Abstract
Salmonellae initiate disease through the invasion of host cells within the intestine. This ability to invade requires the coordinated action of numerous genes, many of which are found within Salmonella pathogenicity island 1 (SPI-1). The key to this process is the ability of the bacteria to respond to the environment, thereby upregulating the necessary genes under optimal conditions. Central to the control of SPI-1 is the transcriptional activator hilA. Work has identified at least 10 different activators and 8 different repressors responsible for the control of hilA. We have previously shown that hilE is a Salmonella-specific negative regulator that is able to repress hilA expression and invasion. Additionally, fimZ, a transcriptional activator responsible for the expression of type I fimbriae as well as flagellar genes, has also been implicated in this process. fimZ is homologous to response regulators from other two-component regulatory systems, although a sensor for the system has not been identified. The phoPQ and phoBR regulons are both two-component systems that negatively affect hilA expression, although the mechanism of action has not been determined. Our results show that PhoBR is capable of inducing fimZ expression, whereas PhoPQ does not affect fimZ expression but does upregulate hilE in an FimZ-dependent manner. Therefore, phosphate (sensed by PhoBR) and magnesium (sensed by PhoPQ) levels are important in controlling hilA expression levels when Salmonella is in the intestinal environment.
INTRODUCTION
Salmonellae have caused disease for many years. These Gram-negative bacteria can be transmitted through meat, dairy products, or eggs, from animals through the fecal-oral route, and indirectly via fecally contaminated water (1). The CDC tracks two forms of the disease, salmonellosis and typhoid fever. Salmonellosis is a mild form of disease that is typically confined to the gastrointestinal tract. It produces symptoms of fever, abdominal cramps, nausea, and diarrhea (2). A common feature of gastroenteritis or typhoid fever is the ability of Salmonella to invade host cells.
Salmonella contains an island known as pathogenicity island 1 (SPI-1). This island is responsible for encoding both the structural proteins necessary for creating a type III secretion needle complex as well as some of the secreted effectors responsible for the manipulation of host cells (3, 4). Central to the control of this island is the transcriptional activator hilA, which needs to be upregulated for invasion to occur. This upregulation leads to the increased expression of all the other genes contained within SPI-1 (5, 6). Many different activators and repressors of hilA have been identified. These activators respond to a myriad of environmental signals, specifically, osmolarity, oxygen, pH, growth state, short-chain fatty acids, bile, and temperature (6–12), leading to precise control of hilA expression. The transcriptional activators HilD and HilC play an important role in controlling hilA expression. Work has shown that both genes are encoded within SPI-1 (13–15), bind directly to promoter sequences upstream of hilA, and are required for hilA induction even in the absence of multiple repressors (16–19). In addition, RtsA, a transcriptional activator encoded outside SPI-1, works in conjunction with HilD and HilC in a feed-forward loop (20, 21). The interactions of these three activators lead to the upregulation of hilA. Many other transcriptional activators have also been identified as being involved in this process. These include the genes csrAB, sirA-barA, fis, fliZ, fadD, fur, mlc, dsbA, and ompR-envZ (22–31).
Studies of hilA regulation have also identified many different repressors of hilA expression. Some of these genes include hha, lon, hilE, ams, rtsB, and pag (21, 32–34). In addition, two-component regulators have been shown to impact hilA expression as well. These regulators are typically composed of a histidine kinase that responds to specific extracellular signals by being autophosphorylated. The phosphorylation of the sensor initiates a phosphorelay in which phosphate is transferred to its cognate response regulator. This phosphorylation causes the response regulator to activate multiple genes (35).
The phoPQ two-component system is an important regulator of hilA expression (36, 37). The sensor protein PhoQ resides in the membrane of the bacterial cell and stops dephosphorylating the response regulator PhoP when magnesium levels drop to micromolar levels. When PhoP is constitutively expressed and phosphorylated, hilA expression is reduced by 9-fold, which correlated to a 63-fold decrease in HEp-2 cell invasion (36, 37). The molecular mechanism for hilA repression by PhoPQ has not been characterized.
The phoBR two-component system also represses hilA expression. This system detects the levels of phosphate in the extracellular environment. When phosphate levels are low, the system is activated by the autophosphorylation of the sensor PhoR followed by the activation of the response regulator PhoB. The activation of PhoB leads to the induction of more than 21 genes within Salmonella enterica serovar Typhimurium. Many of these genes are involved in transporting phosphate from the environment into the bacterial cell (38). PstS is a protein that represses the PhoR sensor under conditions of high environmental phosphate. When pstS was mutated, it led to a 5-fold decrease in hilA expression, which subsequently reduced HEp-2 cell invasion by 5-fold (29). As is the case with phoPQ, the nature of how the PhoB signal leads to hilA repression is not understood.
Previous work by our research group has shown that HilE interacts with HilD, which prevents the activation of hilA by HilD (39). Due to the importance of HilE in mediating repression of Salmonella invasion genes, we undertook a search for genes that activate hilE expression. This search identified the transcriptional activator fimZ, which has been shown to be responsible for the activation of type I fimbriae and whether bacteria adhere to a surface or are motile (40–42). In our studies, we showed that FimZ upregulates hilE expression, thereby playing a significant role in whether hilA is expressed or not. The fimZ gene is homologous to other response regulators found within two-component systems, yet a specific sensor has not been identified (43). We therefore hypothesized that the signals from the PhoPQ and PhoBR two-component regulators are processed through FimZ, leading to the repression of hilA. The following studies show that PhoPQ and PhoBR regulate hilE expression via the fimZ gene.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. Bacteria were routinely grown in Lennox broth (LB; Gibco-BRL) containing the appropriate antibiotics added at the following concentrations: ampicillin at 100 μg/ml, tetracycline at 25 μg/ml, kanamycin at 25 μg/ml, and chloramphenicol at 25 μg/ml. For the β-galactosidase analysis, S. enterica serovar Typhimurium strains were grown in LB overnight shaken at 225 rpm at 37°C. For conditions in which the levels of magnesium were manipulated, the bacterial cultures were grown in an N-salts minimal medium following a previously established protocol, except for the changes indicated by Hmiel et al. and Nelson and Kennedy (44, 45). For bacterial growth in medium that induces hilA expression via the increase in acetate, we followed the protocol outlined by Lawhon et al. (9). Plasmid purifications were performed utilizing Qiagen DNA purification kits, and all other molecular manipulations were conducted using previously established protocols (46).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or phenotypea | Reference |
|---|---|---|
| Salmonella enterica serovar Typhimurium strains | ||
| BJ644 | SL1344 phoP mutant Tetr | 70 |
| BJ704 | SL1344 phoP mutant | This work |
| BJ2462 | SL1344 hilE-cam Camr | 39 |
| BJ3100 | SL1344 pstS55::Tn5 hilE-cam pLS31 (hilA::lacZY) Kanr Camr Tetr | This work |
| BJ3106 | LT2 pho-24 hilE-cam pLS31 (hilA::lacZY) Camr Tetr | This work |
| BJ3179 | LT2 pho-24 fimZ-cam pMAB69 (hilE::lacZY) Camr Tetr | This work |
| BJ3184 | SL1344 pstS55::Tn5 ΔfimZ Kanr | This work |
| BJ3185 | LT2 pho-24 fimZ::cam Camr | This work |
| BJ3371 | SL1344 phoB::cam Camr | This work |
| BJ3372 | SL1344 phoP mutant phoB::cam Camr | This work |
| EE251 | Invasive LT2 derivative, ΔrpsL | 55 |
| RL291 | SL1344 pstS55::Tn5 Kanr | 29 |
| SL1344 | Wild-type virulent strain | 71 |
| SL1344 fimZ::kan | Kanr | 50 |
| TA2367 | LT2 pho-24 | 72 |
| TBW19812 | LT2 phoB1::cat Camr | 73 |
| Plasmids | ||
| pISF239 | pMC1403 vector containing an fimZ::lacZY reporter, Ampr | 43 |
| pLS31 | Low-copy-number vector pRW50 containing an hilA::lacZY reporter, Tetr | 15 |
| pMAB69 | Low-copy-number vector pRW50 containing an hilE::lacZY reporter, Tetr | 50 |
Tetr, tetracycline resistance; Ampr, ampicillin resistance; Camr, chloramphenicol resistance; Kanr, kanamycin resistance.
Creation of defined chromosomal mutations within the hilE and fimZ genes.
In an effort to create defined chromosomal mutations within the S. enterica serovar Typhimurium LT2 phoQc strain TA2367, we utilized the linear transformation procedure (47). Briefly, PCR primers were synthesized with 50 bp of homology to the 5′ and 3′ ends of the hilE gene. In addition, the hilE5W′ primer (5′-TTATAGCAGATTGTCGGTATTTAATCTGGTATACAGAGACACCAACGAACATATGAATATCCTCCTTA-3′) was synthesized so that it carried priming site 2 of pKD3 (47), and the hilE3W′ primer (5′-ATTTCGCTATACAGCATCGCCCACTGCGAGTCCGCAAGCTTGTTTTGTCCGTGTAGGCTGGAGCTGCTTC-3′) was synthesized so that it carried priming site 1 of pKD3. PCR amplification was performed with these primers using plasmid pKD3 as the template, and the expected 1.1-kb fragment was obtained. The linear PCR fragment was purified and electroporated into SL1344 carrying pKD46, and mutants were selected on L-CAM plates at 37°C. Several Camr Amps colonies were purified and found by PCR to have the transformed fragment recombined into the hilE gene on the chromosome. The procedure for creating the defined fimZ::cam mutations followed the above-described protocol utilizing the primers fimZ5W (5′-TGACGCTTATTATAAAACGAAGGACGCATAACAGTCTGAGGCATACAACACATATGAATATCCTCCTTA-3′) and fimZ3W (5′-ATTAGTGTCCGTTATTGTGGCTCCCGAACGATAATTCGCCGGGAGTACATGTGTAGGCTGGAGCTGCTTC-3′).
β-Galactosidase assays.
β-Galactosidase assays were conducted on bacterial cultures using the standard method described by Miller (48).
P22-mediated transductions.
The P22 HT int phage was used to move mutations marked by antibiotic-resistant genes between strains as described previously (49). Transductants were selected on LB agar containing the necessary antibiotic and 10 mM EGTA to prevent P22 reinfection. Transductants were purified twice on LB EGTA agar prior to use of the colonies.
RESULTS
The effects of a phoQc mutation on hilA can be alleviated by the deletion of hilE.
The phoPQ regulon has been demonstrated to exert a powerful influence on the expression of hilA (37). However, the means by which phoPQ exerts its effect on hilA have not been characterized. In our previous studies, we identified hilE as a Salmonella-specific repressor of hilA (39). To determine the effects of HilE on the regulation of hilA via PhoPQ, we conducted a β-galactosidase assay examining the effect of an hilE mutation on hilA::lacZY expression when phoQ is constitutively expressed. As seen in Fig. 1, normal expression of hilA::lacZY from pLS31 within the S. Typhimurium EE251 strain was at 577.9 ± 19.0 Miller units. When the constitutive phoQ mutation is introduced, hilA::lacZY expression is reduced 7.5-fold to 76.4 ± 3.5 Miller units. The introduction of a defined hilE::cam mutation within the chromosome to the constitutive phoQ mutation increased hilA::lacZY expression by 4-fold to 305.7 ± 12.4 Miller units. Although repression of hilA::lacZY within the phoQc strain was not completely eliminated, most of the hilA expression could be restored by deletion of hilE. This indicates that HilE mediates a substantial portion of the repressing activity that a phoQc mutation has on hilA expression.
FIG 1.
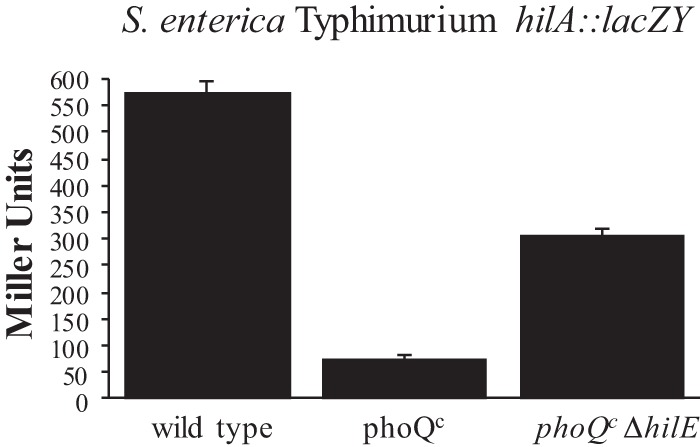
The effects of constitutive phoQ expression on hilA are reduced by deletion of the Salmonella hilE gene. Strains were grown with shaking in LB broth to late stationary phase. The wild-type strain is S. enterica serovar Typhimurium LT2 strain EE251 carrying the hilA::lacZY plasmid reporter pLS31. The TA2367 strain contains the phoQc mutation and the hilA::lacZY reporter plasmid pLS31. BJ3106 is the TA2367 strain containing a defined hilE::cam mutation and the hilA::lacZY reporter plasmid pLS31. Expression levels were determined by lacZ output as measured by β-galactosidase activity. The experiment is representative of an assay which was repeated in triplicate on three separate days.
The signal from the phoPQ regulon is transmitted through the transcriptional activator fimZ.
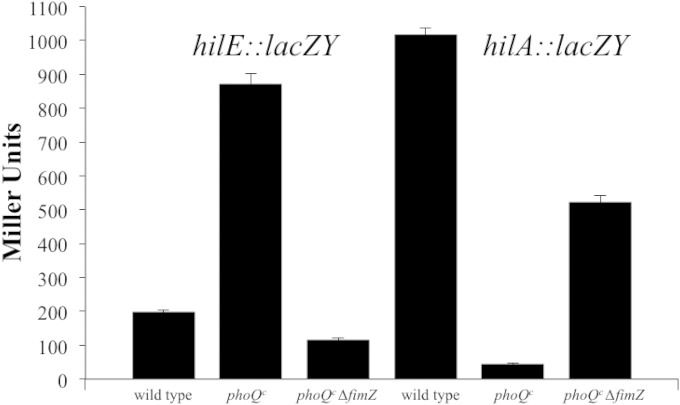
We have previously shown that hilE is regulated by the transcriptional activator FimZ (50). FimZ is a transcriptional activator of type 1 fimbriae genes (41, 43) and also exerts regulatory effects on motility (40), invasion gene expression, and biofilm formation (50). Analysis of the FimZ amino acid sequences reveals that this activator has substantial similarity to response regulators from two-component signaling systems, yet a sensor partner for FimZ has not been identified (43). In this work, we hypothesize that FimZ is responsible for responding to signals from the PhoPQ regulon, which causes an increase in hilE expression. We conducted β-galactosidase assays measuring the levels of hilE::lacZY and hilA::lacZY expression in the presence or absence of a functional fimZ gene. As shown in Fig. 2, the hilE::lacZY reporter expressed at 197.3 ± 5.9 Miller units. When phoQ is constitutively expressed, hilE::lacZY expression increased by 4.4-fold to 873.2 ± 28.8 Miller units. When an fimZ::cam mutation was present in this strain and tested under the same conditions, hilE::lacZY expression was reduced 7.4-fold to 117.3 ± 3.9 Miller units. This indicated that the effect of a phoQc mutation on an hilE::lacZY reporter was being mediated by FimZ. This result was confirmed by measuring the effects an fimZ mutation has on hilA::lacZY expression when the phoQc mutation is also present. Control levels of hilA::lacZY were at 1,017.1 ± 20.8 Miller units. Upon introduction of a phoQc mutation, hilA::lacZY expression was reduced 23-fold to 44.2 ± 3.5 Miller units. When the fimZ::cam mutation was introduced, hilA::lacZY expression increased by 11.8-fold to 522.3 ± 19.7 Miller units. These results confirmed our hypothesis that the effect of PhoPQ on hilA follows a signaling pathway through FimZ and HilE. Since complete alleviation of hilA::lacZY repression by the phoQc mutation did not occur with deletion of fimZ, it seems likely that the signal from the phoQc mutation is also being processed by other pathways.
FIG 2.
The effect of constitutive phoQ expression on hilE and hilA expression is mediated through fimZ. Strains were grown with shaking in LB broth to late stationary phase. The wild-type strain is S. enterica serovar Typhimurium LT2 strain EE251 carrying either the hilA::lacZY plasmid reporter pLS31 or the hilE::lacZY plasmid reporter pMAB69. The strain TA2367 contains the phoQc mutation and either the hilA::lacZY reporter plasmid pLS31 or the hilE::lacZY plasmid reporter pMAB69. BJ3179 is the TA2367 strain containing a defined fimZ::cam mutation and carries the hilE::lacZY reporter pMAB69. BJ3185 is the TA2367 strain containing a defined fimZ::cam mutation and carries the hilA::lacZY reporter pLS31. Expression levels were determined by lacZ output as measured by β-galactosidase activity. The experiment is representative of an assay which was repeated in triplicate on three separate days.
Expression of fimZ is not affected by phoPQ.
Since the PhoPQ signal processes through fimZ to regulate hilA, we wanted to determine whether PhoPQ regulates fimZ transcription. To do so, we measured the expression of an fimZ::lacZY reporter in the wild-type S. Typhimurium LT2 strain and in an LT2 strain in which the phoQc mutation was introduced. Wild-type S. Typhimurium expressed fimZ::lacZY at 334.8 ± 8.0 Miller units, whereas a phoQc strain expressed the fimZ::lacZY reporter at 297.8 ± 6.9 Miller units. Examination of the FimZ primary sequence indicates that the protein contains motifs that are homologous to proteins that are phosphorylated, although phosphorylation of specific residues has not been demonstrated for FimZ (43). From these results, we hypothesize that FimZ is activated in response to the PhoPQ signal (likely by phosphorylation), which leads to induction of hilE expression.
hilA::lacZY expression can be altered by various magnesium concentrations.
As our work has demonstrated that the overexpression of phoQ strongly represses hilA using a signaling pathway that includes FimZ and HilE, we next determined whether hilA::lacZY expression could be altered within S. Typhimurium solely by altering magnesium levels, the primary signal for the PhoPQ two-component regulator. This was done by measuring hilA::lacZY expression in N-salts minimal medium that was either inducing for PhoPQ signaling (8 μM magnesium) or repressing for PhoPQ signaling (10 mM magnesium). Analysis of wild-type S. Typhimurium strain SL1344 with the hilA::lacZY plasmid reporter pLS31 showed that inducing levels of magnesium for phoPQ expression reduced hilA::lacZY expression 28.2-fold from 1,139.7 ± 20.1 to 40.4 ± 0.8 Miller units (Fig. 3). Next, the effects of an hilE::cam mutation on hilA::lacZY expression when magnesium was either inducing or repressing for PhoPQ were measured. Even in the absence of hilE, hilA::lacZY plasmid reporter levels still showed a 25.9-fold reduction (4,098.7 ± 173.4 to 158.3 ± 5.6 Miller units) when the PhoPQ regulators were activated by magnesium (Fig. 3). These results suggest that the effects of magnesium on hilA are not solely mediated through the FimZ/HilE signaling pathway. It is possible that another regulatory system within Salmonella responds to magnesium levels and that these secondary pathways affect hilA expression levels, independent of hilE, under other conditions that do not induce invasion-associated genes.
FIG 3.
Various magnesium concentrations will alter the levels of hilA expression independently of hilE. Strains were grown with shaking in LB broth to late stationary phase. The wild-type S. enterica serovar Typhimurium strain SL1344 containing the hilA::lacZY reporter plasmid pLS31 was compared to the BJ2462 strain, which is an SL1344 strain containing an hilE::cam deletion and carries the same reporter plasmid. Expression levels were determined by lacZ output as measured by β-galactosidase activity. The experiment is representative of an assay which was repeated in triplicate on three separate days. High magnesium was at a concentration of 10 mM, whereas low magnesium was at 8 μM.
hilE::lacZY expression can be induced upon the induction of phoB.
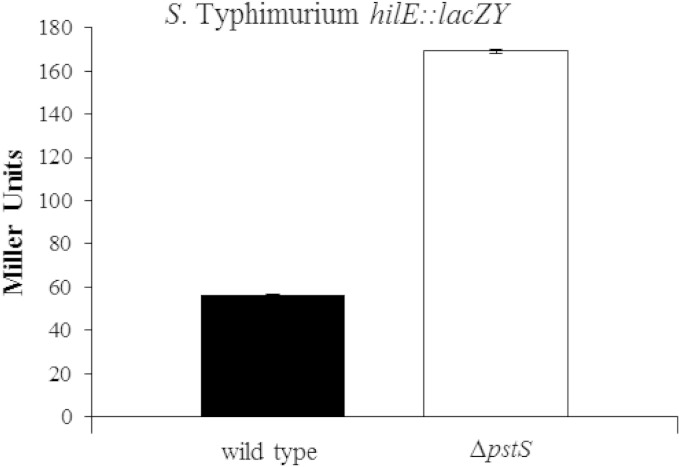
A mutation in the pstS gene was previously identified as causing a reduction in hilA expression (29). Previous work has shown that PstS is responsible for the repression of the two-component regulator PhoBR, which is responsible for the activation of scavenger genes that move phosphate into the cell under low phosphate conditions (38). Since our studies indicated that PhoPQ plays a role in inducing hilE expression, we examined whether the phoBR regulon also regulates hilA via HilE. A β-galactosidase assay was conducted measuring the amount of hilE::lacZY expression in wild-type S. Typhimurium SL1344 and the mutant strain RL291 (an SL1344 derivative with a pstS mutation). Utilizing the hilE::lacZY plasmid reporter pMAB69, we measured wild-type hilE expression at 56.6 ± 0.2 Miller units (Fig. 4). When the pstS gene was disrupted, increasing PhoB activation, expression of hilE::lacZY increased to 169.1 ± 1.0 Miller units. This 3-fold increase in hilE expression indicated that hilE responds to signals from both PhoPQ and PhoBR.
FIG 4.
Overexpression of phoB leads to the activation of hilE. The wild-type strain is S. enterica serovar Typhimurium SL1344. The mutant tested is the SL1344 strain RL291, which contains a pstS deletion leading to the constitutive activation of phoB. Each strain contained the hilE::lacZY reporter plasmid pMAB69. The strains were grown in LB overnight with shaking, and expression levels were determined by measuring lacZ output as measured by β-galactosidase activity. The experiment is representative of an assay which was repeated in triplicate on three separate days.
The effects of a pstS mutation on hilA are alleviated by the deletion of hilE.
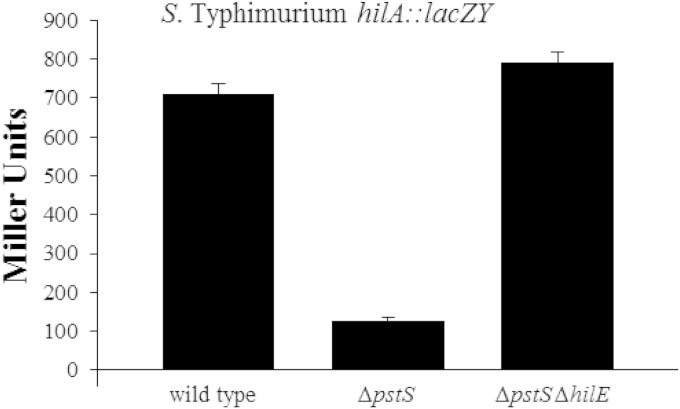
Since the deletion of hilE partially reverses the effects of a phoQc mutation on hilA, we also examined the effect an hilE deletion has on hilA expression when PhoBR is activated by the pstS mutation. Utilizing the hilA::lacZY plasmid reporter pLS31, hilA expression levels were measured in wild-type S. Typhimurium SL1344, the pstS mutant RL291, and BJ3100, a pstS mutant containing a defined hilE::cam insertion. Wild-type SL1344 expressed hilA::lacZY at 711.4 ± 25.2 Miller units (Fig. 5). Deletion of pstS decreased hilA expression by 5.6-fold to 127.2 ± 8.6 Miller units. When BJ3100 (ΔpstS hilE::cam) was assayed, hilA::lacZY expression increased 6.2-fold to 793 ± 24.0 Miller units. The deletion of hilE completely reversed the effects of a pstS mutation on hilA expression, indicating that the pstS mutation, which activates PhoBR signaling, exerts its effect via hilE, to regulate hilA transcription.
FIG 5.
The deletion of hilE reverses the repression of hilA in a constitutive phoB-expressing strain. The wild-type strain is S. enterica serovar Typhimurium SL1344. RL291 is an SL1344 derivative that contains a pstS mutation. BJ3100 is an RL291 strain containing a defined hilE::cam mutation within the chromosome. Each strain contained the hilA::lacZY reporter pLS31. The strains were grown in LB overnight with shaking, and expression levels were determined by measuring lacZ output as measured by β-galactosidase activity.
The activation of phoB increases fimZ expression.
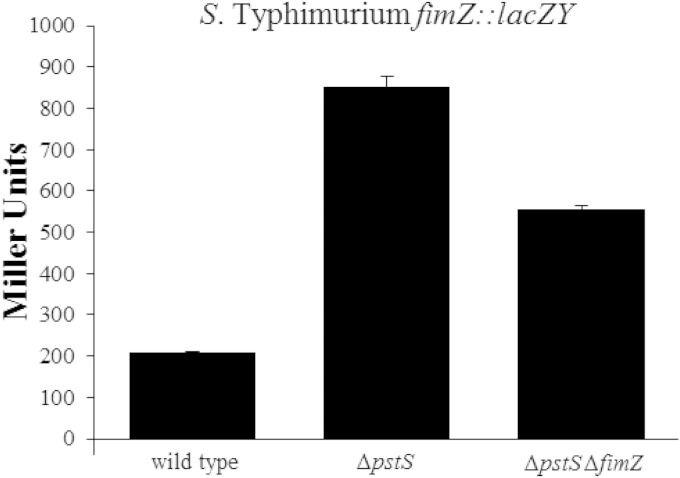
Since the PhoBR signal regulates hilA via hilE, it was logical to examine whether the regulatory signal was transmitted through fimZ similar to that seen with PhoPQ. Utilizing the fimZ::lacZY reporter pISF239, fimZ expression levels were measured in wild-type S. Typhimurium SL1344 and in a pstS mutant. In wild-type SL1344, fimZ expressed at 208.8 ± 0.8 Miller units (Fig. 6). A pstS mutation increased fimZ expression 4-fold to 853.2 ± 25.4 Miller units. One possible explanation of this finding is that the pstS mutation increases protein levels of FimZ, potentially activating transcription of its own gene, consistent with previous work by Yeh et al. (43). Therefore, to rule out the possibility that the induction of fimZ transcription was solely due to the self-induction of the transcriptional activator, we constructed strain BJ3184, which is an SL1344 derivative containing the pstS mutation and a defined fimZ::cam insertion. Within this strain that lacks functional FimZ protein, fimZ::lacZY expression was 555.1 ± 10.6 Miller units, which is still a 2.7-fold increase in fimZ expression compared to that of the wild type (Fig. 6). These results demonstrate that the activation of the PhoB response regulator leads to increased fimZ expression, in the absence of functional FimZ, although the presence of FimZ further increases fimZ expression due to autoactivation. This increase in FimZ activates hilE expression, leading to the repression of hilA.
FIG 6.
Overexpression of phoB increases the level of fimZ expression. The strains were shaken overnight in LB growing at 37°C. The wild-type bacterium is S. enterica serovar Typhimurium strain SL1344. RL291 is an SL1344 derivative that contains a pstS mutation that causes the overexpression of phoB. The BJ3184 strain is the RL291 strain containing a defined fimZ::cam mutation. Each strain tested contained the fimZ::lacZY reporter plasmid pISF239. The strains were grown in LB overnight with shaking, and expression levels were determined by measuring lacZ output as measured by β-galactosidase activity. The experiment is representative of an assay which was repeated in triplicate on three separate days.
DISCUSSION
The process of invasion in Salmonella requires the coordinated control of many different genes responding to a myriad of environmental signals. For invasion to occur, the bacteria must induce the expression of genes within SPI-1 as well as genes encoding the effectors that are secreted by the SPI-1 type III secretion system. The combined functions of these gene products cause the mammalian host cell cytoskeleton to ruffle outward around the invading organism so that it is internalized into the host cells via macropinocytosis (3, 4, 26, 51). Salmonella species have developed a complex regulatory network that determines whether the bacterium has entered an environment that is conducive for invasion. If conditions are not optimal, invasion gene expression is repressed, whereas entry into a more conducive environment leads to activation of the invasion genes. Currently, many different environmental signals have been identified that impact invasion gene expression. These activating signals include oxygen-limiting conditions, high osmolarity, temperature, and growth in a near neutral pH (6–8, 11). In addition, the bacteria downregulate invasion gene expression as the organisms reaches the stationary phase of growth (52). Additional signals, such as the concentrations of short-chain fatty acids (i.e., acetate, propionate, and butyrate), as well as the presence of bile salts, impact gene expression (9, 12, 53), with recent evidence showing that propionyl coenzyme A (propionyl-CoA) specifically regulates HilD posttranslationally, possibly by propionylation of the HilD protein (54).
Induction of SPI-1 requires the expression of hilA and invF, two transcriptional activators found within SPI-1. In the absence of these regulators, the proteins required for formation of the type III secretion system and the secreted effectors will not be produced (5, 55). Work in many different laboratories has identified additional genes that regulate expression of hilA and invF. Currently, csrAB, sirA-barA, fis, fliZ, fadD, ompR-envZ, fur, mlc, dsbA, rtsA, hilC, and hilD have all been shown to positively upregulate hilA expression (13–15, 22–31, 56). In addition, a number of repressors have also been identified that are important in controlling hilA expression. These repressors include lon, hha, ams, pag, phoQc, phoB, rtsB, and hilE (21, 29, 32–35, 37, 39). Our group has characterized the negative regulatory hilE gene and its impact on hilA expression. We have shown by two-hybrid analysis that HilE interacts with HilD to repress hilA transcription (39). Other work has shown that hilE is a Salmonella-specific gene that is not expressed by Escherichia coli (39). Work from several groups has identified factors that regulate Salmonella invasion gene expression through the HilE repressor. The Mlc global regulator has been shown to downregulate an hilE promoter (27). The small noncoding RNA isrM targets the hilE transcript to reduce the repressing activity of hilE (57). The LysR-type regulator LeuO has been shown to activate hilE transcription to repress HilD activity (58).
In an effort to contribute to our understanding of Salmonella SPI-1 virulence gene regulation, we conducted a search for genes that induce hilE::lacZY expression. This search identified fimZ, an important transcriptional activator of type 1 fimbriae (42). FimZ has been implicated in the control of other regulatory systems in Salmonella. Prior to our demonstration of the involvement of fimZ in Salmonella invasion gene regulation, work was published showing that the overexpression or deletion of fimZ inversely controlled motility and fimbrial gene expression (40). Recent work has shown that these three Salmonella properties (i.e., adherence [type 1 fimbriae], motility [flagella], and invasion [SPI-1 gene expression]) are all dynamically regulated via a cross talk mechanism utilizing posttranscriptionally modified FliZ, which is proposed to monitor the bacterial growth state (28, 59–61). Additional work has demonstrated that the flhDC genes, the master operon of the flagellar hierarchy, activate transcription of the hilD gene at early stages of growth, while the HilD regulator activates promoter 5 of the flhDC genes at later stages of growth (62). FimZ is of interest, as it has homology to response regulators (highest homology to bvgA of Bordetella), yet no sensor kinase has been identified as its partner (43). Analysis of mutations that resulted in repression of hilA showed that two of these regulators were either part of a two-component regulatory system (phoQc) or involved in the function of a two-component system (pstS and phoBR). In this work, we have explored how these mutations impact hilA expression by examining their interactions with FimZ and HilE.
In this work, we have studied how PhoPQ, HilE, and FimZ function together to regulate the S. Typhimurium SPI-1 transcriptional activator hilA. We have employed strains with mutations in genes of interest as well as manipulation of magnesium concentrations in the growth medium to ask experimental questions. We have found that most, but not all, of the PhoPQ effects are mediated by FimZ and HilE. The exception was that deletion of hilE did not completely reverse the effects of phoQc on hilA expression. Consistent with our data, recent work has shown that the positive hilA activator DsbA reduces phoPQ expression (63). Our results contribute to the evolving story that multiple environmental signals are processed by various Salmonella two-component regulators to increase or decrease invasion gene expression. Since phoPQ seems to exert its effect at FimZ posttranscriptionally, a likely mechanism is via a phosphorelay mechanism from PhoPQ to FimZ, which would fit the established mechanism of activation of two-component regulatory systems. The likelihood that FimZ functions by receiving phosphorylation signals from multiple two-component signals provides a model to understand how this gene can regulate genes involved in motility, biofilm formation, invasion gene repression, and type I fimbrial gene expression (64). Recent work by Golubeva et al. suggests that PhoPQ is a class III regulator and exerts its regulatory influence by acting directly on hilA (65, 66). This is possible, since the hilE deletion does not completely eliminate repression of hilA by the phoQc mutation. Future efforts will be required to resolve these points.
Additionally, we investigated the effects of the PhoBR regulon on fimZ and hilE expression. Unlike what was observed with PhoPQ, we found that PhoBR directly effects fimZ expression but that its ability to control hilA expression is mediated entirely by HilE. Accordingly, these two-component regulators (PhoPQ and PhoBR) alter hilE and hilA expression by different mechanisms in that one is via a posttranscriptional mechanism and the other is via transcriptional control of hilE.
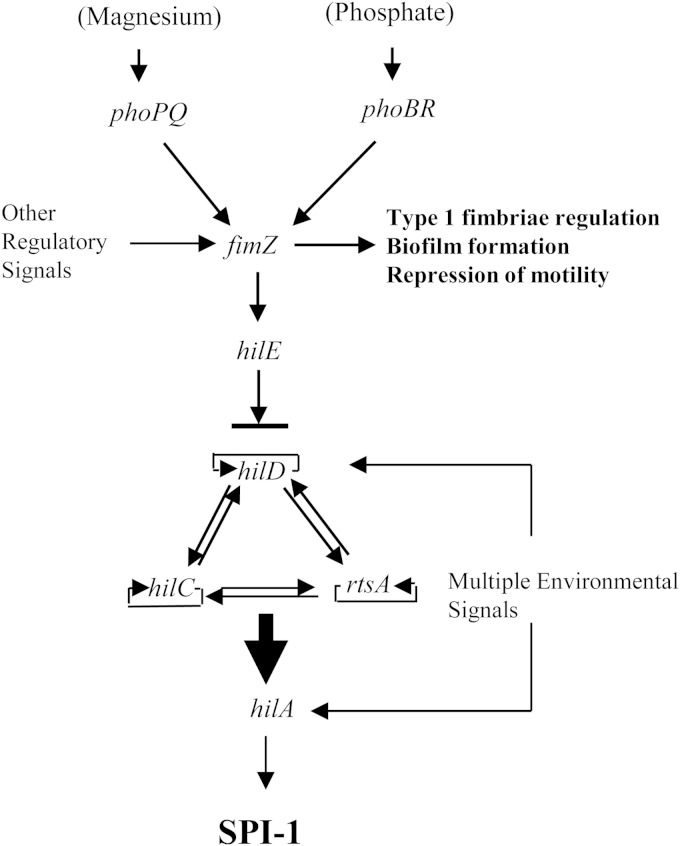
The concept that multiple two-component regulatory systems interact in an overlapping fashion to control a biological pathway is not new. Previous work has shown that Salmonella has three different two-component regulators that control the synthesis of the ugd gene, which is involved in both polymyxin B resistance and capsule biosynthesis (67). An overlapping regulatory network has also been described for the pho regulon of Bacillus subtilis (68). A study analyzing the regulons of known two-component systems in E. coli concluded that there are three possible regulatory schemes that can occur. One, a single sensor can directly interact with a single response regulator. Two, a single sensor can interact with or activate multiple response regulators of DNA-binding activators. Three, multiple sensor proteins can converge onto a single response regulator and the genes that it controls (69). We have contributed data here that we believe help to further define the regulatory hierarchy of regulation of the Salmonella invasion genes and have shown how two different two-component sensing systems interact with other activators and repressors to control expression of Salmonella virulence (Fig. 7). The involvement of fimZ in this process demonstrates that Salmonella has evolved to coordinate the expression or repression of the invasion phenotype with expression of type 1 fimbriae and motility. Future efforts will be aimed at determining the molecular details of this highly coordinated network of gene expression in this important bacterial pathogen.
FIG 7.
Model of the regulatory cascade that transfers environmental signals into changes in Salmonella gene expression. Environmental signals such as magnesium or phosphate concentration increase or decrease fimZ expression. Under conditions of low magnesium concentration, the PhoPQ regulon is activated, leading to the phosphorylation of FimZ with the subsequent increase in hilE expression. Under conditions of low phosphate, PhoBR is activated, which increases fimZ expression, which upregulates hilE expression. A FimZ-activating signal additionally leads to increased type 1 fimbrial expression by direct binding of the FimZ activator to the type 1 fimbrial gene operon. Increased fimZ expression also decreases expression of the flhDC master regulatory proteins for the flagellar regulon by an unknown mechanism. Other regulatory signals generated from the cross talk between the flagellar system or other environmental signals may further influence the expression of fimZ. The increase in FimZ leads to the subsequent increase in HilE protein levels, which limits the availability of HilD to activate the hilA promoter due to HilE-HilD binding. A FimZ-deactivating signal would have the opposite effects. Collectively, these regulatory pathways control hilA expression and downstream expression of SPI-1 (22). In addition, it is likely that there are FimZ-independent signals that affect the expression and cross talk between all the systems described (motility, adherence, and invasion), which allows Salmonella to dynamically control when these various systems respond (60).
ACKNOWLEDGMENTS
We thank Jennifer Boddicker and Nate Ledeboer for careful review of the manuscript.
B.D.J. is supported by NIH grant 2PO1 AI044642.
REFERENCES
- 1.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerg Infect Dis 5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. 2010. Salmonella. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/salmonella/index.html. [Google Scholar]
- 3.Collazo CM, Galan JE. 1997. The invasion-associated type III system of Salmonella Typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol 24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 4.Darwin KH, Miller VL. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev 12:405–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajaj V, Hwang C, Lee CA. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella Typhimurium invasion genes. Mol Microbiol 18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj V, Lucas RL, Hwang C, Lee CA. 1996. Co-ordinate regulation of Salmonella Typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol 22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 7.Ernst RK, Dombroski DM, Merrick JM. 1990. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella Typhimurium. Infect Immun 58:2014–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galan JE, Curtiss R III. 1990. Expression of Salmonella Typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun 58:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawhon SD, Maurer R, Suyemoto M, Altier C. 2002. Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 10.Olekhnovich IN, Kadner RJ. 2007. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol 189:6882–6890. doi: 10.1128/JB.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono S, Goldberg MD, Olsson T, Esposito D, Hinton JC, Ladbury JE. 2005. H-NS is a part of a thermally controlled mechanism for bacterial gene regulation. Biochem J 391:203–213. doi: 10.1042/BJ20050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prouty AM, Gunn JS. 2000. Salmonella enterica serovar Typhimurium invasion is repressed in the presence of bile. Infect Immun 68:6763–6769. doi: 10.1128/IAI.68.12.6763-6769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichelberg K, Hardt WD, Galan JE. 1999. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella Typhimurium pathogenicity island 1. Mol Microbiol 33:139–152. doi: 10.1046/j.1365-2958.1999.01458.x. [DOI] [PubMed] [Google Scholar]
- 14.Rakeman JL, Bonifield HR, Miller SI. 1999. A HilA-independent pathway to Salmonella Typhimurium invasion gene transcription. J Bacteriol 181:3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schechter LM, Damrauer SM, Lee CA. 1999. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol 32:629–642. doi: 10.1046/j.1365-2958.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 16.Boddicker JD, Knosp BM, Jones BD. 2003. Transcription of the Salmonella invasion gene activator, hilA, requires HilD activation in the absence of negative regulators. J Bacteriol 185:525–533. doi: 10.1128/JB.185.2.525-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas RL, Lee CA. 2001. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J Bacteriol 183:2733–2745. doi: 10.1128/JB.183.9.2733-2745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olekhnovich IN, Kadner RJ. 2002. DNA-binding activities of the HilC and HilD virulence regulatory proteins of Salmonella enterica serovar Typhimurium. J Bacteriol 184:4148–4160. doi: 10.1128/JB.184.15.4148-4160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schechter LM, Lee CA. 2001. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella Typhimurium hilA promoter. Mol Microbiol 40:1289–1299. doi: 10.1046/j.1365-2958.2001.02462.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellermeier CD, Ellermeier JR, Slauch JM. 2005. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- 21.Ellermeier CD, Slauch JM. 2003. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J Bacteriol 185:5096–5108. doi: 10.1128/JB.185.17.5096-5108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmer BM, van Reeuwijk J, Watson PR, Wallis TS, Heffron F. 1999. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol 31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 23.Altier C, Suyemoto M, Lawhon SD. 2000. Regulation of Salmonella enterica serovar Typhimurium invasion genes by csrA. Infect Immun 68:6790–6797. doi: 10.1128/IAI.68.12.6790-6797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altier C, Suyemoto M, Ruiz AI, Burnham KD, Maurer R. 2000. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol Microbiol 35:635–646. [DOI] [PubMed] [Google Scholar]
- 25.Ellermeier JR, Slauch JM. 2008. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190:476–486. doi: 10.1128/JB.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston C, Pegues DA, Hueck CJ, Lee A, Miller SI. 1996. Transcriptional activation of Salmonella Typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol 22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Yun J, Yoon H, Park C, Kim B, Jeon B, Kim D, Ryu S. 2007. Mlc regulation of Salmonella pathogenicity island I gene expression via hilE repression. Nucleic Acids Res 35:1822–1832. doi: 10.1093/nar/gkm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin D, Rao CV, Slauch JM. 2008. The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J Bacteriol 190:87–97. doi: 10.1128/JB.01323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas RL, Lostroh CP, DiRusso CC, Spector MP, Wanner BL, Lee CA. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol 182:1872–1882. doi: 10.1128/JB.182.7.1872-1882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, Hassan HM. 2011. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J Bacteriol 193:497–505. doi: 10.1128/JB.00942-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson RL, Libby SJ, Freet AM, Boddicker JD, Fahlen TF, Jones BD. 2001. Fis, a DNA nucleoid-associated protein, is involved in Salmonella Typhimurium SPI-1 invasion gene expression. Mol Microbiol 39:79–88. doi: 10.1046/j.1365-2958.2001.02192.x. [DOI] [PubMed] [Google Scholar]
- 32.Fahlen TF, Mathur N, Jones BD. 2000. Identification and characterization of mutants with increased expression of hilA, the invasion gene transcriptional activator of Salmonella Typhimurium. FEMS Immunol Med Microbiol 28:25–35. doi: 10.1111/j.1574-695X.2000.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 33.Fahlen TF, Wilson RL, Boddicker JD, Jones BD. 2001. Hha is a negative modulator of transcription of hilA, the Salmonella enterica serovar Typhimurium invasion gene transcriptional activator. J Bacteriol 183:6620–6629. doi: 10.1128/JB.183.22.6620-6629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaya A, Tomoyasu T, Tokumitsu A, Morioka M, Yamamoto T. 2002. The ATP-dependent lon protease of Salmonella enterica serovar Typhimurium regulates invasion and expression of genes carried on Salmonella pathogenicity island 1. J Bacteriol 184:224–232. doi: 10.1128/JB.184.1.224-232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson VL, Buckler DR, Stock AM. 2000. A tale of two components: a novel kinase and a regulatory switch. Nat Struct Biol 7:626–633. doi: 10.1038/77915. [DOI] [PubMed] [Google Scholar]
- 36.Behlau I, Miller SI. 1993. A PhoP-repressed gene promotes Salmonella Typhimurium invasion of epithelial cells. J Bacteriol 175:4475–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegues DA, Hantman MJ, Behlau I, Miller SI. 1995. PhoP/PhoQ transcriptional repression of Salmonella Typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol 17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 38.Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon. Escherichia coli and Salmonella Typhimurium: cellular and molecular biology, 2nd ed. ASM Press, American Society for Microbiology, Washington, DC. [Google Scholar]
- 39.Baxter MA, Fahlen TF, Wilson RL, Jones BD. 2003. HilE interacts with HilD and negatively regulates hilA transcription and expression of the Salmonella enterica serovar Typhimurium invasive phenotype. Infect Immun 71:1295–1305. doi: 10.1128/IAI.71.3.1295-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clegg S, Hughes KT. 2002. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J Bacteriol 184:1209–1213. doi: 10.1128/jb.184.4.1209-1213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swenson DL, Clegg S. 1992. Identification of ancillary fim genes affecting fimA expression in Salmonella Typhimurium. J Bacteriol 174:7697–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh KS, Hancox LS, Clegg S. 1995. Construction and characterization of a fimZ mutant of Salmonella Typhimurium. J Bacteriol 177:6861–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh KS, Tinker JK, Clegg S. 2002. FimZ binds the Salmonella Typhimurium fimA promoter region and may regulate its own expression with FimY. Microbiol Immunol 46:1–10. doi: 10.1111/j.1348-0421.2002.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 44.Hmiel SP, Snavely MD, Miller CG, Maguire ME. 1986. Magnesium transport in Salmonella Typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol 168:1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson DL, Kennedy EP. 1971. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem 246:3042–3049. [PubMed] [Google Scholar]
- 46.Maniatis T, Fritsch EF, Sambrook J. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 47.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Press, Cold Spring Harbor, NY. [Google Scholar]
- 49.Davis RW, Botstein D, Roth JR. 1980. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 50.Baxter MA, Jones BD. 2005. The fimYZ genes regulate Salmonella enterica serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect Immun 73:1377–1385. doi: 10.1128/IAI.73.3.1377-1385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 52.Lee CA, Falkow S. 1990. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A 87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, Hinton JC, Van Immerseel F. 2006. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol 72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hung CC, Garner CD, Slauch JM, Dwyer ZW, Lawhon SD, Frye JG, McClelland M, Ahmer BM, Altier C. 2013. The intestinal fatty acid propionate inhibits Salmonella invasion through the posttranslational control of HilD. Mol Microbiol 87:1045–1060. doi: 10.1111/mmi.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee CA, Jones BD, Falkow S. 1992. Identification of a Salmonella Typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A 89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Liu B, Wang Q, Wang L. 2013. Genome-wide analysis of the Salmonella Fis regulon and its regulatory mechanism on pathogenicity islands. PLoS One 8:e64688. doi: 10.1371/journal.pone.0064688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong H, Vu GP, Bai Y, Chan E, Wu R, Yang E, Liu F, Lu S. 2011. A Salmonella small non-coding RNA facilitates bacterial invasion and intracellular replication by modulating the expression of virulence factors. PLoS Pathog 7:e1002120. doi: 10.1371/journal.ppat.1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Espinosa E, Casadesus J. 2014. Regulation of Salmonella enterica pathogenicity island 1 (SPI-1) by the LysR-type regulator LeuO. Mol Microbiol 91:1057–1069. doi: 10.1111/mmi.12500. [DOI] [PubMed] [Google Scholar]
- 59.Chubiz JE, Golubeva YA, Lin D, Miller LD, Slauch JM. 2010. FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar Typhimurium. J Bacteriol 192:6261–6270. doi: 10.1128/JB.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kage H, Takaya A, Ohya M, Yamamoto T. 2008. Coordinated regulation of expression of Salmonella pathogenicity island 1 and flagellar type III secretion systems by ATP-dependent ClpXP protease. J Bacteriol 190:2470–2478. doi: 10.1128/JB.01385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saini S, Slauch JM, Aldridge PD, Rao CV. 2010. Role of cross talk in regulating the dynamic expression of the flagellar Salmonella pathogenicity island 1 and type 1 fimbrial genes. J Bacteriol 192:5767–5777. doi: 10.1128/JB.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mouslim C, Hughes KT. 2014. The effect of cell growth phase on the regulatory cross-talk between flagellar and Spi1 virulence gene expression. PLoS Pathog 10:e1003987. doi: 10.1371/journal.ppat.1003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardenal-Munoz E, Ramos-Morales F. 2013. DsbA and MgrB regulate steA expression through the two-component system PhoQ/PhoP in Salmonella enterica. J Bacteriol 195:2368–2378. doi: 10.1128/JB.00110-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams P, Fowler R, Kinsella N, Howell G, Farris M, Coote P, O'Connor CD. 2001. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics 1:597–607. doi:. [DOI] [PubMed] [Google Scholar]
- 65.Golubeva YA, Sadik AY, Ellermeier JR, Slauch JM. 2012. Integrating global regulatory input into the Salmonella pathogenicity island 1 type III secretion system. Genetics 190:79–90. doi: 10.1534/genetics.111.132779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. 2005. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci U S A. 102:2862–2867. doi: 10.1073/pnas.0408238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mouslim C, Groisman EA. 2003. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol Microbiol 47:335–344. doi: 10.1046/j.1365-2958.2003.03318.x. [DOI] [PubMed] [Google Scholar]
- 68.Sun G, Birkey SM, Hulett FM. 1996. Three two-component signal-transduction systems interact for Pho regulation in Bacillus subtilis. Mol Microbiol 19:941–948. doi: 10.1046/j.1365-2958.1996.422952.x. [DOI] [PubMed] [Google Scholar]
- 69.Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, Wanner BL, Mori H, Mizuno T. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol 46:281–291. doi: 10.1046/j.1365-2958.2002.03170.x. [DOI] [PubMed] [Google Scholar]
- 70.Miller SI, Kukral AM, Mekalanos JJ. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella Typhimurium virulence. Proc Natl Acad Sci U S A 86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wray C, Sojka WJ. 1978. Experimental Salmonella Typhimurium infection in calves. Res Vet Sci 25:139–143. [PubMed] [Google Scholar]
- 72.Miller SI, Mekalanos JJ. 1990. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol 172:2485–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang W, Metcalf WW, Lee KS, Wanner BL. 1995. Molecular cloning, mapping, and regulation of Pho regulon genes for phosphonate breakdown by the phosphonatase pathway of Salmonella Typhimurium LT2. J Bacteriol 177:6411–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]