Abstract
Brucella abortus is a Gram-negative bacterium that infects humans and cattle, causing a chronic inflammatory disease known as brucellosis. A Th1-mediated immune response plays a critical role in host control of this pathogen. Recent findings indicate contrasting roles for lipid mediators in host responses against infections. 5-Lipoxygenase (5-LO) is an enzyme required for the production of the lipid mediators leukotrienes and lipoxins. To determine the involvement of 5-LO in host responses to B. abortus infection, we intraperitoneally infected wild-type and 5-LO-deficient mice and evaluated the progression of infection and concomitant expression of immune mediators. Here, we demonstrate that B. abortus induced the upregulation of 5-LO mRNA in wild-type mice. Moreover, this pathogen upregulated the production of the lipid mediators leukotriene B4 and lipoxin A4 in a 5-LO-dependent manner. 5-LO-deficient mice displayed lower bacterial burdens in the spleen and liver and less severe liver pathology, demonstrating an enhanced resistance to infection. Host resistance paralleled an increased expression of the proinflammatory mediators interleukin-12 (IL-12), gamma interferon (IFN-γ), and inducible nitric oxide synthase (iNOS) during the course of infection. Moreover, we demonstrated that 5-LO downregulated the expression of IL-12 in macrophages during B. abortus infection. Our results suggest that 5-LO has a major involvement in B. abortus infection, by functioning as a negative regulator of the protective Th1 immune responses against this pathogen.
INTRODUCTION
Brucella abortus is a Gram-negative bacterium that survives inside host cells as a facultative intracellular pathogen (1). It infects humans and cattle, causing a chronic inflammatory disease known as brucellosis. In humans, brucellosis symptoms include undulant fever, endocarditis, arthritis, and osteomyelitis (2). In cattle, B. abortus causes miscarriage and infertility, leading to serious economic losses (3). The host immune response to B. abortus is initiated through innate immune mechanisms that recognize bacterial components and provide the necessary signals for the induction of an adaptive immune response (4). This specific adaptive immune response, which is Th1 mediated, plays a critical role in host control of B. abortus (5). The Th1-driven immune response to B. abortus involves CD4+ and CD8+ T lymphocytes, macrophages, dendritic cells (DCs), and proinflammatory cytokines such as gamma interferon (IFN-γ) and interleukin-12 (IL-12) (6–9).
There is a growing interest in the immunoregulatory role of lipid mediators in infectious diseases (10–12). 5-Lipoxygenase (5-LO) is an enzyme required for the biosynthesis of two important groups of lipid mediators, leukotrienes (LTs) and lipoxins (LXs), both derived from arachidonic acid. 5-LO is made in several cell types, including neutrophils, eosinophils, monocytes/macrophages, dendritic cells, mast cells, and lymphocytes (13). To produce LTs, 5-LO acts on arachidonic acid, converting it to leukotriene A4 (LTA4). LTA4 is then converted into leukotriene B4 (LTB4) or leukotriene C4 (LTC4), molecules that are exported from the cell (14). LTs are produced at the initial steps of the acute inflammatory response and act predominantly as proinflammatory lipid mediators. LTB4, for example, attracts neutrophils, monocytes, and lymphocytes to the site of inflammation and induces edema formation by increasing vascular permeability and plasma leakage at the site of inflammation (15–18). In contrast to LTs, production of LXs is dependent on cell-cell interaction by a process known as transcellular biosynthesis and requires the interaction between 5-LO and 15- or 12-lipoxygenase (15-LO or 12-LO, respectively) (19). Production of LXs by 5-15-LOs begins in eosinophils, monocytes, or epithelial cells (18). The 15-LO metabolic product, 15S-hydroperoxyeicosatetraenoic acid (15S-HPETE), is released by these cells, taken up by polymorphonuclear cells or monocytes, and then processed by 5-LO into bioactive lipoxin A4 (LXA4) or lipoxin B4 (LXB4). Production of LXs by 15-12-LO occurs after arachidonic acid conversion to LTA4 by 5-LO in leukocytes. LTA4 is taken up by platelets and transformed to LXA4 and LXB4 via 12-LO activity (20). LXs act as anti-inflammatory and proresolution lipid mediators, by inhibiting both neutrophil and eosinophil transmigration into sites of infection and by promoting the noninflammatory infiltration of monocytes that is required for resolution and wound healing (21–23). LXs also stimulate macrophages to ingest and clear apoptotic neutrophils and elevate levels of the anti-inflammatory cytokine transforming growth factor beta 1 (24, 25).
The role of 5-LO in the immune response to pathogens has been investigated in a growing list of models with contrasting outcomes regarding host control of infection (26). Paracoccidioides brasiliensis infection is fatal in 5-LO-deficient mice, due to the development of an aggravated lung injury and higher pulmonary fungal burden (10). Although parasite burden is reduced (27), Toxoplasma gondii-infected 5-LO-deficient mice also succumb due to an exacerbated proinflammatory response. In contrast, Trypanosoma cruzi-infected 5-LO-deficient mice have a higher survival rate that is likely related to a downregulation of the inflammatory response and a transiently increased parasitemia (28). Mycobacterium tuberculosis-infected 5-LO-deficient mice also display enhanced resistance; however, survival of mice correlates with an upregulation of the inflammatory response and reduced pathogen burden (29).
Whether the activity of 5-LO in B. abortus infection contributes to pathogen elimination or disease enhancement is unknown. The aim of this study was to investigate if 5-LO-dependent mechanisms can control the immune response against this pathogen. Here, 129 Sv/Ev wild-type and 5-LO-knockout (5-LO KO) mice were infected intraperitoneally (i.p.) with B. abortus. We evaluated the progression of infection and concomitant expression of immune mediators. Our results indicate an involvement of 5-LO in the regulation of the Th1 immune response against B. abortus. We demonstrated that the generation of LTB4 and LXA4 by B. abortus infection in wild-type mice occurs in a 5-LO-dependent manner. In the absence of 5-LO, reduced production of LXA4 and enhanced production of proinflammatory cytokines may lead to increased resistance to B. abortus in mice. 5-LO KO mice displayed reduced Brucella burden in spleen and liver and less severe liver pathology than did 129 Sv/Ev wild-type mice. Our results suggest that 5-LO functions as a negative regulator of the host inflammatory response to B. abortus infection.
MATERIALS AND METHODS
Mice.
5-Lipoxygenase-knockout (5-LO KO) mice were kindly provided by João Santana da Silva (Department of Biochemistry and Immunology, School of Medicine of Ribeirão Preto, University of São Paulo, Brazil). Wild-type 129 Sv/Ev mice were obtained from the Federal University of Minas Gerais (Belo Horizonte, Brazil). 5-LO KO and 129 Sv/Ev mice between 6 and 8 weeks of age were used in all experiments and were maintained at the Federal University of Minas Gerais (Belo Horizonte, Brazil). All procedures described in this study had prior approval from the local animal ethics committee.
Bacterial strain.
The B. abortus strain 2308 was grown in Brucella broth (BB) liquid medium (Difco) at 37°C under constant agitation (200 rpm). After 3 days of growth, the bacterial culture was centrifuged, and the pellet was resuspended in saline solution (NaCl, 0.8%) and frozen at −80°C in 20% glycerol. For counting of CFU, B. abortus was serially diluted, plated on BB medium containing 1.5% bacteriological agar, and incubated for 72 h at 37°C.
Mouse infection and bacterial burden.
Mice were infected intraperitoneally (i.p.) with 106 CFU of B. abortus strain 2308. To assess bacterial burden in infected mice, mice were euthanized 1, 2, 3, or 6 weeks after infection and spleens and livers were collected. Spleens and livers from individual animals were homogenized in phosphate-buffered saline (PBS), and serial dilutions were plated on Brucella broth agar (Difco). The number of CFU was determined after 3 days of incubation at 37°C.
Eicosanoid and cytokine measurements.
To determine serum levels of eicosanoids, blood was collected from B. abortus-infected mice via the retro-orbital plexus 1, 3, and 6 weeks after infection, and the LTB4 and LXA4 in the collected serum were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Cayman Chemical Company and Neogen Corporation, respectively). For cytokine detection in spleens, 100 mg of each spleen was homogenized in 1 ml of cytokine extraction solution (0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.1 mM benzethonium chloride, 10 mM EDTA, 20 kallikrein inhibitor units aprotinin A, and PBS) and 0.05% Tween 20. After homogenization, samples were centrifuged at 300 × g for 20 min at 4°C, and the supernatants were used immediately for ELISA (kit from R&D Systems), according to the manufacturer's instructions.
Measurement of mRNA expression of IL-12p35, IFN-γ, iNOS, and 5-LO genes in spleens.
Total RNA from mouse spleens was isolated using TRIzol reagent (Invitrogen). The cDNA was synthesized with Illustra Ready-to-Go real-time PCR (RT-PCR) beads (GE Healthcare) according to the manufacturer's instructions. Expression of mRNA encoding IL-12, IFN-γ, inducible nitric oxide synthase (iNOS), 5-LO enzyme, and β-actin was analyzed by RT-PCR. RT-PCR was performed on an ABI 7900 real-time PCR system (Applied Biosystems) using SYBR Green master mix (Applied Biosystems). The relative level of gene expression was determined by the comparative threshold cycle (CT) method as described by the manufacturer. The following primer pairs were used: 5-LO (kindly provided by Daniel Cisalpino, Federal University of Minas Gerais), AGCTGCCTGCTGTGCATCCC (forward) and CCCGGTGGCATTGGCCTTGT (reverse); IL-12p35, TGGTGTCTCCACTCAAAGAGTCTGAGG (forward) and AGCAGCAGATGTGAGTGG (reverse); IFN-γ, TCTGGAGGAACTGGCAAAAG (forward) and TTCAAGACTTCAAAGAGTCTGAGG (reverse); and iNOS, CAGCTGGGCTGTACAAACCTT (forward) and CATTGGAAGTGAAGCGTTTCG (reverse).
FACS analysis.
Briefly, spleens were collected and treated with Liberase (1 mg/ml; Roche, Basel, Switzerland). The number of cells was adjusted to 106/well, and brefeldin A (1 μg/well; Sigma-Aldrich, Saint Louis, MO, USA) was added for 4 h. Samples were then exposed for 20 min to Fc-block anti-mouse CD16/32 clone 2.4G2 (BD Biosciences, Franklin Lakes, NJ, USA) in fluorescence-activated cell sorting (FACS) buffer (PBS, 0.25% bovine serum albumin [BSA], 1 mM NaN3) and stained for the following surface markers for 20 min: fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD8a clone 53-6.7, phycoerythrin (PE)-conjugated CD11c clone HL3, and allophycocyanin (APC)-Cy7 anti-mouse CD11b clone M1/70 (all from BD) or biotin-conjugated F4/80 clone BM8 (eBioscience, San Diego, CA, USA). Samples that were stained with biotin-conjugated antibodies were incubated with streptavidin-peridinin chlorophyll protein (PerCP) (BD) for 20 min. Cells were stained for intracellular marker using the BD Cytofix/Cytoperm method and APC-conjugated anti-mouse IL-12p40/p70 clone C15.6 (BD). After 30 min of incubation, samples were washed with FACS buffer and resuspended in PBS. Events were collected using a BD FACSCanto II flow cytometer, and data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Histopathology.
Liver medial lobes from infected mice were collected at 1, 2, 3, and 6 weeks postinfection; fixed in 10% buffered formaldehyde; and embedded in paraffin. Histological sections (4 μm) were mounted on a slide and stained with hematoxylin and eosin (HE). Granulomas were counted by microscopy (Axiolab; Carl Zeiss) using a 10× objective lens. All slides were digitalized by scanner (HP Scanjet 2400) at 300-dpi resolution. The pixels of each histological section were subjected to a binary image, and the total area of the slide was calculated. The results were expressed as the number of granulomas per area of liver (mm2). The area of a granuloma was obtained using the KS300 software contained in the Carl Zeiss image analyzer. With a 20× microscopic objective lens, 40 granulomas from each mouse were randomly chosen and scanned using a microcamera (Olympus U-CMAD 3). The total area was measured, and results were expressed in square micrometers (μm2). Ten hepatic granulomas per animal (n = 5) were randomly chosen for evaluation of their cellularity. Lymphocytes and cells morphologically similar to either macrophages or epithelioid cells were counted using the KS300 software. Results were expressed as number of cells per granuloma.
Statistical analysis.
Statistical significance was assessed by the unpaired Student t test, and P values of ≤0.05 were considered significant.
RESULTS
5-LO deficiency increases resistance to Brucella abortus infection in mice.
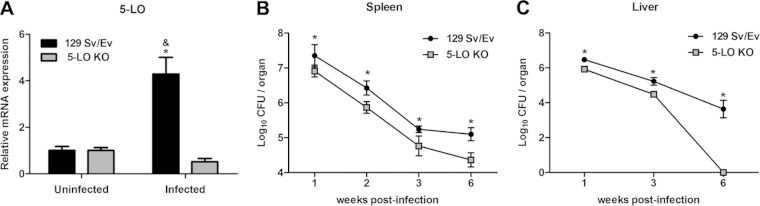
To investigate whether 5-LO metabolism was altered during B. abortus infection, expression of 5-LO was measured in the spleens of 129 Sv/Ev infected mice. 5-LO KO mice were used as a control. Upregulation of 5-LO mRNA was assessed in mice 1 week postinfection and in noninfected (week 0) mice by RT-PCR. As expected, no significant upregulation of 5-LO mRNA was detected in 5-LO KO mice before or after B. abortus infection (Fig. 1A). In contrast, B. abortus-infected 129 Sv/Ev mice strongly upregulated 5-LO mRNA after infection.
FIG 1.
5-LO is induced by B. abortus infection in 129 Sv/Ev mice and downregulates host resistance to this pathogen. 129 Sv/Ev and 5-LO KO mice were infected with B. abortus 2308 (1 × 106 CFU/mouse). (A) Splenocytes were obtained 1 week after infection, and the relative expression of 5-LO mRNA was analyzed by RT-PCR. Uninfected mice were used as controls. (B and C) The bacterial load was analyzed in the spleens of mice (1, 2, 3, and 6 weeks postinfection) and in livers (1, 3, and 6 weeks postinfection) by CFU counting. Results are expressed as means ± standard deviations and are representative of 3 independent experiments (n = 5). *, P ≤ 0.05 compared to 5-LO KO infected mice; &, P ≤ 0.05 compared to 129 Sv/Ev uninfected mice.
To determine the involvement of 5-LO in the control of B. abortus infection, the bacterial burdens of 5-LO KO and 129 Sv/Ev mice at 1, 2, 3, and 6 weeks postinfection were evaluated by quantifying CFU in mouse spleens and at 1, 3, and 6 weeks postinfection in mouse livers. As early as 1 week after infection, 5-LO KO mice (6.9 ± 0.2 log10 CFU/spleen) had a significantly (P < 0.05) reduced bacterial burden compared to 129 Sv/Ev mice (7.4 ± 0.3 log10 CFU/spleen) (Fig. 1B). Similarly, at other time points evaluated, bacterial burdens from 5-LO KO mice continued to be significantly lower than those in 129 Sv/Ev mice in both organs, demonstrating that 5-LO KO mice displayed enhanced resistant to B. abortus infection (Fig. 1B and C). The greatest difference between the two groups of mice was detected 6 weeks postinfection. 5-LO KO mice had 4.4 ± 0.2 log10 CFU/spleen, and 129 Sv/Ev mice displayed 5.1 ± 0.2 log10 CFU/spleen. Interestingly, in livers at 6 weeks postinfection 5-LO KO mice had cleared the infection compared to wild-type animals.
Brucella abortus infection upregulates production of LTB4 and LXA4 in a 5-LO-dependent manner.
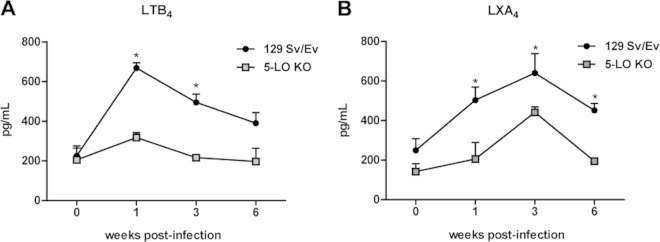
To determine whether B. abortus infection altered 5-LO metabolic pathways, the levels of two 5-LO products, LTB4 and LXA4, were measured in the serum of 5-LO KO and 129 Sv/Ev mice at 1, 3, and 6 weeks postinfection. B. abortus infection of 129 Sv/Ev mice induced LTB4 production, with the highest level detected at the first week postinfection (Fig. 2A). At the third week of infection, the LTB4 levels were still significantly higher in wild-type than in 5-LO KO mice, although LTB4 production was followed by a gradual decrease in serum concentration. Infected 5-LO KO mice did not show significant LTB4 serum production above basal levels (week 0) at any of the times evaluated, suggesting that LTB4 production is dependent on 5-LO during B. abortus infection. With regard to LXA4, 129 Sv/Ev mice infected with B. abortus also induced LXA4 production, albeit differently from LTB4, as the highest level was detected at the third week postinfection (Fig. 2B). Infected 5-LO KO mice had lower LXA4 serum levels than wild-type mice, indicating that LXA4 production is also partially dependent on 5-LO in this infection model. Taken together, these data demonstrate that B. abortus infection activates the 5-LO metabolic pathway, upregulating production of LTB4 and LXA4.
FIG 2.
B. abortus mouse infection upregulates the production of LTB4 and LXA4 in a 5-LO-dependent manner. The blood of 129 Sv/Ev and 5-LO KO mice infected with B. abortus 2308 (1 × 106/mouse) was collected 1, 3, and 6 weeks after infection for the measurement of LTB4 (A) and LXA4 (B) in the serum by ELISA. Results are expressed as means ± standard deviations and are representative of 3 independent experiments (n = 3). *, P ≤ 0.05 compared to 5-LO KO mice.
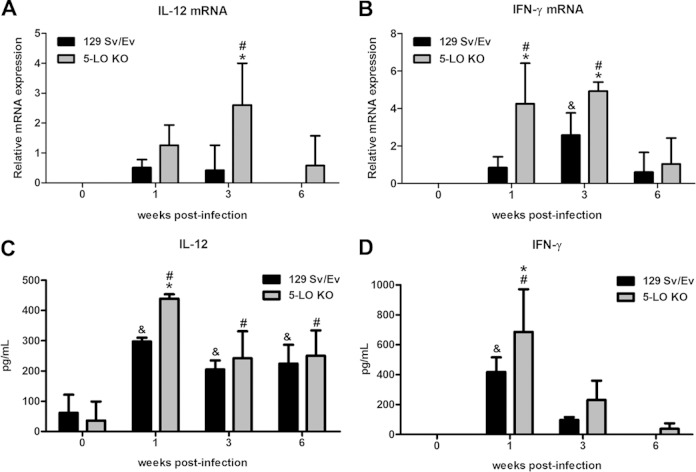
5-LO-deficient mice display increased expression of proinflammatory mediators during Brucella abortus infection.
To investigate whether 5-LO deficiency affects the host immune response during B. abortus infection, gene expression kinetics of the proinflammatory mediators IL-12p35, IFN-γ, and inducible nitric oxide synthase (iNOS) were analyzed. mRNA levels were determined by RT-PCR in spleen homogenates from 129 Sv/Ev and 5-LO KO mice at 1, 3, and 6 weeks after infection. Additionally, IL-12p70 and IFN-γ protein levels were measured in spleen homogenates by ELISA. One week after infection, 5-LO KO mice had elevated protein levels of IL-12p70 (Fig. 3C) and IFN-γ (Fig. 3D) compared to wild-type animals. However, at the third and sixth weeks postinfection, no difference was observed between 5-LO KO and 129 Sv/Ev mice for both cytokines. With regard to transcripts, B. abortus infection induced higher expression of the IFN-γ gene at 1 and 3 weeks postinfection in 5-LO KO mice compared to wild-type animals (Fig. 3B). IL-12p35 mRNA upregulation in 5-LO KO increased relative to wild-type mice only at 3 weeks postinfection, although at 1 week after infection mice had an elevated expression that was not statistically significant (Fig. 3A).
FIG 3.
5-LO-deficient mice display increased expression of proinflammatory cytokines over the course of B. abortus infection. (A and B) RT-PCR analysis of the expression of IL-12p35 (A) and IFN-γ (B) in splenocytes from 129 Sv/Ev or 5-LO KO mice infected with B. abortus 2308 (1 × 106 CFU/mouse) 1, 3, and 6 weeks after infection. Values were normalized using endogenous β-actin as a control and the value of ΔCT from uninfected mouse samples. (C and D) IL-12p70 (C) and IFN-γ (D) protein levels were also measured in mouse spleen homogenates by ELISA. Results are expressed as means ± standard deviations and are representative of 3 independent experiments (n = 3). *, P ≤ 0.05 compared to 129 Sv/Ev mice; &, P ≤ 0.05 compared to uninfected 129 Sv/Ev mice; #, P ≤ 0.05 compared to uninfected 5-LO KO mice.
In terms of iNOS, only at the sixth week after infection did 129 Sv/Ev mice show a significant increase in iNOS mRNA expression, with no upregulation of iNOS transcripts at the first and third weeks postinfection (Fig. 4). 5-LO KO infected mice, however, displayed a strong upregulation of this proinflammatory mediator at the first and third weeks following infection, although at the sixth week of infection, iNOS mRNA levels were similar to those of 129 Sv/Ev mice. Altogether, these data suggest that in the absence of 5-LO, upregulation of the cytokines IL-12 and IFN-γ was observed at 1 week postinfection and the enzyme iNOS occurred at the first and third weeks of B. abortus infection.
FIG 4.
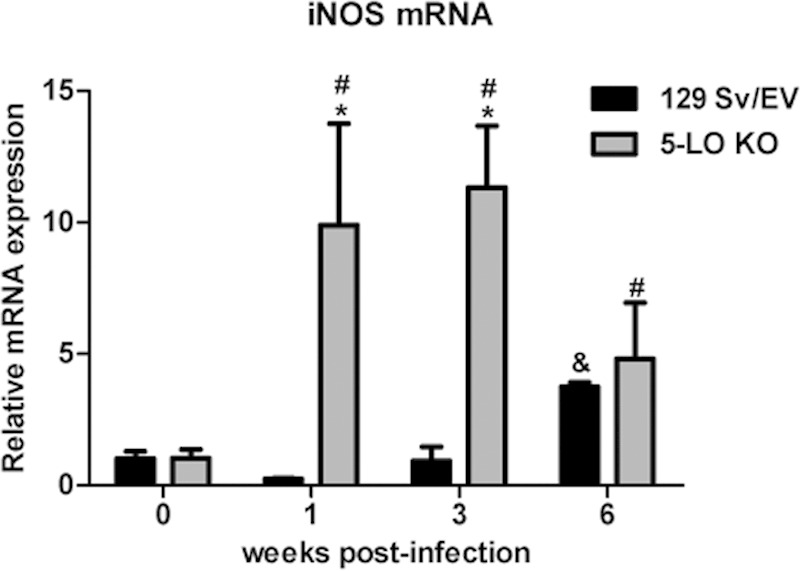
5-LO-deficient mice display increased expression of iNOS mRNA over the course of B. abortus infection. At 1, 3, and 6 weeks after infection of 129 Sv/Ev or 5-LO KO mice with B. abortus (1 × 106 CFU/mouse), iNOS mRNA in mouse splenocytes was measured by RT-PCR. The values were normalized using endogenous β-actin as a control and the value of ΔCT from uninfected mouse samples. Results are expressed as means ± standard deviations and are representative of 3 independent experiments (n = 3). *, P ≤ 0.05 compared to 129 Sv/Ev mice; &, P ≤ 0.05 compared to uninfected 129 Sv/Ev mice; #, P ≤ 0.05 compared to uninfected 5-LO KO mice.
5-LO regulates IL-12 expression in macrophages during Brucella abortus infection.
Because B. abortus-infected mice had an increased proinflammatory response in the absence of 5-LO, we next investigated the cellular source of the proinflammatory cytokine IL-12. One or 3 weeks after infection, splenocytes were analyzed by flow cytometry for the presence of IL-12p70-producing macrophages (CD11b+ F480+ IL-12+) or dendritic cells (CD11c+ CD8a+ IL-12+). Only at the third week postinfection did 5-LO KO mice display a significantly higher percentage of IL-12-positive macrophages than did 129 Sv/Ev mice (Table 1). The frequencies of IL-12-producing DCs were not different between 5-LO KO and wild-type mice. The higher percentage of IL-12-producing macrophages in 5-LO KO mice than in 129 Sv/Ev mice suggests that 5-LO functions as a negative regulator of IL-12 expression in macrophages during B. abortus infection.
TABLE 1.
Percentage of IL-12-producing macrophages or dendritic cells in spleen cells from mice infected with B. abortus
| Wk p.i.a | Mouse genotype | % cell population in the spleen |
|
|---|---|---|---|
| CD11b+ F480+ IL-12p40/p70+b | CD11c+ CD8a+ IL-12p40/p70+ | ||
| 0 | 129 Sv/Ev | 1.2 ± 0.1 | 0.8 ± 0.3 |
| 5-LO KO | 0.6 ± 0.0 | 0.8 ± 0.2 | |
| 1 | 129 Sv/Ev | 1.4 ± 0.0 & | 0.9 ± 0.3 |
| 5-LO KO | 1.5 ± 0.1 # | 1.0 ± 0.4 | |
| 3 | 129 Sv/Ev | 0.8 ± 0.1 & | 1.0 ± 0.3 |
| 5-LO KO | 1.3 ± 0.3 *# | 0.8 ± 0.3 | |
p.i., postinfection.
Statistical significance is indicated by symbols: *, P ≤ 0.05 compared to 129 Sv/Ev mice; &, P ≤ 0.05 compared to noninfected 129 Sv/Ev mice; #, P ≤ 0.05 compared to noninfected 5-LO KO mice (week 0).
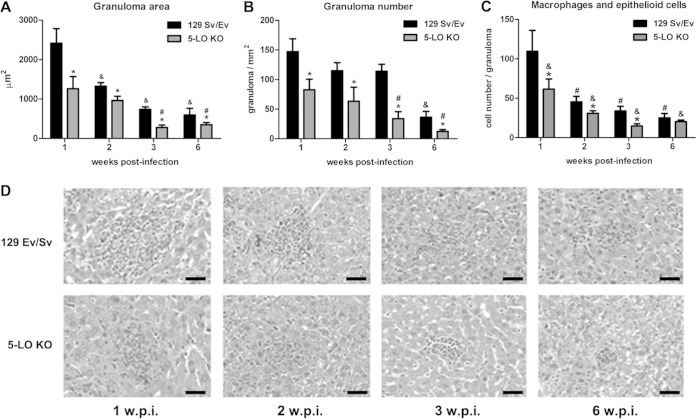
5-LO-deficient mice have reduced liver pathology.
To verify whether the decreased bacterial burden was associated with reduced liver pathology, histological analysis was performed on the livers of infected 5-LO KO and 129 Sv/Ev mice. Hepatic granulomas were counted and the areas of inflammatory infiltrates were measured at 1, 2, 3, and 6 weeks postinfection. Both mouse strains had a gradual decrease in the average area of granulomas as infection progressed (Fig. 5A). However, 5-LO KO mice displayed granulomas with significantly smaller areas than those in 129 Sv/Ev mice (Fig. 5D). Furthermore, at 1, 2, and 3 weeks postinfection 5-LO KO mice presented fewer macrophages and epithelioid cells in liver granuloma than did wild-type mice (Fig. 5C). However, no difference was observed in lymphocyte numbers in liver granulomas between these two mouse strains (data not shown). Additionally, there was a striking difference in the numbers of granulomas between the two mouse strains. As opposed to 5-LO KO mice that displayed reduced numbers of granulomas at all times studied, 129 Sv/Ev mice had a significant reduction in the number of granulomas only at 6 weeks postinfection (Fig. 5B). Together, these data suggest that in the absence of 5-LO, B. abortus-infected mice display less severe liver pathology, accompanied by reduced numbers of granulomas and cellular infiltrates.
FIG 5.
5-LO-deficient mice display less severe liver pathology. (A and B) The average area (A) and total number (B) of hepatic granulomas were quantified in histological slides from the liver of 129 Sv/Ev or 5-LO KO infected mice at 1, 2, 3, and 6 weeks postinfection. (C) The number of macrophages/epithelioid cells in the granulomas was also evaluated. (D) Representative slides from the livers analyzed are shown. Original magnification, ×10. Results are expressed as means ± standard deviations and are representative of 3 independent experiments (n = 5). w.p.i., weeks postinfection; *, P ≤ 0.05 compared to 129 Sv/Ev mice; &, P ≤ 0.05 compared to 129 Sv/Ev mice 1 week postinfection; #, P ≤ 0.05 compared to 5-LO KO mice 1 week postinfection. Bar, 20 μm.
DISCUSSION
In the present study, we addressed the question of whether 5-LO plays a role during B. abortus infection in mice. We observed that B. abortus triggered high levels of 5-LO expression in the spleens of 129 Sv/Ev mice 1 week after infection. To evaluate if this upregulation was associated with the biological function of this enzyme, we measured the levels of its metabolic products, LTB4 and LXA4, in the sera of mice. Both products were produced during B. abortus infection in a 5-LO-dependent manner, but with considerably different kinetics. The peak of LTB4 production was detected 1 week after infection, while the peak of LXA4 production was detected at 3 weeks after infection. LTB4 is an important chemotactic factor that stimulates migration of macrophages, dendritic cells, and neutrophils to infection sites and strongly activates leukocytes during inflammatory processes (14, 30). Early production of LTB4 is consistent with an initial period of inflammation when cell recruitment and activation are required for pathogen killing. In contrast, LXA4 is a potent anti-inflammatory mediator (31), and LXA4 levels were higher at a later phase of infection when an anti-inflammatory immune response is required to control the host response. Interestingly, others (29) have observed a similar profile in M. tuberculosis-infected mice, in which the highest levels of LTB4 occurred at 10 days postinfection, while LXA4 production peaked 20 days after infection. In the M. tuberculosis infection model, the production of LTB4 and LXA4 was also shown to be 5-LO dependent.
Infection with B. abortus leads to a strong Th1 immune response (5). In this context, the Th1-type cytokines IL-12 and IFN-γ are key molecules that participate in the control of B. abortus infection (8). We investigated the expression profiles of these cytokines in the spleens of 129 Sv/Ev mice. IL-12 and IFN-γ mRNAs, as well as protein levels, were upregulated at the early phase of B. abortus infection. In the absence of 5-LO, the response of mice to B. abortus was dramatically altered. First, LTB4 and LXA4 were produced at much lower levels at all time points evaluated. Second, cytokine profiles revealed that IL-12 and IFN-γ were produced in larger amounts in 5-LO KO mice at 1 week postinfection than in wild-type animals. Additionally, in the absence of 5-LO, spleen and liver CFU counts and liver pathology were reduced with considerably lower granuloma numbers and smaller granuloma areas. An enhanced resistance to intracellular bacteria was also seen in 5-LO-deficient mice infected with M. tuberculosis that, similarly to B. abortus-infected 5-LO KO mice, displayed increased expression of proinflammatory mediators (29).
Interleukin-10 (IL-10) is a potent regulatory cytokine in many infections and inflammatory diseases (32). Our group has recently demonstrated that, in the absence of IL-10, mice display increased resistance to Brucella infection that correlates with enhanced production of proinflammatory cytokines (33). We investigated whether IL-10 protein expression was altered in the spleens of B. abortus-infected 5-LO KO mice, but no difference was detected in comparison to 129 Sv/Ev mice (data not shown). We hypothesize that reduced LXA4 levels in 5-LO KO mice may correlate with an increase in proinflammatory mediators observed in these animals at an early phase of infection. Thus, LXA4 may function as an immunoregulatory factor during B. abortus infection. LXA4 may also regulate cell migration to infection sites during B. abortus infection, because a higher percentage of IL-12p70-producing macrophages were detected in the spleens of B. abortus-infected 5-LO KO mice. Others have reported that LXA4 regulates cell migration to infection sites during T. gondii infection (28).
We also decided to investigate other inflammatory mediators that may contribute to control of the bacteria. We detected considerably higher iNOS mRNA levels in 5-LO KO mice throughout B. abortus infection, with the highest iNOS levels at the first and third weeks postinfection. Nitric oxide is important to control intracellular bacterial infection. Others have reported that B. abortus is inhibited by high levels of NO during early infection (34), and in the absence of iNOS, mice exhibited a delayed control of the bacteria (35). The upregulation of iNOS expression displayed by 5-LO KO mice likely contributed to improved control of B. abortus during early infection.
Taken together, our results demonstrate the involvement of 5-LO in the control of B. abortus. 5-LO directs the generation of the proinflammatory mediator LTB4 at the initial phase of B. abortus infection, which parallels enhanced production of type 1 proinflammatory cytokines. In the absence of 5-LO and at the initial stages of infection, NO may be an important component that enhances the control of B. abortus. At the later phases of infection, 5-LO directs the generation of the anti-inflammatory mediator LXA4 that coincides with the downregulation of liver pathology. For that reason, 5-LO activity is a potential target for the control of Brucella replication in infected patients. 5-LO inhibitors are currently used in the treatment of asthmatic patients, and the present results support the development of new immunopharmacological interventions for the control of brucellosis.
ACKNOWLEDGMENTS
This work was supported by grants from CNPq, CNPq/CONICET, FAPEMIG, FAPEMIG/CNPq (PRONEX), CAPES/PNPD, CNPq/CT-Biotec, CNPq/REPENSA, and INCT-Vacinas.
REFERENCES
- 1.Gorvel JP, Moreno E. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet Microbiol 90:281–297. doi: 10.1016/S0378-1135(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 2.Franco MP, Mulder M, Gilman RH, Smits HL. 2007. Human brucellosis. Lancet Infect Dis 7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 3.Pappas G. 2010. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents 36(Suppl 1):S8–S11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Gomes MT, Campos PC, de Almeida LA, Oliveira FS, Costa MM, Marim FM, Pereira GS, Oliveira SC. 2012. The role of innate immune signals in immunity to Brucella abortus. Front Cell Infect Microbiol 2:130. doi: 10.3389/fcimb.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golding B, Scott DE, Scharf O, Huang LY, Zaitseva M, Lapham C, Eller N, Golding H. 2001. Immunity and protection against Brucella abortus. Microbes Infect 3:43–48. doi: 10.1016/S1286-4579(00)01350-2. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira SC, Splitter GA. 1995. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol 25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence T, Willoughby DA, Gilroy DW. 2002. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol 2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 8.Brandao AP, Oliveira FS, Carvalho NB, Vieira LQ, Azevedo V, Macedo GC, Oliveira SC. 2012. Host susceptibility to Brucella abortus infection is more pronounced in IFN-gamma knockout than IL-12/beta2-microglobulin double-deficient mice. Clin Dev Immunol 2012:589494. doi: 10.1155/2012/589494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. 2008. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol 180:1080–1087. doi: 10.4049/jimmunol.180.2.1080. [DOI] [PubMed] [Google Scholar]
- 10.Santos PC, Santos DA, Ribeiro LS, Fagundes CT, de Paula TP, Avila TV, Baltazar LDM, Madeira MM, Cruz RDC, Dias AC, Machado FS, Teixeira MM, Cisalpino PS, Souza DG. 2013. The pivotal role of 5-lipoxygenase-derived LTB4 in controlling pulmonary paracoccidioidomycosis. PLoS Negl Trop Dis 7:e2390. doi: 10.1371/journal.pntd.0002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado FS, Aliberti J. 2008. Role of lipoxin in the modulation of immune response during infection. Int Immunopharmacol 8:1316–1319. doi: 10.1016/j.intimp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Machado FS, Mukherjee S, Weiss LM, Tanowitz HB, Ashton AW. 2011. Bioactive lipids in Trypanosoma cruzi infection. Adv Parasitol 76:1–31. doi: 10.1016/B978-0-12-385895-5.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radmark O, Samuelsson B. 2010. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun 396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 14.Hedi H, Norbert G. 2004. 5-Lipoxygenase pathway, dendritic cells, and adaptive immunity. J Biomed Biotechnol 2004:99–105. doi: 10.1155/S1110724304310041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tager AM, Luster AD. 2003. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids 69:123–134. doi: 10.1016/S0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 16.Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. 2003. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol 4:974–981. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- 17.Bjork J, Hedqvist P, Arfors KE. 1982. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation 6:189–200. doi: 10.1007/BF00916243. [DOI] [PubMed] [Google Scholar]
- 18.Stables MJ, Gilroy DW. 2011. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res 50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Romano M. 2010. Lipoxin and aspirin-triggered lipoxins. ScientificWorldJournal 10:1048–1064. doi: 10.1100/tsw.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano M, Serhan CN. 1992. Lipoxin generation by permeabilized human platelets. Biochemistry 31:8269–8277. doi: 10.1021/bi00150a021. [DOI] [PubMed] [Google Scholar]
- 21.Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN. 1998. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J 12:487–494. [DOI] [PubMed] [Google Scholar]
- 22.Serhan CN, Takano T, Gronert K, Chiang N, Clish CB. 1999. Lipoxin and aspirin-triggered 15-epi-lipoxin cellular interactions anti-inflammatory lipid mediators. Clin Chem Lab Med 37:299–309. [DOI] [PubMed] [Google Scholar]
- 23.Maddox JF, Serhan CN. 1996. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. 2002. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol 13:2497–2507. doi: 10.1097/01.ASN.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 25.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. 2006. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 26.Machado FS, Aliberti J. 2006. Impact of lipoxin-mediated regulation on immune response to infectious disease. Immunol Res 35:209–218. doi: 10.1385/IR:35:3:209. [DOI] [PubMed] [Google Scholar]
- 27.Aliberti J, Serhan C, Sher A. 2002. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med 196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavanelli WR, Gutierrez FR, Mariano FS, Prado CM, Ferreira BR, Teixeira MM, Canetti C, Rossi MA, Cunha FQ, Silva JS. 2010. 5-Lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microb Infect 12:587–597. doi: 10.1016/j.micinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. 2005. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest 115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters-Golden M, Henderson WR Jr. 2007. Leukotrienes. N Engl J Med 357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 31.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. 2006. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med 12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 33.Corsetti PP, de Almeida LA, Carvalho NB, Azevedo V, Silva TM, Teixeira HC, Faria AC, Oliveira SC. 2013. Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PLoS One 8:e74729. doi: 10.1371/journal.pone.0074729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Qureshi N, Soeurt N, Splitter G. 2001. High levels of nitric oxide production decrease early but increase late survival of Brucella abortus in macrophages. Microb Pathog 31:221–230. doi: 10.1006/mpat.2001.0463. [DOI] [PubMed] [Google Scholar]
- 35.Ko J, Gendron-Fitzpatrick A, Splitter GA. 2002. Susceptibility of IFN regulatory factor-1 and IFN consensus sequence binding protein-deficient mice to brucellosis. J Immunol 168:2433–2440. doi: 10.4049/jimmunol.168.5.2433. [DOI] [PubMed] [Google Scholar]