Abstract
Background:
The present study was designed to evaluate hypertension and dyslipidemia in prediabetic subjects with a family history of type 2 diabetes (first-degree relatives), and they were compared with the normal glucose-tolerance subjects.
Materials and Methods:
Three thousand and eighty-six (788 men and 2298 women) subjects were selected from a consecutive sample of patients with Impaired Glucose Tolerance (IGT), Impaired Fasting Glucose (IFG), and Combined (IFG and IGT), and their first-degree relatives formed the control group. Potential risk factors for diabetes including age, gender, body size, HbA1c, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides, blood pressure (BP), urine microalbumin, and family and personal medical history were assessed.
Results:
The studied participants included 300 IGT patients (9.7%), 625 IFG patients (44.9%), 411 combined patients (13.3%), and 1750 (56.7%) normal subjects. Aging led to increase in hypertension. Increase in body mass index (BMI) led to an increase in the prevalence of hypertension significantly in all groups. The mean triglyceride in the normal group was different in comparison with that of the IGT (P < 0.05) and combined (P < 0.001) groups. Differences in total cholesterol were observed in the normal group when compared with the IGT (P < 0.05) and combined (P < 0.001) groups, and of the combined group in comparison with the IGT (P < 0.05) group. The difference in LDL level was related to the combined group in comparison with IGT, marginally (P < 0.1), and normal in comparison with the combined group (P < 0.05).
Conclusion:
Prevalence of hypertension was not significantly different between the groups, however, in prediabetic patients it was higher than in the normal group, and prevalence of dyslipidemia in prediabetic subjects was significantly higher than in the normal group.
Keywords: Dyslipidemia, glucose tolerance, hypertension, prediabetic
INTRODUCTION
Prevalence of diabetes, which is associated with increase in morbidity and mortality, is increasing and it is one of the major healthcare problems in the world.[1] According to the pathogenesis and natural history of diabetes, it has a prolonged prediabetic phase.[2] Studies have shown that heart disease and atherogenic progression in diabetic patients have presented in the prediabetic phase.[3] Prediabetes generally refers to an intermediate stage between the clinical entity of type 2 diabetes and normal glucose levels.[4,5]
Prediabetes increases the risk of developing diabetes. Prospective and observational studies showed that diabetes developed approximately in 25-40% of prediabetic patients after three to eight years.[6,7,8] Prediabetes is considered as a risk factor for cardiovascular disease and macrovascular disease development and is not only a significant risk factor for progression of type 2 diabetes. Evidence advocates that prediabetic patients have a significantly greater risk for cardiometabolic disease and death, when compared with normal subjects.[9,10,11,12]
Prediabetic individuals are more likely to be obese than others, and unrelated to their age or body mass index, are additionally expected to have multiple risk factors for cardiovascular disease (CVD), including dyslipidemia and hypertension.[3] The goal of blood pressure is the same in diabetes, as in prediabetic patients. It is noticeable that hypertension and dyslipidemia, as important risk factors of CVD, are common in prediabetic states and should be managed as aggressively.[13]
The exact relationship between prediabetes and CVD is still unclear and controversial. However, studies show the relationship between prediabetes and morbidity and mortality.[14,15,16,17]
Screening program, preventive strategy, and risk factor detection are important for prediabetic patients. Hypertension and dyslipidemia are well-recognized markers of cardiovascular risk. The present study is designed to evaluate hypertension and dyslipidemia in prediabetic subjects with a family history of type 2 diabetes (first-degree relative) and compare them with those of normal subjects.
MATERIALS AND METHODS
This case-control study was carried out on 3086 (788 men and 2298 women) patients, with first-degree relatives with diabetes, in an Outpatient Clinic in Isfahan Endocrinology and Metabolism Research Center (IEMRC), Iran. The Ethics Committee of Isfahan University of Medical Sciences had approved the study and an informed consent was obtained from each participant.
Patients with Impaired Fasting Glucose (IFG) or Impaired Glucose Tolerance (IGT) and patients with a combination of these disorders (IFG and IGT) were selected as the case groups and their first-degree relatives were chosen as the control group. The control group included siblings and children of patients with type 2 diabetes.
The participants completed the laboratory tests, including standard 75 g -two-hour oral glucose tolerance test (OGTT), HbA1c (measured with the help of a spectrophotometer), microalbuminuria, serum creatinine, triglycerides cholesterol, HDL (measured using standardized procedures), LDL (calculated by the Friedwald equation, provided the total triglyceride did not exceed 400 mg/dL),[14] and BP (systolic and diastolic), at registration. In addition, a questionnaire on health status and the various potential risk factors of diabetes was completed. This questionnaire included gender, age at diagnosis, age, educational level, duration of diabetes (time between diagnosis and baseline examination), BMI (weight/height2 (kg/m2)), and smoking status (never, current).
Patients with IGT, IFG, combined, and diabetes were identified from the baseline and follow-up OGTTs were performed according to American Diabetes Association criteria.[17] For the present study, the analyses were limited to the IGT, IFG, combined, and patients’ first-degree relatives groups.
According to American Diabetes Association (ADA) criteria, the participants were divided into groups as follows: Normal glucose tolerance (NGT) was defined as fasting plasma glucose (FPG) of less than 100 mg/dL and two-hour post load glucose of <140 mg/dL.
The prediabetic state was defined as a state with an Impaired Fasting Glucose (IFG) (a fasting plasma glucose (FPG) of 100-125 mg/dL) and/or an impaired glucose tolerance (IGT) (two-hour post load glucose of 140-199 mg/dL).[17] All blood sampling procedures were performed in the Central Laboratory of the Isfahan Endocrine and Metabolism Research Center.
Height and weight were measured in light clothes and without shoes, using the standard apparatus. The weight was measured with a 0.1 kg accuracy on a calibrated beam scale. The height was measured with a 0.5 cm accuracy with a measuring tape. The height was measured only at the start of the study. The systolic and diastolic blood pressures were measured after 10 minutes of resting by using a calibrated mercury sphygmomanometer and standard techniques. All clinical measurements for patients and normal participants were made using the same standardized protocol.
Statistical analysis
The quantitative variables are represented as a mean (±SD or SE), while the qualitative variables as a number (percent). The Chi-square test or linear-by-linear Chi-square test as appropriate, were used for comparing the prevalence rates and the association between the qualitative variables, respectively. For age, sex, and body mass index (BMI), the Multivariate Analysis of Variance (MANOVA) stratified analysis was considered for comparing the quantitative dependent variables along with the Bonferroni post-hoc test. All statistical calculations were carried out with the SPSS for Windows (SPSS Inc., Chicago, IL, USA). The level of significance was considered to be less than 0.05.
RESULTS
According to the baseline characteristics of the 3086 participants, they included 300 (9.7%) IFG patients, 625 (44.9%) IGT patients, 411 (13.3%) combined patients, and 1750 normal subjects; the control group had older participants compared to the others. The number of females was significantly more than men in all the groups in the study. There was no significant difference in BMI across all groups.
Table 1 shows the prevalence of hypertension in the glucose-tolerance groups, in terms of age, sex, and BMI variables. Aging led to an increase in hypertension. However, it was significant only in the normal group. There was a significant association between the BMI and prevalence of hypertension in all groups. An increase in BMI led to an increase in the prevalence of hypertension.
Table 1.
The prevalence of hypertension in the glucose-tolerance groups in terms of age, sex, and BMI variables
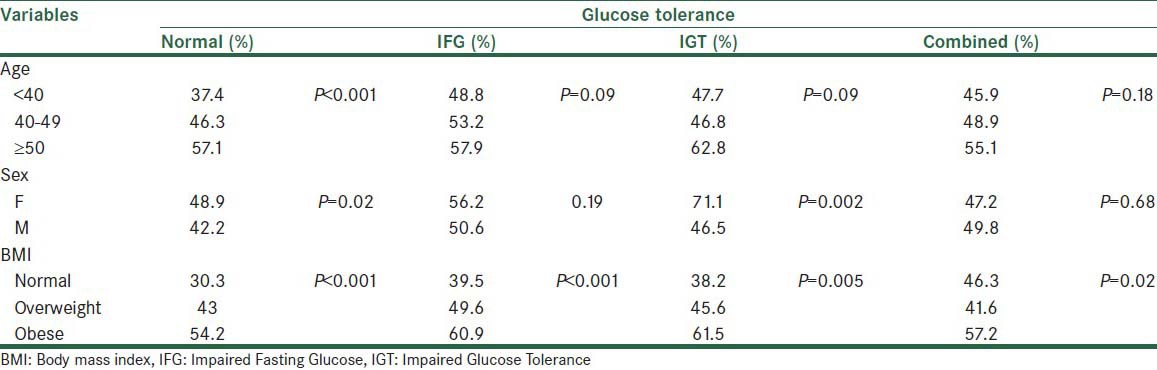
The triglyceride (TG) level in glucose-tolerance groups was significantly more than in the normal group. The effect of the age variable was statistically significant at P < 0.001. An older age led to higher TG levels in all groups. There was no significant interaction effect between the age and glucose tolerance status on the triglyceride level.
Triglyceride level difference in the level of the sex variable was statistically significant (P < 0.001). The mean triglyceride level in women was higher than in men, in all levels of the glucose tolerance variable. The difference was more notable in the combined and IGT groups. There was no significant interaction effect between sex and the glucose-tolerance groups. Also, the BMI affected the TG level significantly in all groups [Table 2].
Table 2.
The mean triglyceride level (standard error) in normal and glucose-tolerance groups in different levels of age, sex and BMI variables
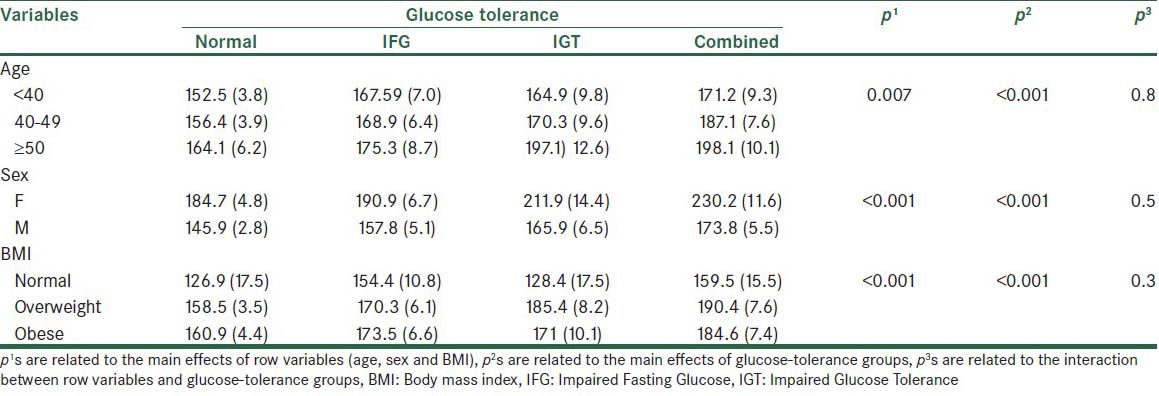
The total cholesterol level was higher in older and obese subjects. The result of Bonferroni post-hoc test demonstrated that the mentioned difference is related to the normal with IGT (P < 0.05), normal with combined (P < 0.001), and combined with IGT (P < 0.05) groups. In addition, the effect of the age variable was statistically significant. No significant interaction effect was found between the age and glucose tolerance status, on the total cholesterol level. The total cholesterol level in women was higher than in men in most levels of the glucose tolerance variable; however, no statistically significant difference was detected. There was no significant interaction effect between sex and the glucose-tolerance groups [Table 3].
Table 3.
The mean total cholesterol levels (standard error) in the normal and glucose-tolerance groups at different levels of age, sex, and BMI variables
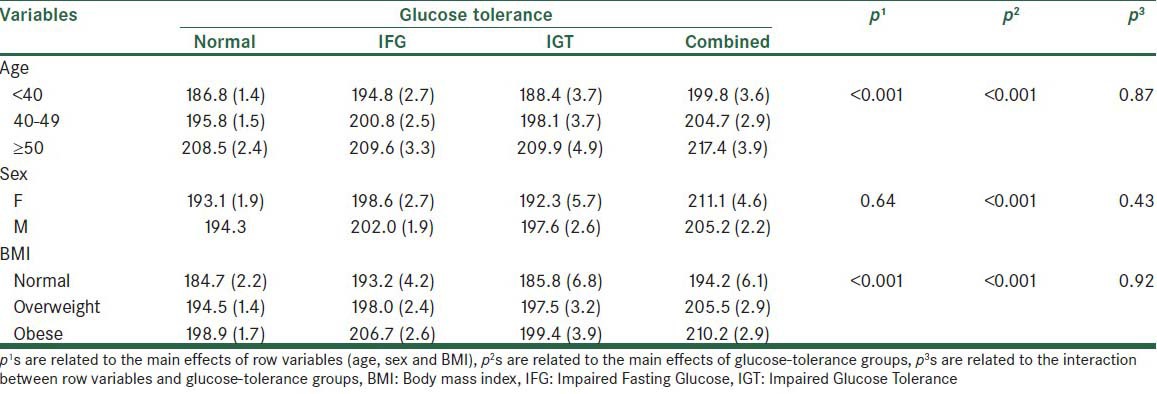
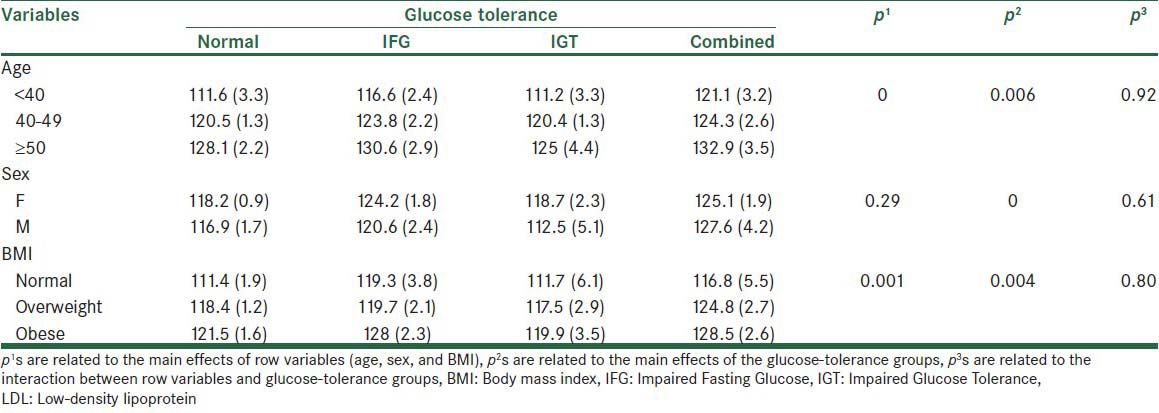
Table 4 shows the mean LDL level in the normal and glucose-tolerance groups at different levels of age, sex, and BMI variables. The effect of the age variable was statistically significant across groups. It was also shown by the Bonferroni test the all pairwise comparison age groups were significantly different. The interaction effect between the age and glucose-tolerance status on the LDL level was not statistically significant. Although the LDL level in all glucose-tolerance groups was higher in men than in women, the differences were not statistically significant. Also, no significant interaction effect between the sex and glucose-tolerance groups was observed. BMI led to a significant effect. The difference was attributed to normal with obese (P < 0.01) and to obese with overweight (P < 0.05) groups, by using the Bonferroni post-hoc test.
Table 4.
The mean LDL levels (standard error) in the normal and glucose-tolerance groups at different levels of age, sex, and BMI variables
DISCUSSION
Prevalence of hypertension and dyslipidemia in prediabetic subjects with a family history of type 2 diabetes (first-degree relative) in comparison with the normal groups was questioned in the present study. Results of our study showed that the prevalence of dyslipidemia in prediabetic subjects was significantly more than in the normal group, but hypertension in prediabetic patients was similar to the normal group. IFG and IGT were associated with obesity and dyslipidemia, such as, high triglycerides, high total cholesterol, and hypertension.
Findings of the previous studies were inconsistent. Some studies showed lack of difference in the lipid profile between the IFG and normal subjects. However, another reported similar changes of lipid profile in prediabetic patients and normal subjects.[18,19,20]
These wide fluctuations may be partially due to the different cut-off points that were used for defining IFG, according to American Diabetes Association (ADA) and World Health Organization (WHO). However, most studies showed that the IFG group presented significant pro-atherogenic changes in all lipid parameters in comparison with the NGT group.
Our study showed that the prevalence of IFG was lower than IGT. Similarly, IGT was found to be more prevalent compared to IFG in Mauritius (USA) and in the Pima Indians.[21,22]
There are few studies that reported the prevalence of hypertension and dyslipidemia in prediabetic subjects with a family history of diabetes, however, results of the present study showed that aging led to an increase in the prevalence of hypertension, and male patients showed a greater prevalence of hypertension than female patients. With respect to the lipid profile, the triglyceride level was higher in the IGT and combined groups compared to the normal group. Total cholesterol was higher in the IGT and combined groups compared to the normal group, and LDL cholesterol was significant between the IGT and combined groups and the normal group. An important result was that all the assessed variables were similar in the IFG group with the normal group, and it was possible that these differences between the groups were related to the IGT.
Obesity, hypertension, and dyslipidemia are important cardiovascular risk factors in prediabetic patients. Our results showed differences between prediabetic patients and normal subjects, therefore, assessing and treating these risk factors is an important aspect for reducing cardio metabolic risk.
In summary, the prevalence of hypertension and dyslipidemia in prediabetic patients in comparison with normal groups was assessed in the present study, however, prevalence of hypertension was not significantly different between the groups, but in prediabetic patients it was higher than in the normal group. Prevalence of dyslipidemia in prediabetic subjects was significantly higher than in the normal group. However, further studies need to be conducted, to assess hypertension and dyslipidemia in prediabetic subjects based on the glucose-tolerance status in these subjects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Deedwania PC, Fonseca VA. Diabetes, prediabetes and cardiovascular risk: Shifting the paradigm. Am J Med. 2005;11:939–47. doi: 10.1016/j.amjmed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: A systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–7. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association, Position statement: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42–7. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 5.Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: When do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14:933–46. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG. Screening and diagnosis of prediabetes: Where are we headed? Diabetes Obes Metab. 2007;9(Suppl 1):12–6. doi: 10.1111/j.1463-1326.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) trial. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomized controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 8.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–55. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4–36. [PubMed] [Google Scholar]
- 10.Ferrannini E, Gastaldelli A, Iozzo P. Pathophysiology of prediabetes. (vii-viii).Med Clin North Am. 2011;95:327–39. doi: 10.1016/j.mcna.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Grundy SM, Wang W, Smith SC, Jr, Vega GL, Wu Z, et al. Ten-year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J. 2007;153:552–8. doi: 10.1016/j.ahj.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. AACE Task Force for Developing Diabetes Comprehensive Care Plan. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 13.Brunner EJ, Shipley MJ, Witte DR, Fuller JH, Marmot MG. Relation between blood glucose and coronary mortality over 33 years in the Whitehall study. Diabetes Care. 2006;29:26–31. doi: 10.2337/diacare.29.01.06.dc05-1405. [DOI] [PubMed] [Google Scholar]
- 14.Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, Simoons M, et al. Euro Heart Survey Investigators. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004;25:1880–90. doi: 10.1016/j.ehj.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Petersen JL, McGuire DK. Impaired glucose tolerance and impaired fasting glucose-a review of diagnosis, clinical implications and management. Diab Vasc Dis Res. 2005;2:9–15. doi: 10.3132/dvdr.2005.007. [DOI] [PubMed] [Google Scholar]
- 16.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian diabetes obesity, and lifestyle study (AusDiab) Circulation. 2007;116:151–7. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of Medical Care in Diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LK, Lin MH, Chen ZJ, Hwang SJ, Tsai ST, Chiou ST. Metabolic characteristics and insulin resistance of impaired fasting glucose among the middle-aged and elderly Taiwanese. Diabetes Res Clin Pract. 2006;71:170–6. doi: 10.1016/j.diabres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Blake DR, Meigs JB, Muller DC, Najjar SS, Andres R, Nathan DM. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors; Results from the Baltimore Longitudinal Study on Aging. Diabetes. 2004;53:2095–100. doi: 10.2337/diabetes.53.8.2095. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JE, Zimmet PZ, de Courten M, Dowse GK, Chitson P, Gareeboo H, et al. Impaired fasting glucose or impaired glucose tolerance: What best predicts future diabetes in Mauritius? Diabetes Care. 1999;22:399–402. doi: 10.2337/diacare.22.3.399. [DOI] [PubMed] [Google Scholar]
- 21.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 22.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–12. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]


