Abstract
Proteus mirabilis is a common human pathogen causing recurrent or persistent urinary tract infections (UTIs). The underlying mechanisms for P. mirabilis to establish UTIs are not fully elucidated. In this study, we showed that loss of the sigma factor E (RpoE), mediating extracytoplasmic stress responses, decreased fimbria expression, survival in macrophages, cell invasion, and colonization in mice but increased the interleukin-8 (IL-8) expression of urothelial cells and swarming motility. This is the first study to demonstrate that RpoE modulated expression of MR/P fimbriae by regulating mrpI, a gene encoding a recombinase controlling the orientation of MR/P fimbria promoter. By real-time reverse transcription-PCR, we found that the IL-8 mRNA amount of urothelial cells was induced significantly by lipopolysaccharides extracted from rpoE mutant but not from the wild type. These RpoE-associated virulence factors should be coordinately expressed to enhance the fitness of P. mirabilis in the host, including the avoidance of immune attacks. Accordingly, rpoE mutant-infected mice displayed more immune cell infiltration in bladders and kidneys during early stages of infection, and the rpoE mutant had a dramatically impaired ability of colonization. Moreover, it is noteworthy that urea (the major component in urine) and polymyxin B (a cationic antimicrobial peptide) can induce expression of rpoE by the reporter assay, suggesting that RpoE might be activated in the urinary tract. Altogether, our results indicate that RpoE is important in sensing environmental cues of the urinary tract and subsequently triggering the expression of virulence factors, which are associated with the fitness of P. mirabilis, to build up a UTI.
INTRODUCTION
Proteus mirabilis, a common uropathogen, is an infectious agent of pyelonephritis and catheter-associated urinary tract infections (UTIs) (1, 2). P. mirabilis has many virulence factors that contribute to UTIs (1–3). These factors include fimbria-mediated adherence to host urothelial cells and the catheters (2, 4), flagella mediated motility (swarming and swimming) (1, 5), hemolysin (6), and invasion of host tissues and immune evasion (1, 7). Flagella-dependent swarm cell formation contributes to establishing infections by migrating along the catheter (5). Hemolysin is also thought to facilitate bacterial spread within the kidney and the development of pyelonephritis by damaging host tissues (6). Bacteria must successfully evade immune responses to persist within the host. P. mirabilis uses several strategies to avoid immune attacks in the urinary tract. One is to vary the antigenic structures, such as flagellin by flagellar gene rearrangement (8), and fimbriae by fimbrial gene diversity or phase variation to prevent antibody recognition (1, 3, 9). Other immunoavoidance factors for P. mirabilis include capsules (2), IgA proteases (ZapA) (10), and lipopolysaccharides (LPS) (1, 2). Capsules are effective at hiding many bacterial surfaces and preventing opsonization (2). P. mirabilis is an antigenically heterogeneous species due to structural differences of LPS (2). Modified LPS promotes bacterial survival by increasing resistance to cationic antimicrobial peptides and by altering host recognition by Toll-like receptors (TLRs) (11). Moreover, capability of invading urothelial cells to survive intracellularly probably represents another mechanism for immune evasion and persistence (1, 3, 7).
Many studies have reported that the presence of mannose-resistant Proteus-like (MR/P) fimbriae of P. mirabilis is important in UTIs (12, 13). MR/P fimbriae facilitate colonization of the urinary tract, and deficiency of the MR/P fimbriae decreased bacterial loads in the mouse model of UTIs (14, 15). The mrp gene cluster contains two transcripts: mrpABCDEFGHJ (designated the mrp operon) and mrpI (12). The promoter for the mrp operon, which contains all of the genes required for MR/P fimbrial biogenesis, resides on a 251-bp invertible element (IE) (12). The gene mrpI, transcribed divergently from the mrp operon and independent of the mrp promoter, encodes a recombinase capable of switching the IE from either “ON” to “OFF” or from “OFF” to “ON” to control MR/P fimbria expression (12).
Less is known about the host response to uropathogenic P. mirabilis but aspects of the host defense might be similar to uropathogenic E. coli (UPEC) (1–3). Urothelial cells secreted soluble mediators such as soluble IgA, lactoferrin, and bactericidal antimicrobial peptides to inhibit attachment of UPEC (16). Microbes that overwhelm these early defenses contact urothelia and activate an innate inflammatory response through TLRs (17). The inflammatory response consists of three principal steps: (i) urothelial cell activation and the production of distinct inflammatory cytokines, (ii) immune cell recruitment to the infectious site, and (iii) local destruction and elimination of the invading bacteria (16, 18).
The bacterial envelope maintains cell homeostasis and is the site for crucial processes, such as metabolic energy transduction, the transport of nutrients and wastes, signal transduction, and cell-cell communication (19). RpoE, an alternative sigma factor, is essential for the maintenance of cell envelope integrity in Gram-negative bacteria (20–22). In this regard, rpoE gene is important in pathogenesis and stress survival in many Gram-negative bacteria (20–22), but the function of RpoE in uropathogenic P. mirabilis is still not known. RseA, belonging to the rpoE operon, is an anti-RpoE factor under nonstressed conditions. The release of RpoE from RseA binding allows it to combine core RNA polymerase to transcribe RpoE-dependent genes (20). In the present study, we characterized the roles of P. mirabilis RpoE in virulence by in vitro and in vivo assays. This is the first report to show that P. mirabilis RpoE affected multiple traits, including swarming motility, hemolysin activity, bacteria-mediated cytotoxicity, fimbria production, survival in macrophage, invasion ability, induction of cytokine expression, and colonization in mice. It is worth noting that P. mirabilis RpoE could not only regulate expression of MR/P fimbriae through mrpI but also modulate host immune responses. We noticed that fimbria expression, survival in macrophages, invasion ability, and colonization in mice were decreased in the rpoE mutant and that the induction of interleukin-8 (IL-8) by rpoE mutant was higher relative to the wild type. In addition, we found that RpoE was activated by urea, a component in urine. Altogether, RpoE of P. mirabilis could play important roles in establishing UTIs via modulating the appropriate production of virulence factors, including those associated with host immune responses. To our knowledge, this is the first report describing the roles of RpoE in P. mirabilis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. Bacteria were routinely cultured at 37°C in Luria-Bertani (LB) medium. The LSW− agar (LB agar plates with glycerol to prevent swarming) was used to prevent the swarming motility when needed (23).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant phenotypea | Source or reference |
|---|---|---|
| Strains | ||
| P. mirabilis | ||
| Wild type | Wild-type (N2); Tetr | Clinical isolate |
| rseA mutant | Wild type derivative; rseA knockout mutant; Kmr | This study |
| rpoE mutant | Wild typederivative; rpoE knockout mutant; Kmr | This study |
| rseAc | rseA mutant strain containing pGEM-T Easy-rseA; RseA-complemented strain; Ampr | This study |
| rpoEc | rpoE mutant strain containing pGEM-T Easy-rpoE; RpoE-complemented strain; Ampr | This study |
| E. coli | ||
| DH5α | fhuA2 lacΔU169 phoA glnV44 W80′ lacZ ΔM15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | Invitrogen |
| S17-1 λpir | λpir lysogen of S17-1 [thi pro hsdR2 hsdM+ recA RP4 2-Tc::Mu-Km::Tn 7(Tpr Smr)]; permissive host able to transfer suicide plasmids requiring the Pir protein by conjugation to recipient cells | 24 |
| Plasmids | ||
| pGEM-T Easy | High-copy TA cloning vector; Ampr | Promega |
| pUT/mini-Tn5-Km | Suicide plasmid requiring the Pir protein for replication and containing a mini-Tn5 cassette containing the Kmr gene | 24 |
| pACYC184 | Low-copy cloning vector, P15A replicon; Cmr Tetr | 24 |
| pGEM-T Easy-rseA | pGEM-T Easy containing intact rseA sequence, including its ribosome binding site (RBS); Ampr | This study |
| pGEM-T Easy-rpoE | pGEM-T Easy containing intact rpoE sequence, including its ribosome binding site (RBS); Ampr | This study |
| pACYC184-rpoE- xylE | rpoE reporter plasmid, pACYC184 containing intact rpoE promoter sequence before xylE; Cmr | 24 |
Cmr, chloramphenicol resistance; Tetr, tetracycline resistance; Ampr, ampicillin resistance; Smr, streptomycin resistance; Kmr, kanamycin resistance; Tpr, trimethoprim resistance.
Construction of mutants and complementation.
Sequences flanking the rpoE gene were amplified by PCR using the primer pairs rpoE-upF/rpoE-upR and rpoE-downF/rpoE-downR (see Table S1 in the supplemental material), respectively, and cloned into pGEM-T Easy (Promega) to generate prpoEKO-up and prpoEKO-dn. The prpoEKO-up was digested with SalI/XbaI, and the rpoE upstream sequence containing fragment was ligated to the SalI/XbaI-digested prpoEKO-dn to produce the prpoEKOupdn plasmid, which contains both upstream and downstream sequences of rpoE. A kanamycin resistance (Kmr) cassette was inserted in the XbaI-digested prpoEKOupdn plasmid to generate the prpoEKOupdnKm, a plasmid containing the Kmr cassette-disrupted combined upstream and downstream sequence of rpoE. The fragment containing the Kmr cassette-disrupted combined upstream and downstream sequence of rpoE was cleaved by SalI/SphI from prpoEKOupdnKm and ligated into SalI/SphI-cleaved pUT/mini-Tn5(Km) to generate pUTrpoEKO. Gene inactivation mutagenesis by homologous recombination and confirmation of mutants with double-crossover events were performed as described previously (23). The rseA mutant was obtained in the same way using the primer pairs rseA-upF/rseA-upR and rseA-downF/rseA-downR.
For complementation of rpoE mutant, the fragments containing full-length rpoE and rseA were amplified by PCR by using primer pairs rpoEcom-F/rpoEcom-R and rseAcom-F/rseAcom-R and cloned into pGEM-T Easy (Promega), respectively, to generate the plasmids prpoE-com and prseA-com. prpoE-com and prseA-com were transformed into the rpoE and rseA knockout mutants, respectively, to generate the RpoE-complemented and RseA-complemented strains.
Reporter assay.
The rpoE-xylE reporter plasmid (24)-transformed wild-type and mutant strains were grown overnight in LB broth with 20 μg of chloramphenicol/ml and diluted 100-fold in the same medium, and then the XylE activity was monitored at 3, 5, and 7 h and overnight after incubation. For testing signals for rpoE expression, the wild type and mutants containing the rpoE-xylE reporter plasmid were grown overnight in LB broth, diluted 100-fold, cultured to an optical density at 600 nm (OD600) of 0.5 at 37°C, and exposed to 20 μg of polymyxin B (PB)/ml or 0.5 M urea for 2 h. The XylE activity was then measured as described previously (24).
Swarming assay.
The swarming migration assays was performed as described previously (24, 25). Briefly, overnight bacterial cultures (5 μl) were inoculated centrally onto the surface of dry LB swarming plates containing 2% (wt/vol) agar, which were then incubated at 37°C. The swarming migration distance was measured by monitoring the swarm fronts of the bacterial cells at 1-h intervals.
Measurement of the hemolysin activity.
The overnight bacterial cultures (120 μl) were inoculated onto the surface of dry LB swarming plates, which were then incubated at 37°C. The hemolysin activity was determined at 5 and 7 h after inoculation, as described previously (24, 25).
Cytotoxicity assay.
Cells (5 × 103 cells/well) of the human urothelial cell line NTUB1, which was originally derived from a urinary bladder carcinoma (23), were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) under 5.0% CO2 for 2 days using 96-well culture plates. Overnight bacterial cultures (120 μl) were inoculated onto the surface of dry LB swarming plates, and bacteria were collected and washed with phosphate-buffered saline (PBS) at 5 h after incubation at 37°C. The bacterial number were adjusted to 106 CFU/ml by RPMI 1640, 200 μl of P. mirabilis strains were applied to each well at a multiplicity of infection (MOI) of 20, and the plates were incubated at 37°C for 2 h. The culture supernatants were collected, and the lactate dehydrogenase (LDH) release was quantified using a CytoTox 96 nonradioactive cytotoxicity kit (Promega) according to the manufacturer's instructions. Cytotoxicity was determined by calculating the percentage of LDH release relative to the maximum LDH release from uninfected NTUB1 lysed with Triton X-100.
IE assay.
The expression of P. mirabilis MR/P fimbriae, encoded by mrp operon, depends on the promoter orientation (9, 12). The IE “on” means the promoter direction is for mrp operon expression. Genomic DNA from overnight and exponential cultures (OD600 of 0.5) of various P. mirabilis strains were prepared for amplification of the invertible element (IE) element using mrpP1 and mrpP2 primers (see Table S1 in the supplemental material). The PCR product was digested with AflII and resolved on a 2% agarose gel.
Real-time RT-PCR.
To study the effect of the RpoE gene on the mRNA levels of mrpA, mrpJ, mrpI, flhDC, and fliC1, total RNA of the wild type, the rpoE mutant, and the rseA mutant from overnight LB cultures and cells grown on agar plates for 5 h after seeding was extracted, and real-time reverse transcription-PCR (RT-PCR) was performed as described previously (24). The levels of mRNA were normalized against gyrB mRNA.
Expression and purification of the MrpA-His6 fusion protein and preparation of mouse antiserum against MrpA.
The mouse polyclonal antiserum against MrpA was obtained as described previously (26). A 528-bp BamHI/XhoI fragment containing mrpA was cloned into the BamHI/XhoI sites of pET32a (Novagen) to construct pET-MrpA. MrpA was expressed as a fusion protein tagged with a His6 tail from pET-MrpA upon induction with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The MrpA-His6 fusion protein was purified using Ni2+-NTA resin (Invitrogen) according to the protocol provided by the manufacturer. The purified MrpA-His6 fusion protein was desalted on a Ultracel-10K centrifugal filter device (Millipore) buffered with PBS. The protein sample was quantified by a Bio-Rad protein assay. The predicted 40-kDa MrpA-His6 protein was resolved on an SDS–12.5% polyacrylamide gel and subsequently excised from the gel. To raise antiserum against MrpA, the excised gel containing MrpA was emulsified in Freund complete adjuvant and subcutaneously injected into BALB/c mouse (50 μg of protein/mouse). At 2 and 4 week after the primary immunization, each mouse was given a booster injection of 50 μg of protein emulsified in Freund incomplete adjuvant. Serum was collected 2 weeks after the final booster. The specificity of the antiserum was established using P. mirabilis hfq mutant (whose mrp operon is OFF) as the negative control, and the correct size of MrpA was predicted to be ∼18 kDa.
Western blot analysis.
Overnight LB broth cultures (15 ml) of various P. mirabilis strains were washed, and the pellets were collected and resuspended in PBS (1 ml). Total proteins of bacteria were extracted by sonication, quantified by Bio-Rad protein assay, and adjusted to the same concentration. The protein samples were denatured in the sample buffer (100°C, 5 min), electrophoresed on an SDS–12.5% polyacrylamide gel, and then transferred to a Amersham Hybond-P membrane (GE Healthcare). The blot was incubated with mouse polyclonal antiserum against MrpA described above, followed by sheep anti-mouse immunoglobulin G-horseradish peroxidase conjugate (GE Healthcare), and then developed using enhanced chemiluminescence detection reagents (Perkin-Elmer).
Macrophage infection assay.
The assay was performed as described previously (27), with some modifications. Briefly, 12-well plates were seeded with 105 cells (per well) of THP-1 cells, and THP-1 cells were differentiated by using 50 ng of phorbol myristate acetate/ml in RPMI 1640 with 10% FCS under 5.0% CO2 for 24 h. The overnight cultures of P. mirabilis strains were applied to each well at an MOI of 10 (106 CFU/well). Bacteria were brought into contact with macrophages by centrifugation, followed by incubation for 30 min at 37°C. After infection, the cells were washed with PBS and incubated for 1 h in RPMI 1640 with 250 μg of streptomycin/ml to kill extracellular bacteria. Immediately, cells in some wells were lysed by 1% Triton X-100 to determine the CFU of intracellular bacterial cells at t0; others were incubated in medium containing 250 μg of streptomycin/ml for an additional 1 and 4 h to obtain the respective CFU counts.
Cell invasion assay.
The wild-type P. mirabilis and its mutants were cultured in LB broth at 37°C overnight before the cell invasion assay was performed according to the protocol of Jiang et al. (28). Briefly, human urothelial NTUB1 cells were grown and then infected with a bacterial suspension containing 107 cells (MOI = 10) for 1 h. Urothelial cells were then washed and incubated at 37°C in 1 ml of RPMI 1640 medium containing streptomycin for another 1 h. The cells were washed again with PBS and then lysed using 1 ml of lysis solution. Cell lysates were diluted serially and viable bacteria were counted by plating on LSW− agar plates. Cell invasion ability was determined as the percentage of viable bacteria that survived the streptomycin treatment versus the total inoculum, and the relative invasion was expressed.
Cytokine expression of NTUB1 cells.
Determination of the relative levels of selected human cytokines and chemokines was performed using Human Cytokine Array Panel A (R&D Systems, USA) according to the manufacturer's instructions. In brief, the NTUB1 cells were grown in the 12-well plate (106 cells/well) and incubated with overnight bacterial cultures in RPMI 1640 (107 cells/well) at an MOI of 10 for 3 h at 37°C. The culture supernatant and NTUB1 cells were then subjected to analyses of the cytokine array and real-time RT-PCR, respectively. A mixture of NTUB1 cell culture supernatant and the detection antibody was added to the membrane spotted with capture antibodies. After incubation, the membrane was washed, and the chemiluminescence produced by the sample/antibody hybrid on the membrane was detected after the addition of streptavidin-labeled horseradish peroxidase. NTUB1 cells were washed, the total RNA was extracted, and real-time RT-PCR was performed as described previously (24, 29) using the primers listed in Table S1 in the supplemental material to examine the mRNA expression of IL-8, MIF, PAI-1, and CXCL1 against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA. To study the effect of LPS on the expression of IL-8 mRNA, LPS (0.1, 1, and 5 μg/ml) extracted from P. mirabilis strains as described previously (24, 28) was applied to wells with NTUB1 cells, the plates were incubated for 3 h, and the total RNA was isolated for real-time RT-PCR.
Infection of mice.
The C57BL/6 mouse model of UTIs was used as described previously (29). Briefly, 6-week-old female mice were injected transurethrally with a 50-μl overnight culture suspension of P. mirabilis strains at a dose of 107 CFU per mouse. On days 3 and 6 after injection, the mice were sacrificed, and bladder and kidney samples were collected, weighed, suspended in 0.5 ml of PBS, and then homogenized to determine the viable bacterial count by plating on LSW− agar plates. All animal experiments were performed in strict accordance to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Laboratory Animal Center (Taiwan), and the protocol was approved by the Institutional Animal Care and Use Committee of National Taiwan University College of Medicine. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
Hematoxylin and eosin staining.
One and six days after the transurethral inoculation of PBS, the wild type, the rseA mutant, and the rpoE mutant, respectively, C57BL/6 mice (as described above) were sacrificed, and for each mouse, bladder and the kidney samples were collected, preserved in 10% formalin (pH 7.2), embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined microscopically. The extent of renal pathology was scored as follows (30): 0, no inflammation; 1, polymorphonuclear neutrophils (PMNs) confined to the peripelvic region; 2, PMN clusters detectable in the papilla or peripelvic cortex; and 3, widespread extension of PMNs into the cortex or outer medulla. The severity of histological modifications of each bladder was scored as follows (30): 0, no histological modifications; 1, occasional submucosal immune cell infiltrates; 2, widespread submucosal immune cell infiltration with minimal spread to the muscularis or epithelium; and 3, widespread inflammation with dense perivascular cuffs, transmural distribution, and intraepithelial inflammatory cells. The investigator was blind to the identity of the bacterial strain.
Nucleotide sequence accession numbers.
The nucleotide sequences of P. mirabilis intergenic region between mrpA and mrpI and the region containing rpoE and rseA genes have been deposited in GenBank under accession numbers KJ814628 and KJ814629, respectively.
RESULTS
Identification and characterization of the P. mirabilis rpoE operon.
To investigate the roles of rpoE in P. mirabilis, we first searched in the released genome of P. mirabilis HI4320 (accession no. AM942759) and found that the rpoE operon of P. mirabilis, like that of E. coli, consists of rpoE, rseA, rseB, and rseC genes. Using primers annealing to conserved sequences, we cloned and sequenced the fragment containing rpoE, rseA, and the region upstream of rpoE in P. mirabilis N2. The nucleotide sequences of rpoE and rseA were both found to be 99% identical to those of P. mirabilis HI4320. The amino acid sequences of RpoE and RseA were 87 and 57%, respectively, identical to UPEC strain CFT073. Analysis of the upstream sequence of the rpoE operon revealed a putative binding site for RpoE, 5′-AACttcattctattttaatgcTCtaA-3′, based on the conserved binding sequence (5′-AACtt-N16-TCnaA-3′) (20). Capital and lowercase letters represent the relative frequencies of each base at that position; the sequences in >80% are in capital letters. Knowing RseA is an anti-RpoE factor and RpoE is autoregulated (20), we hypothesized that mutation in rseA might cause RpoE overexpression in P. mirabilis. Therefore, the rpoE and rseA mutants were constructed, and a reporter assay was performed in the wild type, the rseA mutant, and the rpoE mutant containing the rpoE-xylE plasmid. As shown in Fig. 1, the promoter activity of rpoE (XylE activity) was decreased in the rpoE mutant but increased in the rseA mutant compared to the wild type at 3, 5, and 7 h and overnight after incubation. The XylE activities of both the wild type and the rseA mutant, but not the rpoE mutant, exhibited a growth-phase-dependent pattern, which was most pronounced during the stationary phase. Thus, we concluded RpoE was positively autoregulated and that rseA mutant was an rpoE overexpression strain of P. mirabilis.
FIG 1.
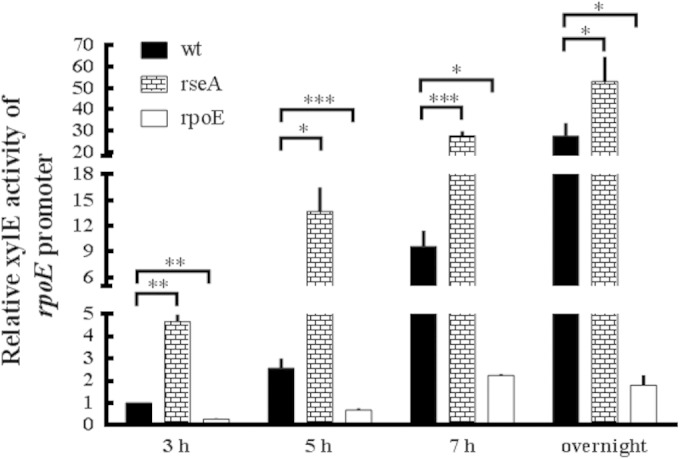
Promoter activity of rpoE in wild-type P. mirabilis, rseA mutant, and rpoE mutant strains. The activities of XylE in the rpoE-xylE reporter plasmid-transformed wild-type, rseA mutant, and rpoE mutant strains were determined by reporter assay at 3, 5, and 7 h and overnight after incubation. The XylE activity of the wild type at 3 h was set at 1, and other data are presented relative to this value. The data are averages and standard deviations of three independent experiments. Significant differences were determined by using the Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). wt, wild type; rseA, rseA mutant; rpoE, rpoE mutant.
P. mirabilis RpoE regulated swarming motility, hemolysin activity, and cytotoxicity to urothelial cells.
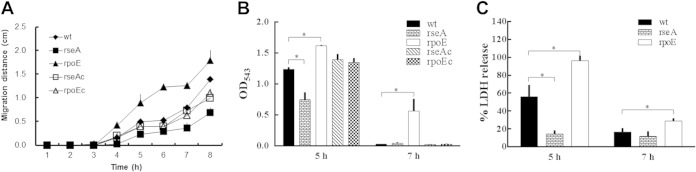
The swarming phenomenon is a metabolically complicated process, so any factors that affect this phenomenon would likely affect the fitness of the organism (1, 2). We thus tested the swarming behavior of wild-type and mutant strains and found that the swarming distance was increased for the rpoE mutant but decreased for the rseA mutant at 4, 5, 6, 7, and 8 h after inoculation onto the swarming plate (Fig. 2A). Both complemented strains (designated rseAc and rpoEc) restored almost wild-type swarming. Previous studies have shown that multiple virulence factors are coordinately expressed during P. mirabilis swarming migration (31, 32). P. mirabilis hemolysin was a pore-forming cytotoxin, contributing to the damage of human renal cells (6). We then tested the hemolysin activity. As shown in Fig. 2B, the hemolysin activity was decreased in the rseA mutant but increased in the rpoE mutant compared to the wild-type strain after growing on swarming plates for 5 h. At 7 h after inoculation, the rpoE mutant exhibited significant hemolysin activity in contrast to other strains. Accordingly, we found that the rpoE mutant differentiated into more swarmer cells at 7 h after incubation than did the other strains (data not shown). These data indicated that RpoE could inhibit swarming motility, swarm cell differentiation, and swarming-related hemolysin activity. Knowing P. mirabilis RpoE inhibited hemolysin activity, we hypothesized that RpoE might influence cytotoxicity. To identify bacterium-induced cytotoxicity, the wild-type and mutant (rseA and rpoE) strains were cocultured with NTUB1 cells, respectively, and the LDH released was measured. As shown in Fig. 2C, the rpoE and rseA mutants displayed higher and lower cytotoxicities, respectively, relative to the wild type. Thus, mutation of rpoE increased the cytotoxicity of P. mirabilis.
FIG 2.
Effect of P. mirabilis RpoE on swarming, hemolysin activity, and cytotoxicity. (A) Swarming migration of the wild-type, rseA (rseA) and rpoE (rpoE), and complemented rseA (rseAc) and rpoE (rpoEc) strains. Aliquots (5 μl) of overnight culture were inoculated centrally onto LB swarming plates. The plates were incubated at 37°C, and the migration distance was measured hourly after inoculation. (B) Hemolysin activities of the wild-type, rseA and rpoE mutants, and complemented rseA and rpoE strains. The hemolysin activity was determined by measuring the OD543 at 5 and 7 h after seeding the bacteria on LB swarming plates. (C) Cytotoxicity of wild-type, rseA mutant, and rpoE mutant strains to NTUB1 cells. Bacterial strains after seeding onto LB swarming plates for 5 and 7 h were cocultured with NTUB1 cells. After 2 h, the cytotoxicity was determined by quantifying the percentage of LDH release relative to the maximum LDH release from uninfected NTUB1 lysed with Triton X-100. All data are the averages, and standard deviations of three independent experiments are given. Significant differences were determined by using the Student t test (*, P < 0.05).
Regulation of MR/P fimbria expression by RpoE and the effect of RpoE-regulated mrpJ on expression of flhDC and fliC1.
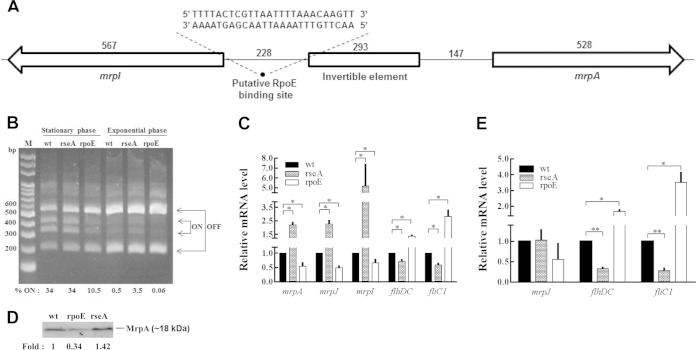
P. mirabilis MR/P fimbriae have been shown to be important in UTIs (12–15). We investigated whether RpoE affects expression of MR/P fimbriae in DNA, mRNA, and protein levels. The IE assay using exponential-phase and overnight LB broth cultures was performed to determine the ON (fragments of 336 and 407 bp) or OFF (fragments of 204 and 539 bp) position of the IE. The IE was located on an intergenic region between mrpA and mrpI (Fig. 3A). Both ON and OFF orientations were found in stationary-phase cultures of the wild type, the rseA mutant, and the rpoE mutant, with less “ON” in the rpoE mutant (Fig. 3B). Exponential-phase cultures of all strains appeared to favor the OFF position compared to the stationary phase and almost no “ON” in the rpoE mutant. Based on ImageJ software quantification, the relative proportions of DNA in the ON phase of stationary wild-type, rseA mutant, and rpoE mutant strains were 34, 34, and 10.5%, respectively, while those of exponential-phase cells were 0.5, 3.5, and 0.06%, respectively. Real-time RT-PCR and Western blot analysis were used to further confirm the results of the invertible element assay. Accordingly, the mrpA and mrpJ mRNA levels of the rpoE mutant were lower, but those of the rseA mutant were significantly higher than the that of the wild type (Fig. 3C). Figure 3D indicates that the MrpA protein level of rpoE mutant was also lower than that of the wild type and rseA mutant. These results indicated that RpoE regulated the expression of the mrp operon. Therefore, we searched the consensus sequence of RpoE binding site in the intergenic region between mrpA and mrpI and found the putative binding sequence, 5′-AACttgtttaaaattaacgagTaaaA-3′, only upstream of mrpI (Fig. 3A). It has been shown that switching to the ON orientation of IE occurred to a greater extent when expression of MrpI was induced by IPTG (9). To demonstrate whether the expression of mrpI gene is regulated by RpoE, which further influences the ON/OFF of the mrp operon, we tested for the expression of mrpI in wild-type, rseA mutant, and rpoE mutant strains. As shown in Fig. 3C, the mRNA amount of mrpI was decreased in the rpoE mutant but increased in the rseA mutant. In summary, the data revealed P. mirabilis RpoE regulated mrpI expression and suggested that RpoE affected mrp expression through regulating mrpI. In addition, we found that excess MrpI does not contribute more to turn on the mrp operon, based on the observation that the ON percentages of wild-type and rseA mutant were almost the same (Fig. 3B and C).
FIG 3.
Regulation of mrp operon, mrpI, flhDC, and fliC1 by P. mirabilis RpoE. (A) Schematic drawing of the intergenic region between mrpA and mrpI. The sequence of the putative RpoE binding site upstream of mrpI is “5′-AACttgtttaaaattaacgagTaaaA-3”. The numbers indicate the sizes (in bp) of each DNA fragment. (B) Promoter orientation of mrp operon in wild-type, rseA mutant, and rpoE mutant strains determined by the IE assay. The assay was performed as described in Materials and Methods using overnight and exponential cultures. “ON” means the direction of mrp promoter is for mrp operon expression. The “% ON” is indicated by the band intensity of the ON band compared to those of the ON and OFF bands. M, marker. (C) Expression of mrpA, mrpJ, mrpI, flhDC, and fliC1 in wild-type, rseA mutant, and rpoE mutant strains cultured in LB broth. Total RNA was extracted from overnight cultures of wild-type, rseA mutant, and rpoE mutant strains, and the mRNA amounts of mrpA, mrpJ, mrpI, flhDC, and fliC1 were quantified by real-time RT-PCR. (D) Effect of RpoE on the protein level of MrpA. The total proteins were extracted from overnight cultures of wild-type, rseA mutant, and rpoE mutant strains, and Western blot analysis with antiserum against MrpA was performed as described in Materials and Methods. The fold change of the mutants is given relative to the band intensity of the wild type. (E) Expression of mrpJ, flhDC, and fliC1 in wild-type, rseA mutant, and rpoE mutant strains cultured on LB agar plates. Total RNA was extracted from bacterial cells grown on LB agar plates at 5 h after seeding, and the mRNA amounts of mrpJ, flhDC, and fliC1 were quantified by real-time RT-PCR. In panels B and D, the representative result from three independent experiments is shown. In panels C and E, the value obtained for the wild-type cells was set at 1, and all other data were expressed relative to this value after being normalized to a housekeeping gene (gyrB). The data are the averages and standard deviations of three independent experiments, and significant differences were determined using the Student t test (*, P < 0.05; **, P < 0.01). wt, wild type; rseA, rseA mutant; rpoE, rpoE mutant.
Given that the P. mirabilis mrp operon is off in agar-grown cells (9, 12), MrpJ is a repressor of flhDC (encoding the flagellar transcriptional regulator) (26, 33), and RpoE regulated the expression of the mrp operon (Fig. 3B and D), we compared the mRNA expression of mrpJ, flhDC, and fliC1 (encoding flagellin) in wild-type, rseA mutant, and rpoE mutant strains cultured in LB broth and on plates to clarify the effect of rpoE-mediated mrpJ expression on the expression of flhDC and fliC1. We found that mrpJ was positively regulated by RpoE in broth cultures but that alterations of RpoE had no effect on the expression of mrpJ in plate cultures, a phase-off condition for mrp (Fig. 3C and E). In addition, flhDC and fliC1mRNA levels were decreased in the rseA mutant and increased in the rpoE mutant from both broth and plate cultures (Fig. 3C and E). In this regard, we found that the rseA mutant displayed a significant reduction in swimming (see Fig. S1 in the supplemental material) and swarming (Fig. 2A) compared to the wild type. These results indicate that RpoE-mediated repression of swarming and the expression of flhDC or fliC1 in cells from plate cultures is not MrpJ dependent but that MrpJ contributes to the RpoE-mediated reduction of swimming motility and fliC1 expression in cells from broth cultures.
Role of P. mirabilis RpoE in survival in macrophages, invasion ability, and induction of cytokine expression.
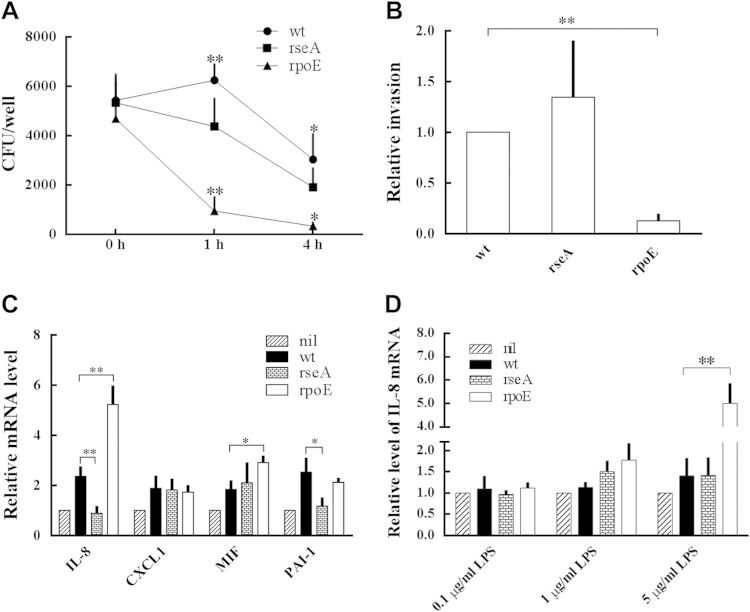
To understand whether RpoE is involved in the innate response of macrophages (34) to eliminate P. mirabilis, we challenged THP-1 cells with wild-type and mutants, killed external bacteria with streptomycin, and assessed the survival of internalized P. mirabilis. We found that there was no significant difference between the intramacrophage survival of wild-type and mutant cells immediately after streptomycin treatment. However, the CFU counts of intramacrophage rpoE mutant were reduced significantly after further incubation for 1 and 4 h compared to the wild type (Fig. 4A), indicating that the loss of rpoE impaired the survival of P. mirabilis in macrophages. The rseA mutant exhibited a survival pattern similar to that of the wild type.
FIG 4.
Effect of P. mirabilis RpoE on survival in macrophages, cell invasion, and cytokine expression. (A) Survival of the wild-type, rseA mutant, and rpoE mutant strains in macrophages. THP-1 cells were infected with bacteria for 30 min at an MOI of 10, and the survival of the intracellular bacteria was determined by the streptomycin protection assay at 0, 1, and 4 h. Asterisks indicate significant differences between wild-type and rpoE mutant strains at 1 and 4 h (*, P < 0.05; **, P < 0.01). (B) Invasion abilities of wild-type, rseA mutant, and rpoE mutant strains. The ability to invade NTUB1 cells was determined as described in Materials and Methods. The ability of the wild type was set at 1, and other data are presnted relative to this value. **, Significant difference between wild-type and rpoE mutant strains (P < 0.01). (C) Expression of IL-8, CXCL1, MIF, and PAI-1 in NTUB1 cells treated with wild-type, rseA mutant, or rpoE mutant strains. IL-8, CXCL1, MIF, and PAI-1 mRNA levels of NTUB1 cells after challenging with the bacteria were determined by real-time RT-PCR. (D) IL-8 expression of NTUB1 cells treated with LPS extracted from the wild-type, rseA mutant, or rpoE mutant strains. LPS at 0.1, 1, or 5 μg/ml was coincubated with NTUB1 cells, and the mRNA amounts of IL-8 were measured 3 h after incubation by real-time RT-PCR. In panels C and D, the value of NTUB1 cells without treatment (nil) was set at 1, and other data are given relative to this value. The data are averages and standard deviations of three independent experiments, and significant difference were determined by using the Student t test (*, P < 0.05; **, P < 0.01). wt, wild type; rseA, rseA mutant; rpoE, rpoE mutant.
Given that UPEC evaded the host defense mechanism by invading into the epithelium (2, 18, 35) and P. mirabilis also can invade into urothelial cells (7, 28), we then assessed the role of RpoE in invasion into NTUB1 cells. As shown in Fig. 4B, the rpoE mutant was significantly less able to invade cells than was the wild type.
The production of cytokines and chemokines from epithelial cells initiates the innate immune responses in UTIs, and the immune cells such as neutrophils would be attracted to the infection site to eliminate the pathogens (16). Therefore, we next examined the role of RpoE in inducing the expression of cytokines and chemokines. To investigate a variety of cytokines and chemokines produced by NTUB1 cells after coculture with P. mirabilis wild-type or rpoE mutant strains, a human cytokine array was used. We found that spots of IL-8, CXCL1, MIF, and PAI-1 appeared more intense from supernatants of NTUB1 cells treated with the rpoE mutant (data not shown). To confirm the expression of IL-8, CXCL1, MIF, and PAI-1, real-time RT-PCR was performed using NTUB1 cells treated with various P. mirabilis strains. Both wild-type and rpoE mutant strains can induce IL-8 mRNA, though significantly more was induced by the rpoE mutant than by the wild type (Fig. 4C). rseA mutant (RpoE overexpression)-treated cells expressed IL-8 mRNA similarly to the untreated control (Fig. 4C). In addition, the rpoE mutant induced a significantly higher level of MIF mRNA than did the wild type, and the rseA mutant inhibited the mRNA level of PAI-1 (a cytokine supporting IL-8-mediated neutrophil migration) relative to the wild type (Fig. 4C). It has been reported that the innate immune response relies on recognition of pathogen-associated molecular patterns (PAMPs) (17). LPS is a prominent feature of Gram-negative bacteria, being one of the most potent PAMPs known and responsible for the inflammatory response (17). Moreover, RpoE regulates biogenesis and the modification of LPS in Gram-negative bacteria (20, 36), so we hypothesized that RpoE-regulated LPS might be one of the bacterial components to affect the IL-8 expression of NTUB1 cells. To this end, we treated NTUB1 cells with LPS (0.1, 1, and 5 μg/ml) extracted from wild-type, rseA mutant, and rpoE mutant strains, respectively, and the IL-8 mRNA level was determined. As shown in Fig. 4D, the IL-8 mRNA amount of NTUB1 cells was induced ∼5-fold by 5 μg of LPS/ml from the rpoE mutant only. Altogether, RpoE-mediated LPS changes could explain the difference of IL-8 expression induced by wild-type P. mirabilis and the rpoE mutant.
Colonization of the urinary tract was attenuated in the P. mirabilis rpoE mutant.
Knowing that P. mirabilis RpoE controlled the expression of diverse virulence factors mentioned above, including swarming, hemolysin, fimbriae, invasion, and modulating the host immune responses, we established the C57BL/6 mouse model for UTIs (29) to determine the effect of inactivation of either rpoE or rseA on the colonization ability of P. mirabilis. As shown in Fig. 5, the rpoE mutant had a severely impaired ability to colonize within the bladders and kidneys relative to the wild type. The colonization of the RpoE-complemented strain showed no significant difference from the wild type, and the rseA mutant did so except on day 3 from bladder samples. A significant difference in the bacterial load was observed between the wild type and the rpoE mutant on days 3 and 6 after transurethral inoculation. This result indicated RpoE is required for the colonization and survival of P. mirabilis in the mouse urinary tract.
FIG 5.
Colonization of wild-type P. mirabilis, rseA mutant, rpoE mutant strains and the RpoE-complemented strain in mice. C57BL/6 mice (ten mice per group) were inoculated transurethrally with overnight cultures of bacterial strains at a dose of 107 CFU per mouse. Bacterial loads were determined in the bladder and kidneys on day 3 (A) and day 6 (B) after inoculation. Horizontal bars indicate median values for each group, and the dotted horizontal line indicates the limit of detection (100 CFU). Circles, squares, triangles, and diamonds represent the wild type, the rseA mutant, the rpoE mutant, and the RpoE-complemented strain retrieved from mice, respectively. Significant differences were determined observed by using the Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). wt, wild type; rseA, rseA mutant; rpoE, rpoE mutant; rpoEc, RpoE-complemented strain.
Histopathologic evaluation of mouse bladders and kidneys.
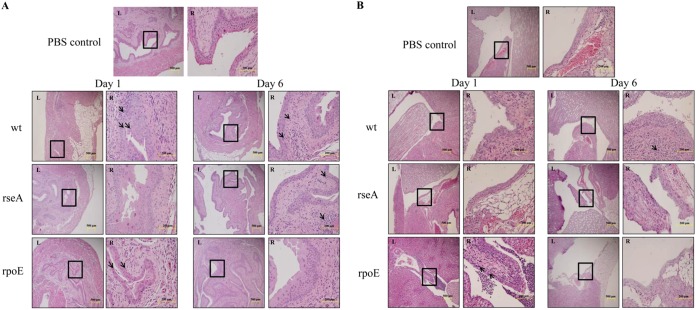
Knowing that the loss of rpoE led to higher mRNA levels of IL-8 and MIF (Fig. 4C), which could attract the immune cells to confine the bacterial loads in vivo, we also evaluated the capacity of the wild type and mutants to induce immune cell infiltration in mouse bladders and kidneys. The histopathology of the bladder and kidneys was evaluated according to the criteria described by Alamuri et al. (30) and was expressed as a semiquantitative score. On day 1 postinoculation, light microscopic evaluation revealed a significant increase of immune cell infiltrates in bladders of the wild type- or rpoE-treated mice compared to the PBS control, while that of the rseA mutant was similar to the PBS control (Fig. 6A). Only the kidneys of rpoE-treated mice exhibited widespread extension of immune cells into the cortex or outer medulla, scored as 3, at 1 day after inoculation (Fig. 6B and Table 2). On day 6 postinoculation, colonization of the bladders by the wild type or rseA mutant was accompanied by occasional submucosal inflammatory cell infiltrates (scored as 1), and those of the rpoE mutant showed almost no sign of inflammatory cell infiltration (Fig. 6A and Table 2). At 6 days after inoculation, the kidneys of rseA or rpoE mutant-inoculated mice displayed almost no sign of immune cell infiltration, in contrast to those of the wild type, with PMN clusters detectable in the papilla or the peripelvic cortex (Fig. 6B and Table 2). Altogether, the rpoE mutant could recruit immune cells in the bladder and kidneys during the early stage of infection (Fig. 6). The wild type induced immune cell infiltration in the bladder but not kidneys on day 1 after inoculation and in both the bladder and the kidneys on day 6 (Fig. 6). Interestingly, the rseA mutant could not recruit immune cells in the kidneys (Fig. 6B).
FIG 6.
Histological images of mice infected with wild-type P. mirabilis, rseA mutant, or rpoE mutant strains. C57BL/6 mice were infected with wild-type, rseA mutant, or rpoE mutant strains as in Fig. 5, and mice were sacrificed on day 1 and day 6 postinfection. Bladder (A) and kidney (B) samples were collected, preserved in formalin, embedded, sectioned, stained, and observed microscopically. The amplified image of the rectangle area of each left panel (×100) is shown on the right at ×400. The site of immune cell infiltration is indicated by arrows. wt, wild type; rseA, rseA mutant; rpoE, rpoE mutant.
TABLE 2.
Histopathological evaluation of mice inoculated with wild-type P. mirabilis, rseA mutant, and rpoE mutant
| Strain | Scorea |
|||
|---|---|---|---|---|
| Day 1 |
Day 6 |
|||
| Bladder | Kidney | Bladder | Kidney | |
| Wild type | 2 | 0 | 1 | 2 |
| rseA mutant | 0 | 0 | 1 | 0 |
| rpoE mutant | 3 | 3 | 0 | 0 |
| PBS control | 0 | 0 | 0 | 0 |
The scores for histological modifications of the kidney are presented: 0, no inflammation; 1, PMNs confined to the peripelvic region; 2, PMN clusters detectable in the papilla or peripelvic cortex; and 3, widespread extension of PMNs into the cortex or outer medulla. The scores for the histological modifications of each bladder are also presented: 0, no histological modifications; 1, occasional submucosal immune cell infiltrates; 2, widespread submucosal immune cell infiltration with minimal spread to the muscularis or epithelium; and 3, widespread inflammation with dense perivascular cuffs, transmural distribution, and intraepithelial inflammatory cells.
Urea and polymyxin B induced promoter activity of the rpoE gene in P. mirabilis.
Based on the fact that P. mirabilis RpoE plays such an important role in virulence factor expression and colonization in mouse, it was tempting to determine what the signals activate the expression of RpoE in the urinary tract, an environment containing antimicrobial peptides and a lot of urea. Therefore, the XylE activities of rpoE-xylE reporter in the wild type and mutants were determined in the presence of urea or polymyxin B, a cationic antimicrobial peptide. The promoter activity of the rpoE gene was induced by 20 μg of PB/ml or 500 mM urea after incubation for 2 h in the wild type but not in the rseA and rpoE mutants (Fig. 7). The results suggested that P. mirabilis RpoE could be activated in the host urinary tract during infection.
FIG 7.
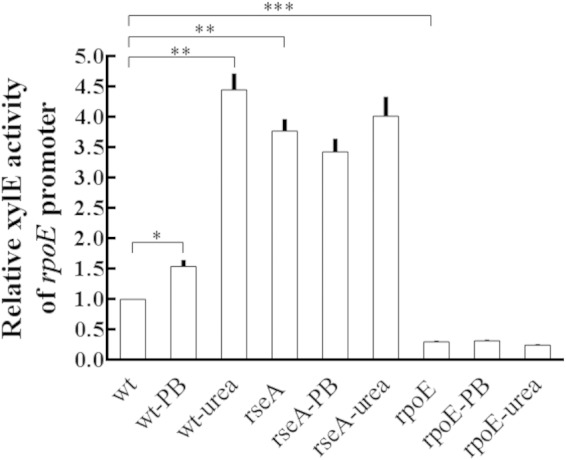
Promoter activity of P. mirabilis rpoE induced by urea and PB. The wild-type, rseA mutant, and rpoE mutant strains containing the rpoE-xylE reporter plasmid were grown overnight in LB broth, diluted, cultured to an OD600 of 0.5 at 37°C, and exposed to 20 μg of polymyxin B (PB)/ml or 0.5 M urea for 2 h. The XylE activity was measured. The XylE activity of wild-type without treatment was set at 1, and other data are given relative to this value. The data are the averages and standard deviations of three independent experiments. Significant differences were determined by using the Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). wt, wild type; rseA, rseA mutant; rpoE, rpoE mutant.
DISCUSSION
RpoE is involved in virulence, surface stress responses, and modulation of the inflammatory response during infections (20–22, 37). RpoE of uropathogenic E. coli plays roles in motility, biofilm formation, and sensitivity to polymyxin B (38). In the present study, we demonstrated the role of RpoE in the regulation of virulence in uropathogenic P. mirabilis. Swarming motility, hemolysin activity, and cytotoxicity were increased, but fimbria expression, cell invasion, and survival in macrophages were decreased in the rpoE mutant (Fig. 2, 3, and 4). The rpoE mutant induced higher mRNA levels of IL-8 and MIF than did the wild type, but RpoE overexpression (in the rseA mutant) decreased the mRNA levels of IL-8 and PAI-1 (Fig. 4C). Moreover, the rpoE mutation significantly impaired the strain's ability to colonize mice (Fig. 5).
Many observations have indicated that MR/P fimbriae are an important virulence factor for uropathogenic P. mirabilis. In a murine model, UTIs with P. mirabilis elicited a strong immune response to MrpA, indicating that MR/P fimbriae were expressed in vivo (39). Increased expression of MR/P fimbriae correlates with higher levels of colonization in the mouse bladder (14, 15). Vaccine trials in mice using MR/P fimbriae reduce bacterial burdens after challenge (40). Transcriptome analysis also revealed that genes encoding MR/P fimbriae were highly upregulated in vivo (13). The phase variation of MR/P fimbriae, mediated by MrpI, has been suggested as a virulence factor for uropathogenic P. mirabilis (12). Given that MrpI switches the IE in both directions, why can expression of mrp operon be almost off (on agar plates) or on (during UTIs in mice) (12)? Selection pressures or environmental signaling in the urinary tract may determine the outcome, while the regulation of MrpI expression and epigenetic modification of IE may be involved. This is the first study to demonstrate that RpoE increased fimbria (mrp operon) expression by regulating mrpI. Several lines of evidence support the notion. First, an RpoE binding site exists upstream of mrpI (Fig. 3A). Second, more OFF positions of IE were observed in the rpoE mutant than in the wild-type and rseA mutant strains (Fig. 3B). Third, RpoE positively regulated the mRNA levels of mrpI and two genes belonging to the mrp operon, mrpA and mrpJ (Fig. 3C). Fourth, the rpoE mutant had less MrpA protein relative to the wild type (Fig. 3D). On swarming agar plates, the IE of wild-type, rseA mutant, and rpoE mutant strains was in the OFF position (data not shown), and both ON and OFF appeared on subculturing in broth, being more “ON” in the stationary phase when the RpoE expression was increased (Fig. 3B and Fig. 1). On the contrary, Lane et al. suggested that MrpI preferentially switches the IE from ON to OFF (41). They found that there was more ON in low-O2 conditions than in atmospheric O2, a condition wherein mrpI expression is higher than under low O2 (41). It is possible that mrpI may be subjected to different regulation in atmospheric-O2 and low-O2 conditions. In this regard, an Escherichia coli two-component regulator, RcsB, influences the piliation state by controlling transcription of fimB and fimE recombinases in response to environmental cues (42).
We found that alterations of RpoE did affect expression of mrpJ in cells from broth cultures but not those from plate cultures, a phase-off condition for mrp (Fig. 3C and E). It is reasonable to infer that the agar surface may be sensed by P. mirabilis to swarm, a condition in favor of downregulating fimbriae and thus repressing expression of MrpJ. It is notable that the flhDC and fliC1mRNA levels were decreased in the rseA mutant from both broth and plate cultures (Fig. 3C and E). Moreover, the rseA mutant displayed a significant reduction in motility of swimming and swarming (see Fig. S1 in the supplemental material; Fig. 2A). These results suggest that RpoE-mediated swarming repression on agar plates is not MrpJ dependent but that RpoE-regulated MrpJ is involved in the reduction of swimming motility. Overexpression of RpoE (rseA mutant) could upregulate MrpI expression; MrpI then switches IE to the ON position to express the mrp operon, and then the production of MrpJ inhibits flhDC and fliC1 expression when P. mirabilis was subcultured from the LB agar plate into LB broth. The reciprocal regulation of MR/P fimbriae and flagella involving RpoE highlights the role of RpoE in establishing a UTI and the necessity for investigating the dynamics of RpoE expression during infection. Mobley et al. also reported elevated expression of MrpJ in P. mirabilis resulted in reduction of FliC1 protein and swimming motility using overnight broth cultures (26). These researchers found the phase ON mutant has a lower level of FlaA than the OFF mutant and the wild type (26). Obviously, other factors and not MrpJ regulated by RpoE account for the repression of swarming and expression of flhDC or fliC1 in cells from plate cultures. It is also possible that rpoE mutation may have other effects, except low expression of MrpJ, to result in higher expression of FliC1 than was seen with the wild type in broth cultures. In this view, De Lay et al. found several small RNAs inhibited motility of E. coli (43). Among them, we found an ArcZ homologue in P. mirabilis and a putative RpoE binding site in its promoter region. The work to characterize the role of P. mirabilis ArcZ in motility has been undertaken.
Several results of the present study suggested P. mirabilis RpoE could modulate immune responses. First, rpoE mutant induced higher levels of IL-8 and MIF (both protein and mRNA), and rseA mutant decreased IL-8 and PAI-1 mRNA relative to the wild type (Fig. 4C). Second, LPS of the rpoE mutant induced high levels of IL-8 mRNA (Fig. 4D). Third, the rpoE mutant exhibited lower survival inside macrophages than did the wild type. Fourth, the rpoE mutant caused more immune cell infiltration in both bladders and kidneys on day 1 after inoculation (Table 2 and Fig. 6), which corresponds to the increased proinflammatory cytokine expression (Fig. 4C and D). This is the first study to demonstrate that P. mirabilis RpoE affected proinflammatory cytokine expression of urothelial cells. IL-8, MIF, and PAI-1 could all induce the migration of immune cells to the infection site (16, 44–47). IL-8 has been shown to be the main neutrophil attractant in humans and is secreted by both bladder and kidney cell lines in response to uropathogenic E. coli (44). Our finding also supported that IL-8 could be important during UTIs caused by P. mirabilis.
The intracellular environment of a phagocyte may protect the bacteria during the early stages of infection or until they develop a full complement of virulence factors. It has been indicated that the deficiency of bactericidal activity of macrophages from cystic fibrosis patients against Pseudomonas aeruginosa is correlated with the increased susceptibility to bacterial infections in these patients (34). Our results that surviving bacteria of the rpoE mutant decreased rapidly inside macrophages indicated that RpoE may improve the fitness of P. mirabilis during the early stage of infection.
Bacterial LPS are capable of inducing bladder inflammation characterized by an increase in the release of proinflammatory cytokines and recruitment of the immune cells (17, 48). Many Gram-negative pathogens modify LPS to alter TLR4 responses (11, 49). For example, Salmonella PhoP/Q (a two-component system) can sense host environments, regulating genes involved in LPS modification (11). Salmonella modified LPS are up to 100-fold less active for TLR4-driven NF-κB activation, and the PhoP-null mutant is immunostimulatory in vivo (11, 50). We found that LPS at up to 5 μg/ml from the rpoE mutant but not from the wild type induced a significant level of IL-8 mRNA (Fig. 4D), suggesting that P. mirabilis RpoE may participate in LPS modification and that the absence of RpoE-mediated LPS modification results in TLR4 activation and increased IL-8 expression. RpoE in E. coli has been shown to be involved in LPS modification (20, 36). LPS profile analysis also revealed a difference in LPS between the P. mirabilis rpoE mutant and the wild type (data not shown). Moreover, knowing LPS modification influences PB resistance in P. mirabilis (23, 24, 28), the finding that the PB MIC for the wild type was higher than that for the rpoE mutant (>50,000 versus 12,500 μg/ml) also supports the notion.
Different authors have proposed that the induction of an innate response could prevent the progress of infection and that TLR4-mediated recognition of LPS was thought to activate the host defense against Gram-negative bacterial pathogens (17). For example, intranasal immunization of mice with MR/P fimbriae protects P. mirabilis from UTIs (40) and activating TLR4 by LPS impairs colonization of Bordetella pertussis (51). Accordingly, we found rpoE mutant increased the proinflammatory cytokines and the immune cell recruitment and had an impaired ability of colonization.
P. mirabilis could cause persistent UTIs despite antibiotic treatment and catheter changes (1, 2, 7). Variation of MR/P fimbriae (9) and flagellar antigens (8), secretion of ZapA protease (10), and the ability of cell invasion are known mechanisms that were utilized by P. mirabilis to contribute to immune evasion during infection (1, 3). The formation of biofilms and urinary stones is also thought to limit bacterial exposure to antibiotics and antibodies (1, 2, 7). We found that the loss of RpoE did not affect biofilm formation and, by reporter assay, that RpoE did not regulate zapA and ureC (mutation of the urease gene, ureC, prevents stone formation [1]) in P. mirabilis (data not shown). This indicates that RpoE might participate in immune evasion and the persistence of P. mirabilis during UTIs by facilitating invasion into urothelial cells and modulating the expression of fimbria and flagella and immune responses.
The ability to perceive changes in different environments and to modify gene activity is important for bacteria. The activation signals of RpoE could be heat/cold shock, oxidative stresses, osmotic stresses, stationary phase, and growth in vivo in Gram-negative bacteria (20). In the present study, we demonstrated three signals—PB, urea, and the stationary phase (Fig. 1)—for RpoE activation in P. mirabilis. We found that urea, a component of urine, and PB (a cationic antimicrobial peptide) can serve as signals to induce expression of RpoE, reinforcing the role of P. mirabilis RpoE in establishing UTIs. In 2011, Pearson et al. detected gene expression of P. mirabilis from infected mice by microarray analysis (microarray data accession number GSE25977) (13). These researchers found that genes encoding MR/P fimbriae were upregulated and that those for flagella were downregulated compared to an in vitro overnight broth culture. Accordingly, we observed that RpoE positively regulated mrp operon (fimbria expression) and negatively regulated fliC1 (flagellin) in the present study. We suggest that urea could maintain the appropriate level of activated RpoE in vivo.
We characterized here the significance of P. mirabilis RpoE in building up UTIs (Fig. 8). RpoE could sense the component of urine to regulate multiple virulence factors, including fimbria expression, swarming, cell invasion, survival in macrophages, colonization in mice, and modulation of host immune responses. Given the diverse regulons of RpoE, it is not surprising for such global effects of RpoE. Altogether, RpoE could help P. mirabilis adapt to its unique environmental niches for building up a UTI. Our discovery adds RpoE into the complex network of pathogenesis in uropathogenic P. mirabilis and deepens our understanding of the factors affecting the infectious process. The information is expected to contribute to the design of new strategies to improve the control of UTIs caused by P. mirabilis.
FIG 8.
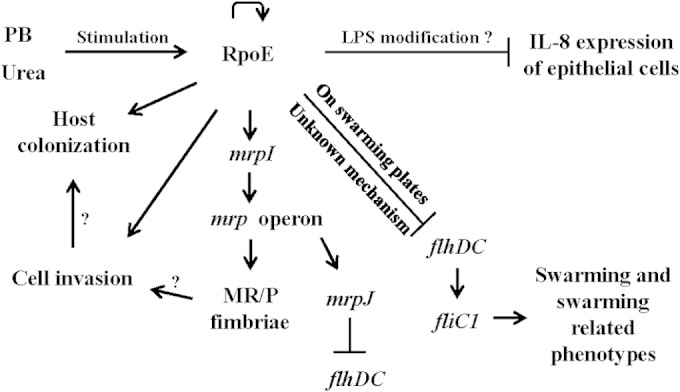
Summary of proposed RpoE roles in P. mirabilis. RpoE is autoregulated (curved arrow) and can be activated by urea or antimicrobial peptides in the urinary tract. Activated RpoE upregulates mrpI expression, and in turn MrpI controls the production of MR/P fimbriae, which are important for cell invasion and subsequent colonization. RpoE inhibits expression of the host cytokine (IL-8; line with a vertical bar). LPS plays a role in the RpoE-mediated IL-8 expression. In cells from broth cultures, mrpJ (one member of the mrp operon) contributes to RpoE-mediated flhDC repression. In cells from swarming agar plates, the regulation of flhDC expression, swarming, and swarming-related phenotypes by RpoE occurs through an MrpJ-independent pathway. A question mark (?) indicates that something was not demonstrated in the present study. PB, polymyxin B.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grant NSC-100-2320-B-002-075 from the National Science Council.
We thank Yeong-Shiau Pu (National Taiwan University Hospital) for providing the NTUB1 cell line and Betty A. Wu-Hsieh and Li-Chung Hsu for providing the THP-1 cells and technique support in the macrophage infection assay.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02232-14.
REFERENCES
- 1.Armbruster CE, Mobley HL. 2012. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol 10:743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielubowicz GR, Mobley HL. 2010. Host-pathogen interactions in urinary tract infection. Nat Rev Urol 7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 4.Jansen AM, Lockatell V, Johnson DE, Mobley HL. 2004. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect Immun 72:7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BV, Young R, Mahenthiralingam E, Stickler DJ. 2004. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect Immun 72:3941–3950. doi: 10.1128/IAI.72.7.3941-3950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mobley HL, Chippendale GR, Swihart KG, Welch RA. 1991. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun 59:2036–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathoera RB, Kok DJ, Verduin CM, Nijman RJ. 2002. Pathological and therapeutic significance of cellular invasion by Proteus mirabilis in an enterocystoplasty infection stone model. Infect Immun 70:7022–7032. doi: 10.1128/IAI.70.12.7022-7032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy CA, Belas R. 1999. Genomic rearrangements in the flagellin genes of Proteus mirabilis. Mol Microbiol 31:679–690. doi: 10.1046/j.1365-2958.1999.01209.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Li X, Johnson DE, Blomfield I, Mobley HL. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol Microbiol 23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]
- 10.Belas R, Manos J, Suvanasuthi R. 2004. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect Immun 72:5159–5167. doi: 10.1128/IAI.72.9.5159-5167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst RK, Guina T, Miller SI. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect 3:1327–1334. doi: 10.1016/S1286-4579(01)01494-0. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lockatell CV, Johnson DE, Mobley HL. 2002. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol Microbiol 45:865–874. doi: 10.1046/j.1365-2958.2002.03067.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearson MM, Yep A, Smith SN, Mobley HL. 2011. Transcriptome of Proteus mirabilis in the murine urinary tract: virulence and nitrogen assimilation gene expression. Infect Immun 79:2619–2631. doi: 10.1128/IAI.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zunino P, Geymonat L, Allen AG, Preston A, Sosa V, Maskell DJ. 2001. New aspects of the role of MR/P fimbriae in Proteus mirabilis urinary tract infection. FEMS Immunol Med Microbiol 31:113–120. doi: 10.1111/j.1574-695X.2001.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 15.Bahrani FK, Massad G, Lockatell CV, Johnson DE, Russell RG, Warren JW, Mobley HL. 1994. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect Immun 62:3363–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weichhart T, Haidinger M, Horl WH, Saemann MD. 2008. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest 38(Suppl 2):S29–S38. doi: 10.1111/j.1365-2362.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A 97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowley G, Spector M, Kormanec J, Roberts M. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat Rev Microbiol 4:383–394. doi: 10.1038/nrmicro1394. [DOI] [PubMed] [Google Scholar]
- 21.Crouch ML, Becker LA, Bang IS, Tanabe H, Ouellette AJ, Fang FC. 2005. The alternative sigma factor sigma is required for resistance of Salmonella enterica serovar Typhimurium to antimicrobial peptides. Mol Microbiol 56:789–799. doi: 10.1111/j.1365-2958.2005.04578.x. [DOI] [PubMed] [Google Scholar]
- 22.Raivio TL. 2005. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol Microbiol 56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang WB, Chen IC, Jiang SS, Chen HR, Hsu CY, Hsueh PR, Hsu WB, Liaw SJ. 2008. Role of RppA in the regulation of polymyxin b susceptibility, swarming, and virulence factor expression in Proteus mirabilis. Infect Immun 76:2051–2062. doi: 10.1128/IAI.01557-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang SS, Lin TY, Wang WB, Liu MC, Hsueh PR, Liaw SJ. 2010. Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrob Agents Chemother 54:2000–2009. doi: 10.1128/AAC.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu MC, Lin SB, Chien HF, Wang WB, Yuan YH, Hsueh PR, Liaw SJ. 2012. 10′(Z),13′(E)-heptadecadienylhydroquinone inhibits swarming and virulence factors and increases polymyxin B susceptibility in Proteus mirabilis. PLoS One 7:e45563. doi: 10.1371/journal.pone.0045563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Rasko DA, Lockatell CV, Johnson DE, Mobley HL. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J 20:4854–4862. doi: 10.1093/emboj/20.17.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rai MN, Balusu S, Gorityala N, Dandu L, Kaur R. 2012. Functional genomic analysis of Candida glabrata-macrophage interaction: role of chromatin remodeling in virulence. PLoS Pathog 8:e1002863. doi: 10.1371/journal.ppat.1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang SS, Liu MC, Teng LJ, Wang WB, Hsueh PR, Liaw SJ. 2010. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob Agents Chemother 54:1564–1571. doi: 10.1128/AAC.01219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang MC, Chien HF, Tsai YL, Liu MC, Liaw SJ. 2014. The RNA chaperone Hfq is involved in stress tolerance and virulence in uropathogenic Proteus mirabilis. PLoS One 9:e85626. doi: 10.1371/journal.pone.0085626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alamuri P, Eaton KA, Himpsl SD, Smith SN, Mobley HL. 2009. Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection. Infect Immun 77:632–641. doi: 10.1128/IAI.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser GM, Hughes C. 1999. Swarming motility. Curr Opin Microbiol 2:630–635. doi: 10.1016/S1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 32.Liaw SJ, Lai HC, Wang WB. 2004. Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect Immun 72:6836–6845. doi: 10.1128/IAI.72.12.6836-6845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson MM, Mobley HL. 2008. Repression of motility during fimbrial expression: identification of 14 mrpJ gene paralogues in Proteus mirabilis. Mol Microbiol 69:548–558. doi: 10.1111/j.1365-2958.2008.06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggio MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, Ascenzioni F. 2011. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One 6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 36.Klein G, Lindner B, Brade H, Raina S. 2011. Molecular basis of lipopolysaccharide heterogeneity in Escherichia coli: envelope stress-responsive regulators control the incorporation of glycoforms with a third 3-deoxy-alpha-d-manno-oct-2-ulosonic acid and rhamnose. J Biol Chem 286:42787–42807. doi: 10.1074/jbc.M111.291799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. 1999. The alternative sigma factor, σE, is critically important for the virulence of Salmonella typhimurium. Infect Immun 67:1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulesus RR, Diaz-Perez K, Slechta ES, Eto DS, Mulvey MA. 2008. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect Immun 76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahrani FK, Johnson DE, Robbins D, Mobley HL. 1991. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun 59:3574–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Lockatell CV, Johnson DE, Lane MC, Warren JW, Mobley HL. 2004. Development of an intranasal vaccine to prevent urinary tract infection by Proteus mirabilis. Infect Immun 72:66–75. doi: 10.1128/IAI.72.1.66-75.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane MC, Li X, Pearson MM, Simms AN, Mobley HL. 2009. Oxygen-limiting conditions enrich for fimbriate cells of uropathogenic Proteus mirabilis and Escherichia coli. J Bacteriol 191:1382–1392. doi: 10.1128/JB.01550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwan WR, Shibata S, Aizawa S, Wolfe AJ. 2007. The two-component response regulator RcsB regulates type 1 piliation in Escherichia coli. J Bacteriol 189:7159–7163. doi: 10.1128/JB.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Lay N, Gottesman S. 2012. A complex network of small non-coding RNAs regulate motility in Escherichia coli. Mol Microbiol 86:524–538. doi: 10.1111/j.1365-2958.2012.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agace WW. 1996. The role of the epithelial cell in Escherichia coli induced neutrophil migration into the urinary tract. Eur Respir J 9:1713–1728. doi: 10.1183/09031936.96.09081713. [DOI] [PubMed] [Google Scholar]
- 45.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 46.Santos LL, Fan H, Hall P, Ngo D, Mackay CR, Fingerle-Rowson G, Bucala R, Hickey MJ, Morand EF. 2011. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis Rheum 63:960–970. doi: 10.1002/art.30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall LJ, Ramdin LS, Brooks T, PC DP, Shute JK. 2003. Plasminogen activator inhibitor-1 supports IL-8-mediated neutrophil transendothelial migration by inhibition of the constitutive shedding of endothelial IL-8/heparan sulfate/syndecan-1 complexes. J Immunol 171:2057–2065. doi: 10.4049/jimmunol.171.4.2057. [DOI] [PubMed] [Google Scholar]
- 48.Saban MR, Saban R, Hammond TG, Haak-Frendscho M, Steinberg H, Tengowski MW, Bjorling DE. 2002. LPS-sensory peptide communication in experimental cystitis. Am J Physiol Renal Physiol 282:F202–F210. [DOI] [PubMed] [Google Scholar]
- 49.Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. 2011. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog 7:e1002454. doi: 10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon DR, Darveau RP. 2005. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res 84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- 51.Errea A, Moreno G, Sisti F, Fernandez J, Rumbo M, Hozbor DF. 2010. Mucosal innate response stimulation induced by lipopolysaccharide protects against Bordetella pertussis colonization. Med Microbiol Immunol 199:103–108. doi: 10.1007/s00430-010-0142-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.