast cancer cells: Evidence for a dose-window effect. Gynecol Oncol. 2010;119:571–8. doi: 10.1016/j.ygyno.2010.08.018. [DOI] [PubMed] [Google Scholar]


