Abstract
The use of animal models has been invaluable for studying the pathogenesis of Mycobacterium tuberculosis infection, as well as for testing the efficacy of vaccines and drug regimens for tuberculosis. Among the applied animal models, nonhuman primates, particularly macaques, share the greatest anatomical and physiological similarities with humans. As such, macaque models have been used for investigating tuberculosis pathogenesis and preclinical testing of drugs and vaccines. This review focuses on published major studies which illustrate how the rhesus and cynomolgus macaques have enriched and may continue to advance the field of global tuberculosis research.
INTRODUCTION
About one-third of the population worldwide is infected with Mycobacterium tuberculosis, while nearly 9 million cases of active tuberculosis (TB) are reported annually (1). Hence, the need for improved preventative and treatment strategies against TB is ever-increasing. In an attempt to globally control TB, researchers have employed multiple animal models (mice, guinea pigs, rabbits, cows, nonhuman primates, and others) for testing novel experimental vaccines and therapies for TB (2, 3). The use of nonhuman primates (NHP), especially cynomolgus macaques (CM; Macaca fascicularis, also called long-tailed macaques, a species of Old World monkeys native to Southeast Asia) and rhesus macaques (RM; Macaca mulatta, a species of Old World monkeys native to Asia; most experimental models are from India or China), has led to significant advances in TB research due to their inherent commonalities with humans, as illustrated in previous reviews in this subject area (4–6). By using the macaque model of TB, we can gain even greater insights into ways to prevent M. tuberculosis infection and disease progression.
JUSTIFICATION FOR MACAQUES IN TB RESEARCH
Macaques exhibit remarkable similarities to humans in virtually every aspect of their anatomy and physiology (7–9). As such, macaques respond similarly to many human immunological, pathological, and drug agents, providing a tremendous advantage over other animal models (6, 10). The literature shows that macaques and humans share extensive clinical manifestations of TB, including pulmonary and extrapulmonary signs and symptoms (6, 10). Clinicians and researchers can monitor the disease course in macaques by measuring nearly identical parameters tested in humans, ranging from skin and blood tests to radiographic imaging and body fluid samples (Table 1). In addition, multidrug chemotherapy for TB provides effective treatment in both humans and macaques (11, 12). Furthermore, as in humans, Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination exhibits variable efficacy in macaques of even the same species (13–15). Table 1 highlights the similarities and differences in M. tuberculosis infection between humans and the rhesus macaques and cynomolgus macaques.
TABLE 1.
Comparison of TB in humans versus rhesus and cynomolgus macaquesa
| Parameter | Humans | Rhesus macaques | Cynomolgus macaques |
|---|---|---|---|
| Clinical manifestations | |||
| Rate of disease progressionb | Acute ≪ latent (10% vs 90%) | Acute ≫ latent (90% vs 10%) | Acute > latent (60% vs 40%) |
| Presence of active/chronic infection symptom | |||
| Cough | + | + | + |
| Bloody sputum | + | + | + |
| Increased body temp | + | + | + |
| Wt loss | + | + | + |
| Presence of latent infection symptoms | |||
| No clinical signs | + | + | + |
| Activated by coinfection (HIV or SIV) | + | + | + |
| Clinical tests | |||
| Skin tests | |||
| PPD | + | + | + |
| Old tuberculin test | + | + | + |
| Blood tests | |||
| ELISA, ELISpot | + | + | + |
| Quantiferon-TB Gold | + | + | + |
| Primagram | − | + | + |
| CBC, ESR, CRP, LT | + | + | + |
| Imaging (chest X-Ray, MRI, PET/CT) | + | + | + |
| Fluid sampling (BAL, gastric aspirate) | + | + | + |
| Pathology | |||
| Caseous granulomas | + | + | + |
| Calcification | +/− | +/− | +/− |
| Fibrous capsule | +/− | +/− | +/− |
| Pulmonary cavities | + | + | + |
| Disseminated lesions | +/− | +/− | +/− |
The majority of macaques, particularly the rhesus species, develop acute or active TB after artificial infection, whereas 90% of infected humans have latent TB. Chronic infection is defined as persistent signs of active disease, radiographic involvement, or culture positivity. Although the PPD and old tuberculin skin tests are used in both humans and macaques, these diagnostic exams are less reliable in macaques than in humans (69). Also, macaques exhibit a more random than apical distribution of pulmonary cavities. Abbreviations: BAL, bronchoalveolar lavage; CBC, complete blood count; CRP, C-reactive protein; LT, lymphocyte transformation; rBCG, recombinant bacillus Calmette-Guérin, +, positive for the indicated finding or functional modality; −, absent finding or unused modality; +/−, a variable finding.
Acute disease lasts weeks to months, and latent disease lasts months to years. Percentages (in parentheses) indicate the global percentage of the species infected with M. tuberculosis.
HISTORICAL OUTLOOK ON MACAQUE MODELS
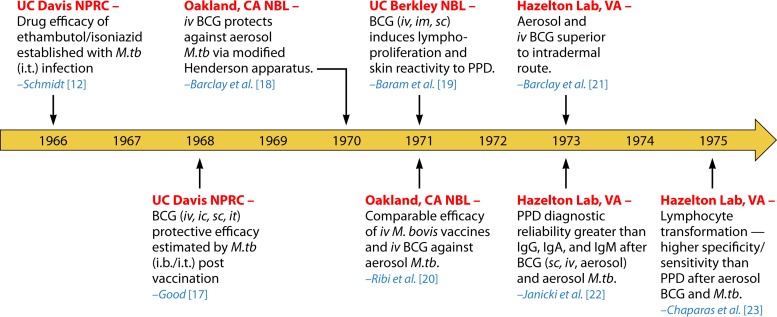
The use of NHP models to study M. tuberculosis infection traces back to published literature from the 1960s. This “Golden Age” of TB research using NHP, performed through the 1970s, generated valuable data on the evolving BCG vaccine (16), as well as one report on the TB drug efficacies of ethambutol and isoniazid (12). The majority of the studies focused on the BCG-induced immune reactions and vaccine efficacy (17–23). All pertinent studies published during this era used Indian RM models, which underwent intrabronchial (i.b.), intratracheal (i.t.), or aerosol infection with M. tuberculosis. The estimated mycobacterial retention rate in mammalian lungs after aerosol exposure was derived from prior animal models, including macaques exposed to anthrax spores, as well as guinea pigs and mice infected with M. tuberculosis (24). Figure 1 recaps the major published articles of NHP models with experimental M. tuberculosis infection during this time period (12, 17–23).
FIG 1.
The “Golden Age” of TB research using rhesus macaques. The timeline illustrates major studies of experimental M. tuberculosis infection in Indian rhesus macaques during the so-called Golden Age from the 1960s to 1970s (12, 17–23). Events are organized chronologically by year of publication. Thus, the 10-year study by Good (17) actually began before the first reported investigation in 1966. Locations of the experiments (red) and the corresponding author (blue) are indicated. Abbreviations: ic, intracutaneous; im, intramuscular; iv, intravenous; sc, subcutaneous; M.tb, M. tuberculosis; NBL, Naval Biological Laboratories; UC, University of California.
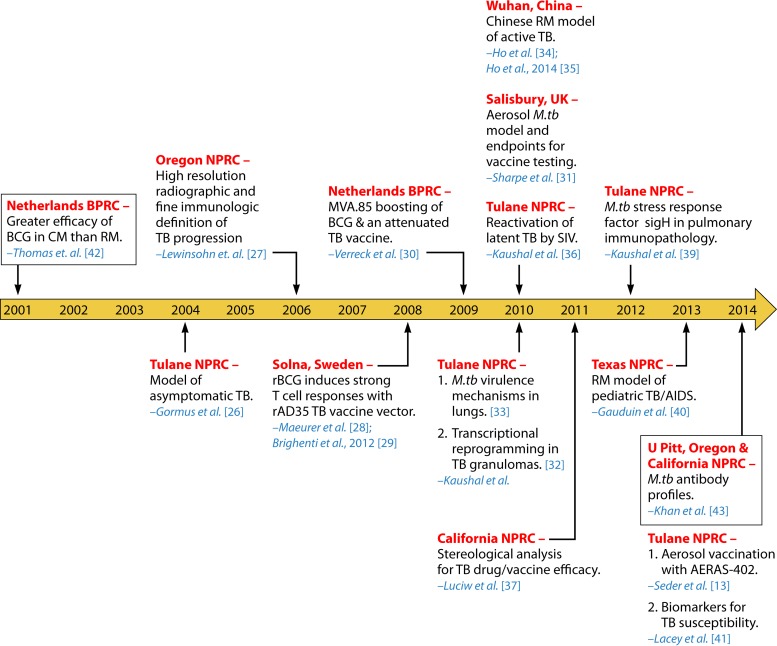
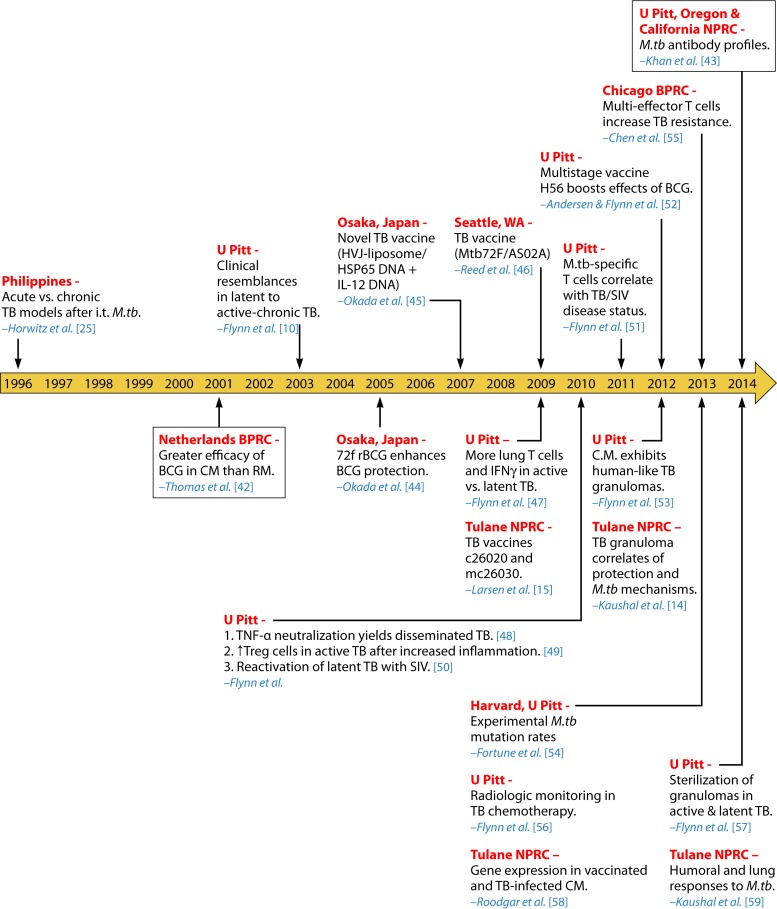
Not until about 20 years after the Golden Age was another study using experimentally infected macaques with M. tuberculosis published (25). This large research gap may be attributed to several factors, including the high maintenance costs and necessary space/equipment for biocontainment to properly conduct such experiments (6). Additionally, the limited animal availability, handling difficulties, and adverse public opinions discouraged TB research with NHP. However, with greater research funding and the emergence of compatible reagents for macaques, further investigations into M. tuberculosis infection using NHP models regained momentum (10). Especially amid the expansion of the National Primate Research Centers (NPRCs), TB investigators revisited the macaque model of experimental M. tuberculosis inoculation with renewed enthusiasm. A series of subsequent investigations were dedicated to macaque research to assess novel TB vaccines and drugs, as well as to gain an understanding of the pathogenesis of M. tuberculosis infection and reactivation. Figures 2 and 3 abridge the RM (13, 26–43) and CM (10, 15, 25, 42–59) models of experimental M. tuberculosis infection after the Golden Age, from 2001 to 2014.
FIG 2.
Twenty-first century TB research using rhesus macaques. The timeline shows important studies of experimental M. tuberculosis infection in rhesus macaques during the 21st century, from 2001 to 2014 (13, 26–34, 36–43). Events are organized chronologically by year of publication. Locations of the experiments (red) and the corresponding author (blue) are specified accordingly. Boxed events refer to comparative TB studies using both cynomolgus and rhesus macaques. All studies, except for those reported in references 28 to 30 and 34, used Indian rhesus macaques. Abbreviations: BPRC, Biomedical Primate Research Center; rBCG, recombinant BCG.
FIG 3.
TB research using cynomolgus macaques. The timeline includes significant studies on cynomolgus macaques experimentally infected with M. tuberculosis from 1996 to 2014 (10, 15, 25, 42–59). Events are organized chronologically by year of publication. Locations of experiments (red) and the corresponding author (blue) are listed. Boxed events refer to comparative TB studies using both cynomolgus and rhesus macaques, as shown in Fig. 2. Abbreviations: BPRC, Biomedical Primate Research Center; IFN-γ, gamma interferon; TNF-α, tumor necrosis factor alpha; Treg, regulatory T cells; U Pitt, University of Pittsburgh.
The majority of NHP models of M. tuberculosis infection have involved either CM or Indian RM, except for at least five reported research projects which used Chinese RM in Wuhan, China (34, 35), Solna, Sweden (28, 29), and Rijswijk, the Netherlands (30). So far within the scientific literature illustrating macaque models of M. tuberculosis infection, the routes of inoculation have included i.b. instillation as well as i.t., aerosol, and intranasal (i.n.) infection. Although zoonotic TB outbreaks have revealed horizontal transmission of M. tuberculosis, an experimental macaque model of natural M. tuberculosis infection has not been reported. Tables 2 to 4 summarize by route the dose and M. tuberculosis strain of infection in each study design, along with the major findings of the listed papers.
TABLE 2.
TB studies using both rhesus and cynomolgus macaquesa
| Exptl design |
Major findings | Reference | |
|---|---|---|---|
| No. of animals | M. tuberculosis strainb (inoculation route), dose(s) (CFU) | ||
| 6 RM, 6 CM | Erdman (i.t.), 3,000 | BCG provides greater protective efficacy against TB in CM than in RM | 42c |
| 8 RM, 15 CM | Erdman (i.t.), 1,000–3,000 | In vitro IFN-γ assays provide reproducible, reliable results while causing less stress than the PPD skin test | 61 |
| 5 RM, 4 CM | Erdman (i.t.), 100 | Synthetic peptides may be used in lieu of full-length ESAT-6 protein in TB diagnostic antibody detection assays | 62d |
| 26 RM | Erdman (i.t.), 30–1,000 | Highest rate of TB detection achieved when skin test is combined with PrimaTB STAT-PAK immunoassay | 63e |
| 4 RM | H37Rv (i.t.), 210 | ||
| 3 RM | Beijing (i.t.), 1,000 | ||
| 16 CM | Erdman (i.t.), 100–1,000 | ||
| 4 RM | H37Rv (i.b.), 1,000 | The multiplex microbead immunoassay profiles M. tuberculosis antibodies at multiple stages of infection/disease | 64 |
| 6 CM | Erdman (i.b.), 25 | ||
| 9 RM, 21 CM | Erdman K01 (aerosol), 30–500 | MRI and stereology provides most accurate, quantifiable measurements of TB disease burden; RM exhibit higher susceptibility to M. tuberculosis than CM | 60 |
| 6 RM | Erdman (i.b.), 500 | M. tuberculosis antibody profiles depend on the NHP species and infecting M. tuberculosis strain but do not significantly change with TB disease progression | 43 |
| 4 RM | H37Rv (i.b.), 1,000 | ||
| 14 CM | Erdman (i.b.), 25 | ||
All studies used Indian RM models.
The Erdman strain is a virulent set of M. tuberculosis isolates existing in two forms, including the laboratory ATCC 35801 isolate and the clinically isolated K01; this strain is most commonly used to study acute tuberculosis. Here, Erdman strain ATCC 35801 was used unless K01 is indicated. H37RV is an attenuated laboratory strain of M. tuberculosis typically used to study latent infection.
A TB vaccine-related study (42).
The study reported in reference 62 also used African green monkeys.
In the study reported in reference 63, additional RM, CM, and African green monkey groups were inoculated with the mycobacteria M. kansaii and M. avium.
RHESUS VERSUS CYNOMOLGUS MACAQUE MODELS OF TB
Both RM and CM models have served to evaluate the efficacy of TB vaccines/drugs, as well as improve our understanding of the immunopathogenesis of M. tuberculosis infection and reactivation. However, there are differences between the two species. For example, one investigation revealed that BCG provides greater protective efficacy in CM than in RM against M. tuberculosis infection (42). Later investigations exploring stereological techniques for measuring the bacterial burden showed that RM are more susceptible to M. tuberculosis infection than are CM (60). As a result, RM are more often used for the study of active TB, whereas CM provide better models of latent or chronic TB (10). Depending on the route and dose of infection, as well as the strain for the inoculum, either CM or RM can develop acute, chronic, or latent TB. Therefore, several research endeavors have employed both species for developing and determining the efficacy of TB screening immunoassays (61–64) (Table 2).
RHESUS MACAQUES
RM have been used extensively to study TB (Table 3). The early investigations during the Golden Age (17–21) specifically showed that M. tuberculosis infection in RM progresses rapidly, within 8 to 9 weeks after aerosol inhalation of the H37Rv strain administered at low doses of up to 62 CFU. In 2004, the Tulane NPRC established a model of asymptomatic, or latent, M. tuberculosis infection of RM (26). The investigators there revealed that RM are a good model for not only active TB but also asymptomatic TB when the investigators used lower doses (30 CFU) of the H37Rv strain. In addition to RM of Indian origin, Chinese RM can also be used as a viable model of acute TB (34). Chinese RM are highly susceptible to M. tuberculosis infection and develop active TB regardless of the dose of strain H37Rv used (34). In an attempt to improve the methods to monitor the progression of TB disease, Helke et al. (27) from the Oregon NPRC showed that use of high-resolution radiographic and fine immunologic studies helped define the disease status in RM as in humans. Namely, computed tomography (CT) evaluation and M. tuberculosis-specific T cell frequencies measured by enzyme-linked immunosorbent spot (ELISPOT) assays correlated well with the bacterial burden and severity of disease (27).
TABLE 3.
TB studies using rhesus macaquesa
| Exptl design |
Major findings | Reference(s) | |
|---|---|---|---|
| No. of RM | M. tuberculosis strainb (inoculation route), dose(s) (CFU) | ||
| 8 | Erdman (i.b.), 10–150 | RM are a good model for latent TB, with use of low doses of H37Rv | 26 |
| 12 | H37Rv (i.b.), 30–6,000,000 | ||
| 4 | H37Rv (i.b.), 1,000 | High-resolution radiographic and fine immunologic studies provide definition of TB disease progression | 27 |
| 18 | Erdman (i.t.), 500 | Recombinant BCG (AFRO-1) induces strong antigen-specific T cell responses with TB vaccine vector (rAD35) | 28, 29c |
| 24 | Erdman (i.t.), 1,000 | MVA.85 boosting of BCG and an attenuated, phoP-deficient TB vaccine show protective efficacy against TB | 30c |
| 16 | Erdman K01 (aerosol), 40–65 | RM may be used as models of M. tuberculosis aerosol challenge; IFN-γ (ELISpot, ELISA) does not correlate with protection against TB; only MRI offers a reliable correlate | 31 |
| NP | NP | Early TB lesions have a highly proinflammatory environment, expressing IFN-γ, TNF-α, JAK, STAT, and C-C/C-X-C chemokines; in contrast, late TB lesions have a silenced inflammatory response | 32 |
| 12 | 326 CDC1551 Himar 1 mutants (i.n.), 100,000 | Virulence mechanisms of M. tuberculosis include transport of lipid virulence factors, biosynthesis of cell wall arabinan and peptidoglycan, DNA repair, sterol metabolism, and lung cell entry | 33 |
| 9 | H37Rv (i.b.), 50–3,000 | Chinese RM are highly susceptible to M. tuberculosis infection and develop active TB regardless of the dose of strain H37Rv or Erdman used | 34, 35 |
| 24 | Erdman (i.b.), 25–500 | ||
| 16 | CDC1551 (i.n.), 50 | RM are an excellent model of TB/HIV coinfection and can be used to study TB latency and reactivation | 36 |
| 6 | Erdman K01 (i.b.), 500 | Stereological analysis quantitative data show a strong correlation between bacterial load and lung granulomas | 37 |
| 13 | CDC1551 (i.n.), 5,000 | The M. tuberculosis stress response factor sigH is required for M. tuberculosis growth and replication in mammalian lungs | 38 |
| 3 | Erdman (i.b./i.n.), 5–50 | Newborn macaques infected with aerosolized M. tuberculosis develop human-like immunologic responses and are a good model for pediatric TB/HIV | 40 |
| 32 | Erdman K01 (i.b.), 275 | RM aerosol vaccination with AERAS-402 elicits transient cellular immune responses in blood and robust, sustained immune responses in BAL fluid but does not protect against high-dose M. tuberculosis infection | 13c |
| 17 | CDC1551 (aerosol), 100 | Clinical profiles vary considerably among RM infected with M. tuberculosis but can help identify predictive biomarkers for TB susceptibility along with gene expression profiles | 41 |
All studies, except for those reported in references 28 to 30 and 33, used Indian rhesus macaques. Abbreviations: i.d., intradermal; i.n., intranasal; NP, not provided.
The Erdman strain is most commonly used to study acute TB. It is a virulent subset of M. tuberculosis and exists in two forms, the laboratory isolate ATCC 35801 and the clinical isolate, K01; the Erdman ATCC 35801 strain was used in most studies, except those for which the K01 strain is indicated. H37RV is an attenuated laboratory strain of M. tuberculosis typically used to study latent TB infection. CDC1551 is a clinical isolate of M. tuberculosis and exhibits a similar degree of virulence as the Erdman strain.
TB vaccine-related study.
CYNOMOLGUS MACAQUES
In addition to RM, CM have aided researchers similarly in the study of TB (Table 4), particularly after the so-called Golden Age (16). The first published CM investigation of controlled M. tuberculosis infection stemmed from Walsh et al.'s (25) work in the Philippines (Fig. 3), in collaboration with the University of California Los Angeles School of Medicine. In this project, the Philippine CM were found to make an excellent model of not only acute TB but also chronic TB. This research pioneered the intratracheal infection of Philippine CM in a TB model. Within 7 years, Capuano et al. (10) from The University of Pittsburgh developed a CM model representing the full spectrum of human M. tuberculosis infection by infecting the macaques after intrabronchial inoculation with a low dose (25 CFU) of the Erdman strain. Interestingly, this low dose of inoculum precipitated various reactions in the CM, ranging from latent TB to active-chronic and even rapidly progressive TB. The CM's pulmonary and even extrapulmonary manifestations of disease within the different stages of TB infection closely resembled the pathological and clinical findings in human TB, as confirmed by laboratory assessments, including purified protein derivative (PPD) tests and erythrocyte sedimentation rate (ESR) profiling (10) (Table 1). A substantial number of the proceeding publications on CM models of M. tuberculosis infection were similarly published by researchers at The University of Pittsburgh (47–50, 52, 56, 57). Therefore, CM models have now been used for evaluating TB drugs and vaccines (42, 46, 52).
TABLE 4.
TB studies using cynomolgus macaquesa
| Exptl design |
Major findings | Reference(s) | |
|---|---|---|---|
| No. of CM | M. tuberculosis strain (inoculation), dose(s) (CFU) | ||
| 28 | Erdman (i.t.), 10–100,000 | Philippine CM provide an excellent model of chronic TB | 25 |
| 17 | Erdman (i.b.), 25 | Low-dose infection of CM represents the full spectrum of human M. tuberculosis infection and provides a model to study latent as well as active-chronic and rapidly progressive TB | 10 |
| 16 | Erdman (i.t.), 500 | CM vaccination with the 72f rBCG vaccine provides better protective efficacy than with BCG | 44b |
| 44 | Erdman (i.t.), 500 | CM vaccination with the HSP65 plus IL-12/HVJ vaccine provides better protective efficacy than BCG | 44, 45b |
| 15 | Erdman (i.t.), dose not reported | CM vaccination with Mtb72F/AS02A provides greater protective efficacy than BCG alone | 46b |
| 24 | Erdman (i.b.), 1,000 | CM vaccination with mc26020 or mc26030 provides less protection than with BCG | 15b |
| 25 | Erdman (i.b.), 25 | At necropsy, CM with active TB have more lung T cells and more IFN-γ from PBMC, BAL fluid, and mediastinal lymph nodes than CM with latent TB | 47 |
| 24 | Erdman (i.b.), 25 | Neutralization of TNF results in disseminated disease in acute and latent TB infection with normal granuloma structure in a CM model | 48 |
| 41 | Erdman (i.b.), 25 | Increased regulatory T cells in active TB occur in response to increased inflammation, not as a causal factor of disease progression | 49 |
| 15 | Erdman (i.b.), 25 | Reactivation of latent TB with SIV is associated with early T cell depletion and not virus load | 50 |
| 7 | Erdman (i.b.), 25 | M. tuberculosis-specific multifunctional T cells are better correlates of antigen load and disease status than of protection | 51c |
| 5 | Erdman (i.b.), 200 | ||
| 33 | Erdman (i.t.), 25–500 | The multistage vaccine H56 boosts effects of BCG to protect CM against active TB and reactivation of latent TB | 52 |
| 14 | Erdman (i.b.), 25–200 | The CM model of M. tuberculosis infection mimics human TB, particularly in granuloma type and structure | 53 |
| 8 | Erdman (i.t.), 250 | M. tuberculosis may modulate protective immune responses via the use of indoleamine 2,3-dioxygenase (an immunosuppressant) found in nonlymphocytic regions of TB granulomas | 14b |
| 9 | Erdman (i.b.), 25 | Experimental and epidemiologic estimates of the M. tuberculosis mutation rate are comparable | 54 |
| 27 | Erdman (i.b.), 500 | Early expansion/differentiation of Vγ2Vδ2 T effector cells during M. tuberculosis infection increases resistance to TB | 55 |
| 26 | Erdman (i.b.), 25–400 | TB granulomas evolve and resolve independently within a single host; individual lesions respond differently to different drugs; overall PET and CT signals can predict successful TB drug treatment | 56 |
| 12 | Erdman (i.b.), 1,000 | Compared to nonvaccinated CM, BCG-vaccinated CM exhibit higher expression levels of TNF-α, IL-10, IL-1b, TLR4, IL-17, IL-6, IL-12, and iNOS in lungs | 58b |
| 39 | Erdman (i.b.), 25 | Sterilization of TB granulomas occurs in both active and latent TB amid the differential killing of M. tuberculosis within a single host | 57 |
| 2 | SNP strains (i.b.), 34 | ||
| 8 | Erdman (i.b.), 240–500 | CM vaccination with BCG transiently increases levels of macrophages and lymphocytes in blood, with later recruitment in the lungs; however, M. tuberculosis continues to replicate in lungs | 59b |
Abbreviations: SNP strains, strains with a single-nucleotide polymorphism mutation; rBCG, recombinant BCG; BAL, bronchoalveolar lavage; PBMC, peripheral blood mononuclear cells; iNOS, inducible nitric oxygen synthase.
TB vaccine-related study.
Animals were coinfected with M. tuberculosis and SIV.
SPECIFIC MACAQUE MODELS OF TB
Macaque models for TB vaccine evaluation.
Using the RM model, researchers from the Swedish Institute of Infectious Disease Control strived to augment, broaden, and prolong immune protection against M. tuberculosis. They showed that a recombinant BCG (AFRO-1) could induce strong antigen-specific T cell responses when combined with the TB vaccine vector rAD35 (28, 29). Two other investigations using RM models of M. tuberculosis infection also focused on evaluating TB vaccines (30, 31). The Biomedical Primate Research Center in Rijswijk, the Netherlands, aimed to develop a model to test TB vaccines before progressing to human clinical trials (30). By using an RM model employing intratracheal inoculation with the Erdman strain of M. tuberculosis, the study revealed that prior immunization with the MVA.85-boosted BCG and an attenuated, phoP-deficient TB vaccine provided effective protection against M. tuberculosis infection. A novel aerosol challenge model effected by Sharpe et al. (31) helped to assess the endpoints for testing the BCG/MVA.85 vaccine. This NHP model used a three-jet collision nebulizer in addition to a modified Henderson apparatus (a device for studying the infectivity and virulence of microorganisms in small air droplets; the 3 components include a continuous aerosol-generating unit (collision spray), exposure unit, and sampling unit), as described by Barclay et al. (18), in a head-out plethysmography chamber. The results of these studies indicated that the gamma interferon (IFN-γ) indices—from ELISPOT assays and enzyme-linked immunosorbent assays (ELISAs)—do not relate to protection against TB; only the magnetic resonance imaging (MRI) readouts offered a reliable correlate, using ex vivo lung samples removed at necropsy (18, 31). Another way to objectively measure the efficacy of TB vaccines and drugs was later developed by Luciw et al. (37) at the California NPRC. The research findings from these studies determined that stereological analysis (i.e., three-dimensional interpretation of planar sections of materials or tissues) from MRI could provide quantitative data, showing a significant correlation between bacterial load and lung granulomas.
Researchers did not use CM for testing TB vaccines until much later than for the early RM models of TB. Between 2005 and 2012, three reported studies using CM models evaluated the efficacy of TB vaccines that had been tested previously with smaller animal models, including guinea pigs and mice (44, 45). The first project evaluated the efficacy of the 72f recombinant BCG vaccine and HASP65 plus interleukin-12 (IL-12)/HVJ vaccine (44), which another team of investigators also tested later in Osaka, Japan (45). Both research investigations showed that the recombinant BCG vaccines were more effective than BCG alone. In subsequent years, the multistage vaccine H56 was found to boost the effects of BCG to protect CM against active TB and the reactivation of latent TB (52). Ultimately, the macaque models have exhibited the potential to help evaluate preventative methods and interventions before reaching human clinical trials, particularly for TB vaccines.
Macaque models for TB drug evaluation.
Finally, another recently reported investigation of experimental M. tuberculosis infection in a CM model was also published from the University of Pittsburgh School of Medicine (56, 57). The purpose of this research endeavor was to determine potential alternative markers for evaluating the efficacy of TB drugs. Specifically, the studies compared the overall metabolic and radiographic changes, as well as the alterations within individual granulomas, in CM infected with M. tuberculosis. Of note, the results revealed that TB granulomas evolve and resolve independently within a single host and that individual lesions respond variably to different drugs (56). Furthermore, the clinical findings concluded that the overall positron emission tomography (PET) and CT signals could be used as prognostic markers to predict successful TB drug therapies. However, the overall metabolic and radiographic changes reported by this study were nonspecific indicators of metabolic activity, measured from [18F]FDG-radiolabeled glucose in PET/CT imaging data (56).
Macaque models for the study of TB pathogenesis.
By gaining a better understanding of the pathogenesis of M. tuberculosis infection and reactivation of latent TB, researchers can further develop and improve treatment strategies. As such, investigators at the Tulane NPRC profiled the TB granuloma transcriptome in an RM model to identify key immune signaling pathways that are activated during M. tuberculosis infection (32). Previously, scientists from the Chicago Center for Biomedical Research characterized gene networks in RM after only BCG vaccination/infection (65). Mechanistic studies of M. tuberculosis delineated more specific factors employed by M. tuberculosis to successfully infect and persist in mammalian lungs (33). The identification of a potential therapeutic target sparked from the discovery of the M. tuberculosis stress response factor SigH as an important player in the growth/replication of M. tuberculosis (38, 39).
Lin et al. (47) characterized the clinical manifestations of TB disease within the three stages: latent, active-chronic, and rapidly progressive TB. The results of these studies showed that at necropsy, CM with active TB had more CD4+ and CD8+ T cells in the lungs and more gamma interferon from peripheral blood mononuclear cells, bronchoalveolar lavage fluid, and mediastinal lymph nodes than CM with latent TB (47). Investigators from the same group also showed that tumor necrosis factor neutralization resulted in disseminated disease in both acute and latent TB with normal granulomatous structures (48). Another CM study examined the role of CD4+ regulatory T cells in active TB; these cells occurred in response to increased inflammation rather than acting as a causative factor in the progression to active disease (49).
In light of the discovery that TB granulomas change uniquely within a host (56), the latest published investigation by Lin et al. (57) aimed to define how these structures vary between CM with latent and active TB. Interestingly, the sterilization of the granulomas occurred in both stages of TB, regardless of the differential killing of bacteria within a single host (57). Moreover, TB vaccine-related studies have elucidated possible genetic mechanisms of host resistance to M. tuberculosis after immunization (58), as well as immunosuppressant mechanisms of M. tuberculosis virulence (59). While adding insight into the molecular and pathological pathways of TB progression, these findings may also help to evaluate the disease status as well as spur the development of novel therapeutic targets.
Macaque models of TB/HIV coinfection.
Macaques also serve as an excellent model of TB/HIV coinfection, which is of importance to understand TB latency and reactivation (36). Upon inoculation with high-dose BCG (36, 66, 67) or low-dose Erdman strain (25 CFU, i.b.) (50), latently infected RM and CM, respectively, had reactivated TB when coinfected with the simian immunodeficiency virus (SIV). Pathogenic SIV-BCG interactions facilitated the development of TB-like disease (67), while antiretroviral agents restored M. tuberculosis-specific T cell immune responses (36). The reactivation of latent TB in CM infected with SIV was associated with early T cell depletion and not the virus load (50). Another particularly innovative RM model of TB was established at the Southwest NPRC (40). The purpose of that study was to institute an NHP model for pediatric TB/HIV coinfection. Newborn macaques were infected with M. tuberculosis Erdman strain intrabronchially or via the aerosol route, using an ultrasonic nebulizer specifically adapted for the newborn macaque nose. Investigators confirmed M. tuberculosis infection by various methods, including chest X-rays, ELISPOT, bronchoalveolar and gastric lavages, and necropsy. Because people with HIV/AIDS carry a high risk of M. tuberculosis infection and disease severity, it is clinically significant to have a model that mimics coinfection.
KEY LESSONS AND FUTURE DIRECTIONS
Collectively, the literature on macaque models of TB has shared four important lessons and opportunities for improvement. First, we recognize that the biosafety requirements, cost of equipment and maintenance, and animal availability have impeded scientists from using NHP models in TB research. To address these issues, researchers may experiment with smaller genera and species of NHPs that still recapitulate human TB, such as the common marmoset (Callithrix jacchus) model (68). Second, we found that different animal species, individually and as a whole, respond differently when exposed to M. tuberculosis in terms of immunopathogenesis of the disease (43, 64) (Table 2). Therefore, investigators must consider the purpose of the study for the appropriate selection of NHP species. Third, the strain of M. tuberculosis used for inoculation can significantly impact the disease outcome. Instead of employing merely the laboratory-adopted Erdman and H37Rv strains, study designs should include clinical isolates of M. tuberculosis. Although this approach may introduce more variability into the NHP studies of TB while making studies performed at different sites more difficult to compare, the findings would likely further mimic human TB disease and add valuable knowledge to the field. Lastly, most of the macaque studies so far have shown only acute TB, which is much less prevalent than latent TB in humans. Hence, it is necessary to establish more clinically relevant NHP models that resemble human passive airway transmission. By creating an NHP model of natural M. tuberculosis infection under experimental conditions, researchers would be able to test new and existing TB vaccines/drugs for protection against M. tuberculosis infection.
CONCLUDING REMARKS
Evidently, the macaque models have served as a valuable tool in TB research over the past several decades. As demonstrated in the literature, the CM and RM TB models have revealed clinically similar manifestations of TB disease or latency through multiple diagnostic and prognostic parameters. Even the variabilities in immune responses to M. tuberculosis infection imitate the diverse human host reactions to the pathogen (41). Although a number of studies on TB vaccine evaluation have been conducted using experimentally infected macaques, the majority of these studies merely showed the reduction of TB disease progression by BCG-based vaccines. Therefore, an urgent need still exists in order to establish macaque models that can demonstrate protection against M. tuberculosis infection besides TB disease progression. Fortunately, some investigators and funding institutions have revamped an interest in further developing NHP models for TB research. Undoubtedly, given the reemerging TB epidemic, many people worldwide should benefit from more advanced treatment and prevention strategies against M. tuberculosis infection and disease progression. Hence, the use of NHP models should be considered a highly effective means of reaching these common goals. In essence, from a global health standpoint, there is truth in the words of the literary science author Chris Roberson: “Everything is improved by the judicious application of primates.”
ACKNOWLEDGMENTS
J.C.P. and W.H. were the sole contributors to the literature analysis and written work. Graphic illustrations were developed by J.C.P. and W.H. and enhanced by Patrick Lane at ScEYEnce Studios.
We received no additional support in the preparation of the manuscript.
Biographies

Juliet C. Peña, M.D., M.P.H., dedicated 1 year (2013 to 2014) as a postdoctoral fellow in WenZhe Ho's laboratory at the Temple University School of Medicine in Philadelphia, PA, where she focused her studies on the immunopathogenesis of tuberculosis and HIV. Her interest in tuberculosis research stemmed from her medical and public health background, as well as her collaborations with Wen-Zhe Ho's laboratory using monkey models of tuberculosis. She earned her M.D. (2012) and M.P.H. (2014) from The University of Arizona College of Medicine and the Mel and Enid Zuckerman College of Public Health. Currently, Dr. Peña is completing a health policy fellowship at the Office of Disease Prevention and Health Promotion within the U.S. Department of Health and Human Services.

Wen-Zhe Ho has 30 years of research experience in understanding the interactions between host innate immunity and the pathogens HIV, hepatitis C virus, and M. tuberculosis. He received his M.D. and clinical training as a pediatrician in infectious diseases at Wuhan University, China in the 1980s. He was a postdoctoral fellow in infectious diseases at the Children's Hospital of Philadelphia and the Wistar Institute of the University of Pennsylvania, where he became a full professor (2005). He also received his M.P.H. at the University of Pennsylvania (2006), through which he gained a greater interest in public health issues of M. tuberculosis and HIV coinfection. In 2009, Dr. Ho moved to Temple University, where he now serves as a tenured professor in the Departments of Pathology and Cell Biology. Additionally, as Director/Professor at the Center for Animal Experiment/ABSL-III Laboratory of Wuhan University (2010 to present), he continues his research on M. tuberculosis infection using monkey models.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report 2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Basaraba RJ. 2008. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis 88(Suppl 1):S35–S47. doi: 10.1016/S1472-9792(08)70035-0. [DOI] [PubMed] [Google Scholar]
- 3.Dharmadhikari AS, Nardell EA. 2008. What animal models teach humans about tuberculosis. Am J Respir Cell Mol Biol 39:503–508. doi: 10.1165/rcmb.2008-0154TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanga CA, Flynn JL. 2014. Modeling tuberculosis in nonhuman primates. Cold Spring Harbor Perspect Med 4:a018564. doi: 10.1101/cshperspect.a018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushal D, Mehra S, Didier PJ, Lackner AA. 2012. The non-human primate model of tuberculosis. J Med Primatol 41:191–201. doi: 10.1111/j.1600-0684.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL. 2006. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Ackermann RR, Baliff J, Foxman S, Helbig J, Lesser J, Mosbacher M, Senturia S, Sklar D, Sothman P. 2003. Long-tailed or crab-eating macaque and rhesus macaque, p 18–24. In Ackermann RR. (ed), A comparative primate anatomy: dissection manual. Washington University in St. Louis, St. Louis, MO: http://web.uct.ac.za/depts/age/people/dissect.pdf. [Google Scholar]
- 8.Carlsson HE, Schapiro SJ, Farah I, Hau J. 2004. Use of primates in research: a global overview. Am J Primatol 63:225–237. doi: 10.1002/ajp.20054. [DOI] [PubMed] [Google Scholar]
- 9.O'Neil RM, Ashack RJ, Goodman FR. 1981. A comparative study of the respiratory responses to bronchoactive agents in rhesus and cynomolgus monkeys. J Pharmacol Methods 5:267–273. doi: 10.1016/0160-5402(81)90094-2. [DOI] [PubMed] [Google Scholar]
- 10.Capuano SV III, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun 71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf RH, Gibson SV, Watson EA, Baskin GB. 1988. Multidrug chemotherapy of tuberculosis in rhesus-monkeys. Lab Anim Sci 38:25–33. [PubMed] [Google Scholar]
- 12.Schmidt L. 1966. Studies on the antituberculous activity of ethambutol in monkeys. Ann N Y Acad Sci 135:747–758. doi: 10.1111/j.1749-6632.1966.tb45520.x. [DOI] [PubMed] [Google Scholar]
- 13.Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, Blanchard JL, Didier PJ, Roy CJ, Rao SS, Hokey DA, Scanga CA, Sizemore DR, Sadoff JC, Roederer M, Seder RA. 2014. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J Immunol 193:1799–1811. doi: 10.4049/jimmunol.1400676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehra S, Alvarez X, Didier PJ, Doyle LA, Blanchard JL, Lackner AA, Kaushal D. 2013. Granuloma correlates of protection against TB and mechanisms of immune modulation by Mycobacterium tuberculosis. J Infect Dis 207:1115–1127. doi: 10.1093/infdis/jis778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, Aye PP, Didier P, Huang D, Shao L, Wei H, Letvin NL, Frothingham R, Haynes BF, Chen ZW, Jacobs WR Jr. 2009. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine 27:4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurray DN. 2000. A nonhuman primate model for preclinical testing of new tuberculosis vaccines. Clin Infect Dis 30(Suppl 3):S210–S212. doi: 10.1086/313885. [DOI] [PubMed] [Google Scholar]
- 17.Good RC. 1968. Biology of the mycobacterioses. Simian tuberculosis: immunologic aspects. Ann N Y Acad Sci 154:200–213. [DOI] [PubMed] [Google Scholar]
- 18.Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. 1970. Aerosol-induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect Immun 2:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baram P, Soltysik L, Condoulis W. 1971. The in vitro assay of tuberculin hypersensitivity in Macaca mulatta sensitized with bacille Calmette Guerin cell wall vaccine and/or infected with virulent Mycobacterium tuberculosis. Lab Anim Sci 21:727–733. [PubMed] [Google Scholar]
- 20.Ribi E, Anacker R, Barclay W, Brehmer W, Harris S, Leif W, Simmons J. 1971. Efficacy of mycobacterial cell walls as a vaccine against airborne tuberculosis in the rhesus monkey. J Infect Dis 123:527–538. doi: 10.1093/infdis/123.5.527. [DOI] [PubMed] [Google Scholar]
- 21.Barclay WR, Busey WM, Dalgard DW, Good RC, Janicki BW, Kasik JE, Ribi E, Ulrich CE, Wolinsky E. 1973. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis 107:351–358. [DOI] [PubMed] [Google Scholar]
- 22.Janicki B, Good R, Minden P, Affronti L, Hymes W. 1973. Immune responses in rhesus monkeys after bacillus Calmette-Guerin vaccination and aerosol challenge with Mycobacterium tuberculosis. Am Rev Respir Dis 107:359. [DOI] [PubMed] [Google Scholar]
- 23.Chaparas S, Good R, Janicki B. 1975. Tuberculin-induced lymphocyte transformation and skin reactivity in monkeys vaccinated or not vaccinated with Bacille Calmette-Guerin, then challenged with virulent Mycobacterium tuberculosis. Am Rev Respir Dis 112:43–47. [DOI] [PubMed] [Google Scholar]
- 24.Harper GJ, Morton JD. 1953. The respiratory retention of bacterial aerosols: experiments with radioactive spores. J Hygiene 51:372–385. doi: 10.1017/S0022172400015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh GP, Tan EV, de la Cruz EC, Abalos RM, Villahermosa LG, Young LJ, Cellona RV, Nazareno JB, Horwitz MA. 1996. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med 2:430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 26.Gormus BJ, Blanchard JL, Alvarez XH, Didier PJ. 2004. Evidence for a rhesus monkey model of asymptomatic tuberculosis. J Med Primatol 33:134–145. doi: 10.1111/j.1600-0684.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 27.Lewinsohn DM, Tydeman IS, Frieder M, Grotzke JE, Lines RA, Ahmed S, Prongay KD, Primack SL, Colgin LM, Lewis AD, Lewinsohn DA. 2006. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect 8:2587–2598. doi: 10.1016/j.micinf.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Magalhaes I, Sizemore DR, Ahmed RK, Mueller S, Wehlin L, Scanga C, Weichold F, Schirru G, Pau MG, Goudsmit J, Kuhlmann-Berenzon S, Spangberg M, Andersson J, Gaines H, Thorstensson R, Skeiky YA, Sadoff J, Maeurer M. 2008. rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime-boost setting with an adenovirus 35 tuberculosis vaccine vector. PLoS One 3:e3790. doi: 10.1371/journal.pone.0003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman S, Magalhaes I, Rahman J, Ahmed RK, Sizemore DR, Scanga CA, Weichold F, Verreck F, Kondova I, Sadoff J, Thorstensson R, Spangberg M, Svensson M, Andersson J, Maeurer M, Brighenti S. 2012. Prime-boost vaccination with rBCG/rAd35 enhances CD8(+) cytolytic T-cell responses in lesions from Mycobacterium tuberculosis-infected primates. Mol Med 18:647–658. doi: 10.2119/molmed.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, van der Werff NM, Kersbergen A, Ottenhoff TH, Heidt PJ, Gilbert SC, Gicquel B, Hill AV, Martin C, McShane H, Thomas AW. 2009. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One 4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. 2010. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol 17:1170–1182. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, Kaushal D. 2010. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS One 5:e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta NK, Mehra S, Didier PJ, Roy CJ, Doyle LA, Alvarez X, Ratterree M, Be NA, Lamichhane G, Jain SK, Lacey MR, Lackner AA, Kaushal D. 2010. Genetic requirements for the survival of tubercle bacilli in primates. J Infect Dis 201:1743–1752. doi: 10.1086/652497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Ye YQ, Wang Y, Mo PZ, Xian QY, Rao Y, Bao R, Dai M, Liu JY, Guo M, Wang X, Huang ZX, Sun LH, Tang ZJ, Ho WZ. 2011. M. tuberculosis H37Rv infection of Chinese rhesus macaques. J Neuroimmune Pharmacol 6:362–370. doi: 10.1007/s11481-010-9245-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Xian Q, Guo M, Huang Z, Rao Y, Wang Y, Wang X, Bao R, Evans TG, Hokey D, Sizemore D, Ho WZ. 2014. Mycobacterium tuberculosis Erdman infection of rhesus macaques of Chinese origin. Tuberculosis 94:634–643. doi: 10.1016/j.tube.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Mehra S, Golden NA, Dutta NK, Midkiff CC, Alvarez X, Doyle LA, Asher M, Russell-Lodrigue K, Monjure C, Roy CJ, Blanchard JL, Didier PJ, Veazey RS, Lackner AA, Kaushal D. 2011. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J Med Primatol 40:233–243. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luciw PA, Oslund KL, Yang XW, Adamson L, Ravindran R, Canfield DR, Tarara R, Hirst L, Christensen M, Lerche NW, Offenstein H, Lewinsohn D, Ventimiglia F, Brignolo L, Wisner ER, Hyde DM. 2011. Stereological analysis of bacterial load and lung lesions in nonhuman primates (rhesus macaques) experimentally infected with Mycobacterium tuberculosis. Am J Physiol Lung Cell Mol Physiol 301:L731–L738. doi: 10.1152/ajplung.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutta NK, Mehra S, Martinez AN, Alvarez X, Renner NA, Morici LA, Pahar B, Maclean AG, Lackner AA, Kaushal D. 2012. The stress-response factor SigH modulates the interaction between Mycobacterium tuberculosis and host phagocytes. PLoS One 7:e28958. doi: 10.1371/journal.pone.0028958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehra S, Golden NA, Stuckey K, Didier PJ, Doyle LA, Russell-Lodrigue KE, Sugimoto C, Hasegawa A, Sivasubramani SK, Roy CJ, Alvarez X, Kuroda MJ, Blanchard JL, Lackner AA, Kaushal D. 2012. The Mycobacterium tuberculosis stress response factor SigH is required for bacterial burden as well as immunopathology in primate lungs. J Infect Dis 205:1203–1213. doi: 10.1093/infdis/jis102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cepeda M, Salas M, Folwarczny J, Leandro AC, Hodara VL, de la Garza MA, Dick EJ Jr, Owston M, Armitige LY, Gauduin MC. 2013. Establishment of a neonatal rhesus macaque model to study Mycobacterium tuberculosis infection. Tuberculosis 93(Suppl):S51–S59. doi: 10.1016/S1472-9792(13)70011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo Q, Mehra S, Golden NA, Kaushal D, Lacey MR. 2014. Identification of biomarkers for tuberculosis susceptibility via integrated analysis of gene expression and longitudinal clinical data. Front Genet 5:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langermans JA, Andersen P, van Soolingen D, Vervenne RA, Frost PA, van der Laan T, van Pinxteren LA, van den Hombergh J, Kroon S, Peekel I, Florquin S, Thomas AW. 2001. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci U S A 98:11497–11502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravindran R, Krishnan VV, Dhawan R, Wunderlich ML, Lerche NW, Flynn JL, Luciw PA, Khan IH. 2014. Plasma antibody profiles in non-human primate tuberculosis. J Med Primatol 43:59–71. doi: 10.1111/jmp.12097. [DOI] [PubMed] [Google Scholar]
- 44.Kita Y, Tanaka T, Yoshida S, Ohara N, Kaneda Y, Kuwayama S, Muraki Y, Kanamaru N, Hashimoto S, Takai H, Okada C, Fukunaga Y, Sakaguchi Y, Furukawa I, Yamada K, Inoue Y, Takemoto Y, Naito M, Yamada T, Matsumoto M, McMurray DN, Cruz EC, Tan EV, Abalos RM, Burgos JA, Gelber R, Skeiky Y, Reed S, Sakatani M, Okada M. 2005. Novel recombinant BCG and DNA-vaccination against tuberculosis in a cynomolgus monkey model. Vaccine 23:2132–2135. doi: 10.1016/j.vaccine.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 45.Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Fukamizu R, Tsunai Y, Inoue R, Nakatani H, Namie Y, Yamada J, Takao K, Asai R, Asaki R, Matsumoto M, McMurray DN, Dela Cruz EC, Tan EV, Abalos RM, Burgos JA, Gelber R, Sakatani M. 2007. Evaluation of a novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomolgus monkey model of TB. Vaccine 25:2990–2993. doi: 10.1016/j.vaccine.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y. 2009. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A 106:2301–2306. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, Flynn JL. 2009. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, Grieser H, Chiosea I, Voitenek NN, Capuano SV, Klein E, Flynn JL. 2010. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum 62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, Flynn JL. 2010. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis 202:533–541. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. 2010. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One 5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattila JT, Diedrich CR, Lin PL, Phuah J, Flynn JL. 2011. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol 186:3527–3537. doi: 10.4049/jimmunol.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P. 2012. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest 122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phuah JY, Mattila JT, Lin PL, Flynn JL. 2012. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol 181:508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ragheb MN, Ford CB, Chase MR, Lin PL, Flynn JL, Fortune SM. 2013. The mutation rate of mycobacterial repetitive unit loci in strains of M. tuberculosis from cynomolgus macaque infection. BMC Genomics 14:145. doi: 10.1186/1471-2164-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen CY, Yao SY, Huang D, Wei HY, Sicard H, Zeng GC, Jomaa H, Larsen MH, Jacobs WR, Wang R, Letvin N, Shen Y, Qiu LY, Shen L, Chen ZW. 2013. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog 9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin PL, Coleman T, Carney JP, Lopresti BJ, Tomko J, Fillmore D, Dartois V, Scanga C, Frye LJ, Janssen C, Klein E, Barry CE III, Flynn JL. 2013. Radiologic responses in cynomolgus macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother 57:4237–4244. doi: 10.1128/AAC.00277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, Sacchettini J, Fortune SM, Flynn JL. 2014. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med 20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roodgar M, Lackner A, Kaushal D, Sankaran S, Dandekar S, Satkoski Trask J, Drake C, Smith DG. 2013. Expression levels of 10 candidate genes in lung tissue of vaccinated and TB-infected cynomolgus macaques. J Med Primatol 42:161–164. doi: 10.1111/jmp.12040. [DOI] [PubMed] [Google Scholar]
- 59.Dutta NK, McLachlan J, Mehra S, Kaushal D. 2014. Humoral and lung immune responses to Mycobacterium tuberculosis infection in a primate model of protection. Trials Vaccinol 3:47–51. doi: 10.1016/j.trivac.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharpe SA, Eschelbach E, Basaraba RJ, Gleeson F, Hall GA, McIntyre A, Williams A, Kraft SL, Clark S, Gooch K, Hatch G, Orme IM, Marsh PD, Dennis MJ. 2009. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis 89:405–416. doi: 10.1016/j.tube.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Vervenne RA, Jones SL, van Soolingen D, van der Laan T, Andersen P, Heidt PJ, Thomas AW, Langermans JA. 2004. TB diagnosis in non-human primates: comparison of two interferon-gamma assays and the skin test for identification of Mycobacterium tuberculosis infection. Vet Immunol Immunopathol 100:61–71. doi: 10.1016/j.vetimm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Kanaujia GV, Motzel S, Garcia MA, Andersen P, Gennaro ML. 2004. Recognition of ESAT-6 sequences by antibodies in sera of tuberculous nonhuman primates. Clin Diagn Lab Immunol 11:222–226. doi: 10.1128/CDLI.11.1.222-226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lyashchenko KP, Greenwald R, Esfandiari J, Greenwald D, Nacy CA, Gibson S, Didier PJ, Washington M, Szczerba P, Motzel S, Handt L, Pollock JM, McNair J, Andersen P, Langermans JA, Verreck F, Ervin S, Ervin F, McCombs C. 2007. PrimaTB STAT-PAK assay, a novel, rapid lateral-flow test for tuberculosis in nonhuman primates. Clin Vaccine Immunol 14:1158–1164. doi: 10.1128/CVI.00230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan IH, Ravindran R, Yee J, Ziman M, Lewinsohn DM, Gennaro ML, Flynn JL, Goulding CW, DeRiemer K, Lerche NW, Luciw PA. 2008. Profiling antibodies to Mycobacterium tuberculosis by multiplex microbead suspension arrays for serodiagnosis of tuberculosis. Clin Vaccine Immunol 15:433–438. doi: 10.1128/CVI.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang D, Qiu L, Wang R, Lai X, Du G, Seghal P, Shen Y, Shao L, Halliday L, Fortman J, Shen L, Letvin NL, Chen ZW. 2007. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J Infect Dis 195:55–69. doi: 10.1086/509895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen Y, Shen L, Sehgal P, Zhou D, Simon M, Miller M, Enimi EA, Henckler B, Chalifoux L, Sehgal N, Gastron M, Letvin NL, Chen ZW. 2001. Antiretroviral agents restore Mycobacterium-specific T-cell immune responses and facilitate controlling a fatal tuberculosis-like disease in Macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J Virol 75:8690–8696. doi: 10.1128/JVI.75.18.8690-8696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y, Zhou DJ, Chalifoux L, Shen L, Simon M, Zeng XJ, Lai XM, Li YY, Sehgal P, Letvin NL, Chen ZW. 2002. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-mycobacterium coinfection. Infect Immun 70:869–877. doi: 10.1128/IAI.70.2.869-877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Via LE, Weiner DM, Schimel D, Lin PL, Dayao E, Tankersley SL, Cai Y, Coleman MT, Tomko J, Paripati P, Orandle M, Kastenmayer RJ, Tartakovsky M, Rosenthal A, Portevin D, Eum SY, Lahouar S, Gagneux S, Young DB, Flynn JL, Barry CE III. 2013. Differential virulence and disease progression following Mycobacterium tuberculosis complex infection of the common marmoset (Callithrix jacchus). Infect Immun 81:2909–2919. doi: 10.1128/IAI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lerche NW, Yee JL, Capuano SV, Flynn JL. 2008. New approaches to tuberculosis surveillance in nonhuman primates. ILAR J 49:170–178. doi: 10.1093/ilar.49.2.170. [DOI] [PubMed] [Google Scholar]