Abstract
Although the importance of alveolar macrophages for host immunity during early Streptococcus pneumoniae lung infection is well established, the contribution and relative importance of other innate immunity mechanisms and of bacterial factors are less clear. We have used a murine model of S. pneumoniae early lung infection with wild-type, unencapsulated, and para-amino benzoic acid auxotroph mutant TIGR4 strains to assess the effects of inoculum size, bacterial replication, capsule, and alveolar macrophage-dependent and -independent clearance mechanisms on bacterial persistence within the lungs. Alveolar macrophage-dependent and -independent (calculated indirectly) clearance half-lives and bacterial replication doubling times were estimated using a mathematical model. In this model, after infection with a high-dose inoculum of encapsulated S. pneumoniae, alveolar macrophage-independent clearance mechanisms were dominant, with a clearance half-life of 24 min compared to 135 min for alveolar macrophage-dependent clearance. In addition, after a high-dose inoculum, successful lung infection required rapid bacterial replication, with an estimated S. pneumoniae doubling time of 16 min. The capsule had wide effects on early lung clearance mechanisms, with reduced half-lives of 14 min for alveolar macrophage-independent and 31 min for alveolar macrophage-dependent clearance of unencapsulated bacteria. In contrast, with a lower-dose inoculum, the bacterial doubling time increased to 56 min and the S. pneumoniae alveolar macrophage-dependent clearance half-life improved to 42 min and was largely unaffected by the capsule. These data demonstrate the large effects of bacterial factors (inoculum size, the capsule, and rapid replication) and alveolar macrophage-independent clearance mechanisms during early lung infection with S. pneumoniae.
INTRODUCTION
Microaspiration of potentially pathogenic bacteria colonizing the nasopharynx is the probable cause of most cases of bacterial pneumonia. Whether microaspiration results in pneumonia will depend on the numbers of bacteria reaching the lung, their virulence, and the host's ability to clear the invading bacteria. Defining the efficacy of different lung immune mechanisms and the bacterial characteristics for successful infection will be essential for understanding the epidemiology of pneumonia and for the design of effective preventative strategies. Initial lung immune defenses include the resident alveolar macrophages (AMs) (1–3), physical removal of microbes by mucociliary clearance, and mucosal antibacterial peptides (e.g., defensins) and proteins (e.g., lysozyme and lactoferrin) (4, 5). The importance of AMs for preventing lung infections due to Streptococcus pneumoniae, the commonest cause of bacterial pneumonia, is well established (2, 6–9). The published data indicate that AM recognition and AM phagocytosis of S. pneumoniae are essential for controlling bacterial numbers during the first few hours of lung infection. Impairment of AM-mediated bacterial clearance during early infection, caused by either prior viral infection or genetic modification to prevent recognition of S. pneumoniae by phagocytic receptors, allows S. pneumoniae to more readily establish pneumonic infection (8, 10). The efficacy of AM-mediated immunity to S. pneumoniae is also affected by bacterial inoculum size, with a large inoculum seemingly saturating bacterial clearance and thereby more likely to result in pneumonia (8, 11). Macrophage phagocytosis of S. pneumoniae is enhanced by antibody (12). However, in mouse models, antibody to S. pneumoniae acquired by previous nasopharyngeal colonization had little effect on S. pneumoniae clearance from the lung at very early time points (13, 14).
In contrast to the extensive data on the role of AMs, there are few data on the relative importance of non-AM-dependent lung immunity against S. pneumoniae. Both smoking and viral respiratory infections increase the risk of developing pneumonia, and this might be partly due to the effects of cigarette smoke or virus-dependent epithelial damage on mucociliary clearance. Furthermore, S. pneumoniae organisms are susceptible to killing by soluble components of the mucosal surfaces, such as defensins and lactoferrin (4). Both mechanisms could contribute toward AM-independent immunity during early lung infection, but how important these are compared to AM-dependent immunity is not known. In addition, the bacterial characteristics necessary for a pathogen to successfully establish early lung infection are poorly described. These potentially include avoidance of AM-mediated phagocytosis and perhaps inhibition of AM-independent clearance mechanisms. The S. pneumoniae capsule inhibits phagocytosis (15, 16) and also prevents mucus-dependent clearance from the nasopharynx and inhibits defensins (17, 18), and therefore it could potentially prevent both AM-dependent and -independent clearance. However, unencapsulated bacteria can cause infective exacerbations of COPD (chronic obstructive pulmonary disease) and adhere to epithelium better than encapsulated bacteria (19, 20), suggesting that the capsule may not be essential for S. pneumoniae lung infection. Another bacterial factor that is potentially important for establishing lung infection is an ability to rapidly replicate despite the limited nutrient availability within the lung. However, although S. pneumoniae must replicate in the later stages of an infection, immediate rapid bacterial growth after microaspiration may not be necessary during the early stages of lung infection when immune evasion maybe more important for bacterial survival. The potential effects of these bacterial factors during early lung infection could help explain why S. pneumoniae is such a dominant pathogen causing pneumonia despite being only one of several hundred bacterial species that colonize the nasopharynx.
Using a murine model of early lung infection, we have investigated the relative importance of inoculum size, bacterial replication, the capsule, and AM-independent and -dependent clearance mechanisms for S. pneumoniae to successfully establish lung infection. A simple mathematical model was used to provide estimates of the clearance half-life for AM-independent and -dependent clearance and bacterial doubling times.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The S. pneumoniae TIGR4 wild-type and unencapsulated (P1672) (constructed using the Janus cassette) strains were gifts from J. Weiser (16). The TIGR4Δpab strain was constructed as previously described and transferred to the unencapsulated P1672 strain using conventional transformation techniques (21). Bacteria were cultured at 37°C in 5% CO2 on blood agar plates or in Todd-Hewitt broth plus 0.5% yeast extract (THY) and stored at −80°C in 10% glycerol as single-use aliquots (optical density at 580 nm [OD580] of 0.4). S. pneumoniae organisms were labeled with 6-carboxyfluorescein–succinimidyl ester (FAM-SE) (Molecular Probes) (10 mg/ml in dimethyl sulfoxide [DMSO]; Sigma-Aldrich) as described previously (14), and flow cytometry confirmed that there were no significant differences in fluorescence intensity between strains. For in vitro growth in bronchoalveolar lavage fluid (BALF) experiments, various inocula of S. pneumoniae CFU were added to 1 ml of murine BALF (obtained as detailed below from uninfected mice) and incubated at 37°C in 5% CO2 for up to 6 h, and numbers of bacterial CFU were calculated by culturing serial dilutions on blood agar plates.
Infection experiments and in vivo phagocytosis assays.
Studies were performed according to UK Home Office and university guidelines for animal research. Six-week-old sex-matched CD1 mice were inoculated intranasally (i.n.) under halothane anesthesia with phosphate-buffered saline (PBS)-washed S. pneumoniae and culled after 0.5, 2, 4, or 24 h, and BALF was obtained using 1 ml of sterile PBS (14). Bacterial CFU were calculated by plating serial dilutions of BALF and spleen homogenates and by carrying out BALF differential cell counts using cytospins (14). Flow cytometry using a FACSCalibur (Becton Dickinson) was used to identify the proportion of AMs in BALF (identified by size and granularity) associated with fluorescent bacteria, as described previously (16). For confocal microscopy, BALF AMs were adhered to chamber slides (BD Falcon), fixed, permeabilized by 15 min incubation in 0.1% Triton, and then stained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) and antibody to F4/80 for 30 min. Images of infected AMs were captured on a Leica SP2 confocal microscope, and intracellular bacteria were identified by Z stacking. For AM depletion experiments, mice were treated with i.n. instillation of 50 μl of 7-mg/ml liposomal clodronate (Cl2MDP) or empty liposomes 72 h before inoculation with S. pneumoniae (23, 24).
Mathematical modeling.
Numbers of bacteria recovered by quantitative culture of BALF were considered to reflect total numbers of viable bacteria in this compartment. The net rate of change in number of bacteria in BALF (dN/dT) was modeled using the formula ρN0 − μN0 − ϕN0, where N is number of bacteria at time t, ρ is rate of bacterial growth, μ is rate of AM-independent clearance of bacteria (also includes loss due to spontaneous bacterial death), and ϕ is rate of AM-dependent bacterial clearance. Exponential growth/decay curves were fitted to the empirical data using Prism4.0 (GraphPad). Half-lives were calculated as (dN/dT)/(ln[2]/ln[10]).
Statistical analysis.
Parametric data were analyzed by using unpaired t tests or 2-way analysis of variance (ANOVA), and nonparametric data were analyzed by using Mann-Whitney U or Kruskal-Wallis tests, with P values of <0.05 being considered significant. Results are means or medians for triplicate samples for in vitro experiments and for at least 5 animals for in vivo experiments and are representative of repeated experiments.
RESULTS
Effects of S. pneumoniae capsule on AM phagocytosis in vivo.
Previously published data have shown that the S. pneumoniae capsule inhibits phagocytosis (16). To confirm that the capsule also prevents AM-mediated phagocytosis during early lung infection, mice were inoculated intranasally (i.n.) with 5 × 106 CFU of fluorescently labeled TIGR4 and its unencapsulated derivative P1672, and the BALF was recovered 4 h after inoculation. Flow cytometry was used to assess the proportion of AMs associated with bacteria. This demonstrated that a greater proportion of AMs were recovered from mice infected with P1672 bacteria than from mice infected with TIGR4 (Fig. 1A). AMs from mice infected with fluorescent P1672 also exhibited higher mean fluorescence intensity (i.e., greater numbers of bacteria per cell) (Fig. 1B). The results were confirmed by additional experiments in which recovered AMs were labeled with anti-F4-80 and examined using confocal microscopy and Z stack analysis to specifically identify internalized bacteria. These experiments demonstrated that a median of 6 P1672 bacteria were present inside each infected AM, compared to a median of 2 TIGR4 bacteria, confirming greater internalization of unencapsulated S. pneumoniae (Fig. 1C and D). Overall, these data show that the capsule does inhibit phagocytosis of S. pneumoniae by AMs during early lung infection.
FIG 1.
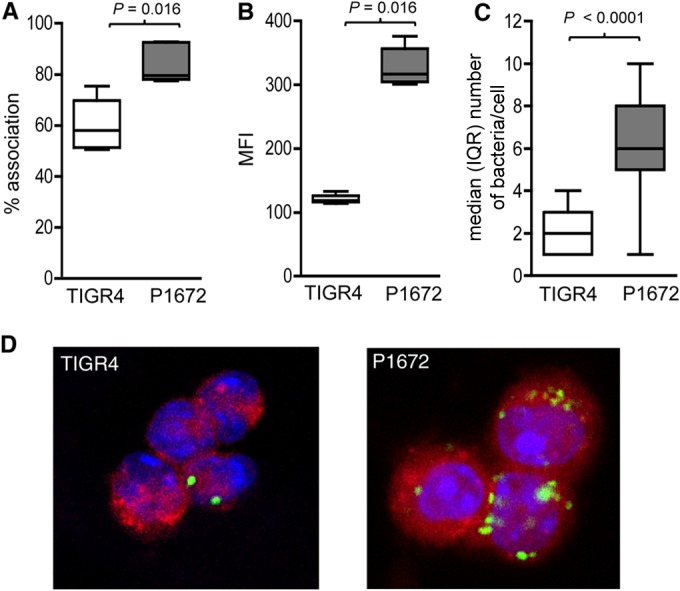
The capsule inhibits AM phagocytosis of S. pneumoniae during early lung infection. (A and B) Association of 6-carboxyfluorescein–succinimidyl ester (FAM-SE)-labeled S. pneumoniae with AMs recovered from mice 4 h after infection by i.n. inoculation with 5 × 106 CFU of TIGR4 (white boxes) or P1672 (gray boxes) assessed by flow cytometry and presented as percentage of fluorescent AMs (A) and the geometric mean fluorescence intensity of fluorescent AMs (B) (14). Representative of data obtained from repeated experiments. (C) Confocal assessment of the internalization of FAM-SE-labeled S. pneumoniae by AMs recovered from mice 4 h after i.n. inoculation with 5 × 106 CFU of TIGR4 or P1672. The data show the median (and interquartile range [IQR]) numbers of intracellular bacteria identified by Z stacking for AMs containing S. pneumoniae. P values were obtained using Mann-Whitney U tests. (D) Representative examples of confocal microscopy of recovered AMs. Blue, DAPI staining of the nuclei; red, F4/80 staining (macrophage marker); green, FAM-SE-labeled bacteria.
Effects of capsule and inoculum size on S. pneumoniae survival during early lung infection.
To investigate whether the effects of the capsule on AM phagocytosis may affect S. pneumoniae recovery from the lungs during early lung infection, BALF bacterial CFU were calculated by plating serial dilutions of BALF recovered from mice infected i.n. with TIGR4 or P1672. The effects of inoculum size on capsule/AM interactions were assessed by inoculating with either 5 × 106 or 5 × 105 CFU of each strain. Both the capsule and inoculum size had large effects on BALF CFU 4 h after inoculation. The numbers of BALF CFU obtained 4 h after mice inoculated with 5 × 106 CFU of TIGR4 were high, representing 55% of the inoculum, whereas numbers in BALF were 2 log10 lower for mice inoculated with 5 × 106 CFU of P1672 (Fig. 2A). In contrast, BALF CFU recovered from mice inoculated with 5 × 105 CFU showed no differences between mice inoculated with P1672 and those receiving TIGR4 and represented <1% of the inoculum (Fig. 2A). The effects of the capsule and inoculum size on establishing successful infection were assessed by calculating BALF and blood CFU 24 h after inoculation. All mice given 5 × 106 CFU of TIGR4 but only around half of mice given 5 × 105 CFU and almost no mice inoculated with 5 × 106 CFU of P1672 had significant numbers of bacteria in BALF and spleen (representing systemic infection) (Fig. 2B and C). Overall, these data demonstrate that although the capsule was essential for establishing lung infection with S. pneumoniae, it had major effects only on S. pneumoniae CFU during early lung infection after mice were given high-dose inocula.
FIG 2.
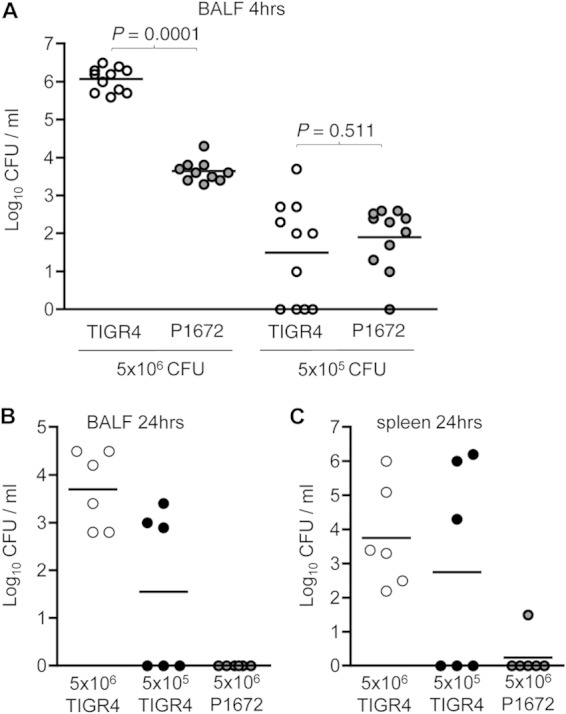
Effects of inoculum size and the capsule on S. pneumoniae BALF CFU during early lung infection. (A) Median BALF CFU (log10/ml) 4 h after i.n. inoculation with 5 × 106 or 5 × 105 CFU of TIGR4 (encapsulated; white circles) and P1672 (unencapsulated; gray symbols) S. pneumoniae. Pooled data are from two separate experiments and representative of repeated experiments. BALF (B) and spleen (C) CFU 24 h after i.n. inoculation with 5 × 106 (white symbols) or 5 × 105 (black symbols) CFU of TIGR4 or 5 × 106 CFU of P1672 (gray symbols) S. pneumoniae. Each symbol represents results for an individual mouse, and bars represent median CFU/ml. P values were obtained using Mann-Whitney U tests.
Effects of depletion of AMs during early lung infection with encapsulated and unencapsulated S. pneumoniae.
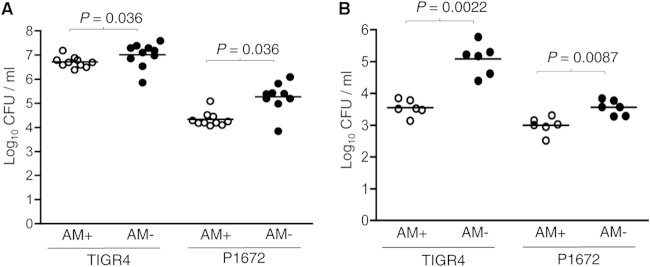
To assess whether differences in 4-h BALF CFU for the P1672 and TIGR4 strains and with the two different sizes of inocula were dependent on AMs, experiments were repeated in mice pretreated with i.n. liposomal clodronate. Treatment with liposomal clodronate depleted at least 80% of AMs (data not shown) (23, 24). Control mice treated with empty liposomes had no statistically significant differences in BALF CFU compared to untreated mice (data not shown). Depletion of AMs resulted in increased BALF CFU at 4 h for both the P1672 and TIGR4 strains for mice given either high- or low-dose inocula (Fig. 3). However, the effects of AM depletion for mice given the high-dose inocula were relatively modest, resulting in 8-fold and 3.7-fold increases in BALF CFU for the P1672 and TIGR4 strains, respectively (Fig. 3A). Similarly, AM depletion resulted in only a 3.6-fold increase in BALF CFU for mice inoculated with low-dose inocula of P1672. In contrast, for mice given 5 × 105 CFU of TIGR4, AM depletion resulted in a 34-fold increase in BALF CFU (Fig. 3B). Hence, AM depletion had marked effects on BALF CFU only for mice given a low-dose inoculum of unencapsulated bacteria. Importantly, after AM depletion, there were still large differences in BALF CFU recovered at 4 h between unencapsulated and encapsulated S. pneumoniae with both sizes of inocula, indicating that the importance of the capsule was not limited to preventing AM-dependent clearance.
FIG 3.
Effects of macrophage depletion on S. pneumoniae CFU in BALF during early lung infection. (A and B) Median BALF CFU (log10/ml) 4 h after i.n. inoculation with 5 × 106 (A) or 5 × 105 (B) CFU of TIGR4 or P1672 S. pneumoniae in mice pretreated with control liposomes (AM+; white symbols) or clodronate liposomes (AM−; black symbols). Each symbol represents results for an individual mouse, bars represents median CFU/ml, and P values were obtained using Mann-Whitney U tests.
Mathematical modeling of BALF S. pneumoniae CFU clearance.
To create a model that could account for factors affecting BALF CFU during early lung infection, exponential growth/decay curves were plotted using log10 BALF CFU data obtained 30, 120, and 240 min after inoculation of clodronate- or empty-liposome-treated mice with 5 × 106 CFU of TIGR4 or P1672. A straight line drawn between the log10 BALF CFU data from 30 and 120 min crossed time zero for all conditions between 6.1 and 6.43 log10 CFU, representing approximately 25 to 50% of the original inoculum (Fig. 4A). These results suggested a constant rate of bacterial clearance during the initial 2 h of infection. Four-hour BALF CFU data did not fit the same exponential decay line, suggesting that bacterial clearance between 2 and 4 h was affected by additional factors, for example, neutrophils, as BALF neutrophilia increased from approximately 20% of BALF cells at 2 h to approximately 60% at 4 h (Fig. 5). The AM-dependent clearance half-life was then calculated by comparing the net change in slope of BALF CFU between clodronate treated and control mice over the first 2 h of infection using the formula dN/dT = ρN0 − μN0 − ϕN0 (Table 1). For mice given 5 × 106 CFU, the AM-dependent clearance half-life for TIGR4 was 135 min, but that for the P1672 strain was only 31 min (Table 1). AM-dependent clearance half-lives were also calculated for mice inoculated with 5 × 105 CFU (Fig. 4B) and were much shorter for the TIGR4 strain at 48 min, with little difference relative to the P1672 strain (42 min) (Table 1). The large differences in AM-dependent clearance rate of the TIGR4 strain relative to inoculum size are compatible with the published data (8) and Fig. 2A, which shows saturation of S. pneumoniae clearance from the lung when given as a high-dose inoculum. The effect of inoculum size seemed to be capsule dependent, as AM-dependent clearance time for P1672 was actually higher for the high-dose inoculum than the low dose; this may reflect the range of error in the calculated clearance rate rather than a true difference.
FIG 4.

Time course of BALF CFU over the initial 4 h of S. pneumoniae lung infection used for mathematical modeling of AM-dependent clearance half-lives. (A) BALF CFU 30, 120, and 240 min after i.n. inoculation with 5 × 106 (log10 6.7) CFU of TIGR4 (circles) or P1672 (diamonds) S. pneumoniae in mice pretreated with control liposomes (white symbols; +AMs) or clodronate liposomes (black symbols; −AMs). Data were pooled from two experiments with 9 to 14 mice each. (B) BALF CFU 120 and 240 min after i.n. inoculation with 5 × 105 (log10 5.7) CFU of TIGR4 (circles) or P1672 (diamonds) S. pneumoniae in mice pretreated with control liposomes (white symbols; +AMs) or clodronate liposomes (black symbols; −AMs). Data were from one experiment that was representative of two different experiments with 6 mice each. The data were plotted assuming a starting CFU within BALF of log10 6.2 (A) or 5.2 (B); symbols represent mean BALF CFU/ml, and error bars represent standard deviations (SDs) (where not shown error bars are contained within symbols).
FIG 5.

Mean (SD) BALF neutrophilia 30, 120, and 240 min after inoculation with 5 × 106 CFU of TIGR4 (gray columns) or P1672 (white columns) S. pneumoniae in mice pretreated with control liposomes (solid columns) or clodronate liposomes (hatched columns). There were no significant differences between groups at each time point (Mann-Whitney U tests). Data are from one experiment that was representative of repeated experiments with 6 mice each.
TABLE 1.
Calculated half-lives for AM-dependent and -independent clearance and doubling time of encapsulated (TIGR4) and unencapsulated (P1672) S. pneumoniae within first 2 h of inoculation with a high- or low-dose inoculuma
| Inoculum (CFU) | Strain | AM-dependent clearance |
Bacterial doubling time |
AM-independent clearance |
|||
|---|---|---|---|---|---|---|---|
| Gradientb | Half-life (min) | Gradientc | Doubling time (min) | Gradientd | Half-life (min) | ||
| 5 × 106 | TIGR4 | −0.0022 | 135 | 0.0183 | 16 | −0.0126 | 24 |
| P1672 | −0.0098 | 31 | 0.0148 | 20 | −0.0217 | 14 | |
| 5 × 105 | TIGR4 | −0.0072 | 42 | 0.0054 | 56 | 0.00144 | NCe |
| P1672 | −0.0062 | 48 | −0.0026 | NC | −0.0044 | 68 | |
Differences in the gradients for change in BALF log10 CFU (log10 CFU min−1, obtained from data in Fig. 4 and 7) used to calculate the clearance half-lives and doubling times.
Difference between clodronate-treated and control mice.
Difference between parental and Δpab strains.
Obtained by subtraction of the BALF CFU gradient of AM-dependent clearance from the gradient for the Δpab strain.
NC, not calculated.
Importance of bacterial replication during early lung infection by S. pneumoniae.
As well as the capsule, S. pneumoniae BALF CFU during early lung infection could potentially be affected by bacterial replication. Hence, the importance of S. pneumoniae growth for establishing infection in the lungs was assessed using Δpab strains, auxotrophic mutants unable to replicate during infection in mice (21). The inability of the TIGR4Δpab strain to grow in BALF was confirmed by culturing bacteria in BALF recovered from uninfected mice (Fig. 6A). Growth of P1672 and TIGR4 was also identical in vitro in murine BALF, with a 6-fold increase in CFU over 2 h equating to a doubling time of over 45 min (Fig. 6B). Within 4 h after i.n. inoculation of 5 × 106 CFU into mice, there were large differences in BALF CFU between TIGR4 and P1672 strains and their Δpab derivatives. For TIGR4Δpab, there was approaching a 4-log10 reduction in BALF CFU compared to TIGR4, and for P1672Δpab, there was a 2-log10 difference compared to P1672 (Fig. 6C). These results confirmed that there was significant S. pneumoniae replication during early lung infection in mice that was important for maintenance of lung infection for mice given a high-dose inoculum.
FIG 6.

Effects of bacterial replication on S. pneumoniae BALF CFU for growth in BALF in vitro and during early lung infection. (A) Growth in 1 ml of BALF in vitro of different inoculum sizes of TIGR4 (stated beneath each column) and 1 × 106 CFU TIGR4Δpab expressed as a percentage of the initial inoculum. Error bars represent standard errors of the means (SEMs). The results for TIGR4Δpab were <1%. (B) Growth in BALF in vitro of S. pneumoniae TIGR4 (white symbols) or P1672 (black symbols) expressed as mean (SEM) CFU/ml over time. (C) Median BALF CFU (log10/ml) 4 h after i.n. inoculation with 5 × 106 of TIGR4 (white symbols), TIGR4Δpab (gray symbols), P1672 (white symbols), or P1672Δpab (gray symbols). Each symbol represents results for an individual mouse, bars represents median CFU/ml, and P values were obtained using Mann-Whitney U tests.
Experiments were repeated to obtain 2-h BALF CFU data for calculation of bacterial doubling times using the mathematical model. The changes in slope between the Δpab and parental strains were compared for BALF CFU 2 h postinoculation, using an estimated CFU for time zero of log10 6.2 (high-dose inoculum) or 5.2 (low-dose inoculum) (Fig. 7). This calculation assumes that AM-dependent and -independent clearance remained the same between parental and Δpab strains. With inocula of 5 × 106 CFU, the calculated TIGR4 and P1672 doubling times were both very rapid at 16 and 20 min (Fig. 7A and Table 1). However, for mice inoculated with 5 × 105 CFU, there was a surprisingly small or no difference in BALF CFU between TIGR4 and TIGR4Δpab, giving a minimum doubling time of 56 min for TIGR4 (Fig. 7B and Table 1). As there was no fall in BALF CFU for P1672Δpab compared to P1672 at 2 h, the doubling time for P1672 in mice given 5 × 105 CFU could not be calculated. Hence, S. pneumoniae replication rate during early lung infection also seemed to be dependent on size of the inoculum, with rapid replication occurring only with a high-dose inoculum. In vitro BALF culture experiments with different inoculum sizes did not show failure of bacterial replication with low-dose inocula (Fig. 6A), suggesting that the effects of inoculum size on S. pneumoniae replication rate were limited to in vivo infection.
FIG 7.

BALF CFU data used for calculation of S. pneumoniae replication times during early lung infection. The data show the numbers of BALF CFU 30 and 120 min after i.n. inoculation with 5 × 106 (log10 6.7) CFU (A) or 120 min after i.n. inoculation with 5 × 105 (log10 5.7) (B) of TIGR4, TIGR4Δpab, P1672, or P1672Δpab. The data are representative of repeated experiments and plotted assuming a starting CFU in BALF of log10 6.2 (A) or 5.2 (B). Symbols represent mean BALF CFU/ml, error bars represent SDs (where not shown, error bars are contained within symbols), and n = 6 to 10.
Calculation of the efficacy of non-AM-dependent immunity.
The persistently greater numbers of TIGR4 than P1672 BALF CFU in clodronate-treated mice suggested that non-AM-dependent mechanisms caused some of the differences in lung clearance between encapsulated and unencapsulated S. pneumoniae. AM-independent immunity represents the effects of multiple immune effectors, including mucociliary clearance and a large number of mucosal factors, and hence is not possible to assess using genetic knockouts or pharmacological inhibition of specific factors. Instead, we estimated the effects of non-AM-dependent immunity indirectly using BALF CFU data obtained after AM depletion and for Δpab strains. We assumed that changes in remaining bacterial BALF CFU for the Δpab strains after subtraction of the AM-dependent clearance rate were due to AM-independent clearance. Using this method, AM-independent clearance half-lives in mice given a high-dose inoculum were 24 min for TIGR4 and 14 min for P1672. These results suggest the AM-independent half-life was considerably shorter than the AM-dependent half-life and seemed to be affected by the capsule (Table 1). In contrast, with the lower-dose inoculum, the AM-independent clearance half-life was 68 min for P1672 but could not be calculated for TIGR4, as changes in BALF CFU could be entirely explained by bacterial replication and AM-dependent clearance (Table 1). Hence, AM-independent clearance of S. pneumoniae dominated AM-dependent clearance after a high-dose inoculum but not after a low-dose inoculum.
DISCUSSION
We have used data from a mouse infection model and mathematical modeling to characterize the relative importance of different factors for S. pneumoniae persistence in the lungs within 4 h of infection. The major new findings are that with a high-dose inoculum, (i) AM-independent clearance mechanisms seem to be more effective than AM-dependent mechanisms for controlling bacterial numbers, (ii) S. pneumoniae replication is rapid even at very early time points and is vital for establishing significant lung infection, and (iii) the S. pneumoniae capsule not only prevents AM-dependent clearance mechanisms but seems to also inhibit AM-independent mechanisms. In contrast, after a lower-dose inoculum, bacterial replication rate and AM-independent clearance mechanisms seem to be much less critical, and AM-dependent clearance of both unencapsulated and encapsulated bacteria occurs at similar rates. Overall, the data indicate that there is a complex dynamic between both bacterial (inoculum size, capsule, and replication) and host (AM-dependent and -independent immunity) factors that affect bacterial persistence during early lung infection and therefore the likely outcome of the infection.
The importance of AMs for lung innate immunity was originally established with Staphylococcus aureus (2, 3), and has been confirmed by multiple papers for S. pneumoniae (8, 9, 11, 25, 26). AMs clear S. pneumoniae from the lungs during early infection by phagocytosis and later in infection by apoptosis (6–8, 26). They also have an important role in regulating inflammatory responses during S. pneumoniae lung infection (27–29). Our data have now provided estimates of AM-dependent half-lives during early lung infection with S. pneumoniae under different conditions. Multiple repeated mouse infection experiments to formally obtain the standard error of the calculated half-lives/doubling times were not performed due to ethical reasons. However, BALF CFU data obtained with an inoculum of 5 × 106 CFU were highly consistent between experiments, providing a high degree of confidence that the calculated half-lives and doubling times were representative. Data obtained by using an inoculum of 5 × 105 CFU were less consistent between experimental conditions, and as a consequence, although the modeling data indicate important trends, the figures for the calculated half-lives/doubling times were probably less precise. After a high-dose inoculum, the AM-dependent clearance half-life of TIGR4 was very high, at over 2 h. In contrast, the AM-dependent clearance half-life of TIGR4 with a lower-dose inoculum was considerably lower, at 41 min. These results support previous publications that show that AM clearance seems to be saturated by higher-dose inocula (8, 9) and provide a figure for how much inoculum size can effect AM function. Clodronate depletes AMs by only 80% (23, 24); hence, the data may partially underestimate AM-dependent clearance, but this would not have affected the relative differences between infection conditions. The flow cytometry and confocal microscopy data showed that the S. pneumoniae capsule does inhibit phagocytosis by AMs after mice were given a high-dose inoculum. This was associated with a much higher AM-dependent rate of clearance of unencapsulated S. pneumoniae in mice given a high-dose inoculum but, interestingly, not after a low-dose inoculum. A potential explanation of this observation maybe the S. pneumoniae capsule's role in preventing complement opsonization (16), which could be relevant for S. pneumoniae interactions with AMs during early lung infection (12, 30, 31). As BALF complement levels are approximately 1/1,000 of blood levels (31), complement-mediated AM phagocytosis could be rapidly saturated by encapsulated but not unencapsulated S. pneumoniae, hence markedly reducing the efficacy of AM-dependent clearance of high-dose inocula of TIGR4. The capsule also prevents nonopsonic phagocytosis, which could inhibit AM phagocytosis of S. pneumoniae mediated by scavenger and lectin receptors (6, 7, 16).
Within the lungs there are a limited availability of cations and of nitrogen and carbohydrate sources and high levels of oxidative stress, which may inhibit bacterial replication. However, using the nonreplicating Δpab strains, we have shown that there can be substantial S. pneumoniae replication within 2 h of lung infection, with a calculated doubling time for TIGR4 of 16 min in mice given a high-dose inoculum. This value is similar to estimated doubling times for growth in blood of 20 to 30 min (32) and lower than in vitro doubling times in complete medium and BALF (both 45 min). The doubling time for the unencapsulated strain was similar despite the much lower numbers of BALF CFU recovered for this strain, indicating that the results were unlikely to have been confounded by the lower BALF CFU of the Δpab strains causing increased immune-mediated clearance. An unexpected observation was the substantially higher doubling time for TIGR4 when it was given as a lower infecting inoculum (56 min). A potential explanation for various doubling times with an inoculum size could be that quorum-sensing mechanisms stimulate growth with the higher inoculum dose; however, S. pneumoniae growth in vitro in BALF did not demonstrate any dose response effects. An alternative explanation that is difficult to investigate is that S. pneumoniae replication can be inhibited by host factors in the lung (e.g., lactoferrin) that are saturated with higher-dose inocula, similar to the effects on AM-dependent clearance.
The persisting differences in BALF CFU between the TIGR4 and P1672 strains in clodronate-treated mice demonstrated that the capsule inhibits AM-independent as well as AM-dependent S. pneumoniae clearance, perhaps through inhibition of mucus-mediated physical clearance and increased resistance to bactericidal peptides (17, 18). Unlike AM-mediated clearance, AM-independent clearance represents the combination of several different immune effectors. Hence, it is difficult to assess directly, and previous modeling of S. pneumoniae lung clearance largely ignored AM-independent clearance mechanisms (32). Data obtained with an inhaled aerosol model indicated a major role for AM-independent clearance during S. aureus lung infection (33), although these findings conflict with other data obtained using a similar model which emphasized the role of AMs (1–3).We reasoned that remaining bacterial clearance for the Δpab nonreplicating strains after AM-dependent effects are taken into account represented AM-independent clearance mechanisms. Using this method to indirectly model AM-independent half-lives suggested that these immune mechanisms had a surprisingly powerful effect after infection with a high-dose inoculum. Under these conditions, the AM-independent clearance half-life was 24 min for TIGR4, less than 20% of the AM-dependent clearance half-life, and was even shorter for the unencapsulated strain. These data may have been artificially decreased by partial depletion of AMs by clodronate and perhaps increased spontaneous bacterial death of the Δpab strains, but despite these caveats the data indicate an important role for AM-independent clearance of S. pneumoniae when a large number of bacteria are aspirated into the lung. This observation has implications for the design of effective preventative strategies. For example, we have used the modeling data to calculate BALF CFU after potential changes in the efficacy of AM-dependent and -independent clearance (Table 2). The results show that altering AM-independent clearance half-life between the values for encapsulated and unencapsulated bacteria and doubling the bacterial replication rate both had larger effects on estimated BALF CFU than improving AM-dependent clearance 4-fold. Hence, a vaccine would have to have powerful effects on AM-dependent clearance in order to promote effective clearance of a substantial inoculum of S. pneumoniae reaching the lungs. This observation helps explain why significant levels of BALF anti-S. pneumoniae antibody do not affect S. pneumoniae clearance from BALF in mouse models of pneumonia (14). Conversely, the importance of AM-independent clearance partially explains the high incidence of pneumonia in patients with impaired mucosal immunity due to chronic lung disease.
TABLE 2.
Predicted S. pneumoniae BALF CFU 2 h after i.n. inoculation with 5 × 106 bacteria
| Efficiency (%) of AM-dependent clearance | No. of CFU after bacterial replication time (min)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| 16 |
20 |
30 |
40 |
|||||
| Slow | Fast | Slow | Fast | Slow | Fast | Slow | Fast | |
| 400 | 5.97 | 4.87 | 5.51 | 4.42d | 4.91 | 3.82 | 4.61 | 3.52 |
| 200 | 6.50 | 5.41 | 6.05 | 4.96 | 5.45 | 4.35 | 5.15 | 4.05 |
| 100b | 6.77c | 5.67 | 6.32 | 5.22 | 5.71 | 4.62 | 5.41 | 4.32 |
| 50 | 6.90 | 5.81 | 6.45 | 5.36 | 5.85 | 4.75 | 5.55 | 4.45 |
Estimated by extrapolation of modeling data for different replication times, variable efficiency of AM-dependent clearance, and the AM-independent clearance half-lives calculated for TIGR4 (24 min [slow]) and P1672 (14 min [fast]). All values are indicated in log10 CFU.
100% corresponds to the AM-dependent half-life for the TIGR4 strain of 135 min.
Represents the actual data obtained with the 5 × 106 CFU inoculum of TIGR4.
Represents the actual data obtained with the 5 × 106 CFU inoculum of P1672.
To conclude, we have shown that after a high-dose inoculum of capsular serotype 4 S. pneumoniae strain, replication and the capsule are necessary for successful lung infection and that AM-independent clearance seems to be more effective than AM-dependent clearance. These data provide a framework for investigating why certain S. pneumoniae capsular serotypes predominate as causes of pneumonia (34, 35), rather than other serotypes or other nasopharyngeal commensal species, and for designing therapeutic strategies to prevent S. pneumoniae lung infection.
ACKNOWLEDGMENTS
This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centre's funding scheme, and was supported by the Medical Research Council UK (project grant G0600410), BBSRC, and Wellcome Trust.
REFERENCES
- 1.Gordon SB, Read RC. 2002. Macrophage defences against respiratory tract infections. Br Med Bull 61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Green GM, Kass E. 1964. The role of the alveolar macrophage in the clearance of bacteria from the lung. J Exp Med 119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M, Goldstein E, Lewis JP, Lippert W, Warshauer D. 1976. Murine pulmonary alveolar macrophages: rates of bacterial ingestion, inactivation, and destruction. J Infect Dis 133:310–320. doi: 10.1093/infdis/133.3.310. [DOI] [PubMed] [Google Scholar]
- 4.Lee H-Y, Andalibi A, Webster P, Moon S-K, Teufert K, Kang S-H, Li J-D, Nagura M, Ganz T, Lim DJ. 2004. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis 4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogan MP, Geraghty P, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG. 2006. Antimicrobial proteins and polypeptides in pulmonary innate defence. Respir Res 7:29. doi: 10.1186/1465-9921-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. 2004. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. 2006. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol 35:474–478. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 9.Sun K, Gan Y, Metzger DW. 2011. Analysis of murine genetic predisposition to pneumococcal infection reveals a critical role of alveolar macrophages in maintaining the sterility of the lower respiratory tract. Infect Immun 79:1842–1847. doi: 10.1128/IAI.01143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoneim HE, Thomas PG, McCullers JA. 2013. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 191:1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, Dickey BF, Tuvim MJ. 2008. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med 177:1322–1330. doi: 10.1164/rccm.200607-1038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon SB, Irving GRB, Lawson RA, Lee ME, Read RC. 2000. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun 68:2286–2293. doi: 10.1128/IAI.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS. 29 October 2014. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J, Khandavilli S, Camberlein E, Hyams C, Baxendale H, Brown J. 2011. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One 6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood WB, Smith MR. 1949. The inhibition of surface phagocytosis by the capsular slime layer of pneumococcus type III. J Exp Med 90:85–96. doi: 10.1084/jem.90.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun 78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun 75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llobet E, Tomás JM, Bengoechea JA. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 154:3877–3886. doi: 10.1099/mic.0.2008/022301-0. [DOI] [PubMed] [Google Scholar]
- 19.Domenech A, Ardanuy C, Calatayud L, Santos S, Tubau F, Grau I, Verdaguer R, Dorca J, Pallares R, Martin R, Liñares J. 2011. Serotypes and genotypes of Streptococcus pneumoniae causing pneumonia and acute exacerbations in patients with chronic obstructive pulmonary disease. J Antimicrob Chemother 66:487–493. doi: 10.1093/jac/dkq480. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez C, Hinojosa C, Shivshankar P, Hyams C, Camberlein E, Brown J, Orihuela C. 2011. Changes in capsular serotype alter the surface exposure of pneumococcal adhesins and impact virulence. PLoS One 6:e26587. doi: 10.1371/journal.pone.0026587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chimalapati S, Cohen J, Camberlein E, Durmort C, Baxendale H, De Vogel C, Van Belkum A, Brown J. 2011. Infection with conditionally virulent Streptococcus pneumoniae Δpab strains induces antibody to conserved protein antigens but does not protect against systemic infection with heterologous strains. Infect Immun 79:4965–4976. doi: 10.1128/IAI.05923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuste J, Sen A, Truedsson L, Jönsson G, Tay L-S, Hyams C, Baxendale HE, Goldblatt F, Botto M, Brown JS. 2008. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect Immun 76:3761–3770. doi: 10.1128/IAI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maus U, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, Lohmeyer J. 2002. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am J Physiol Lung Cell Mol Physiol 282:L1245–L1252. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- 24.Leemans JC, Juffermans NP, Florquin S, van Rooijen N, Vervoordeldonk MJ, Verbon A, van Deventer SJ, van der Poll T. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J Immunol 166:4604–4611. doi: 10.4049/jimmunol.166.7.4604. [DOI] [PubMed] [Google Scholar]
- 25.Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MKB. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol 171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- 26.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. 2010. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun 78:1214–1220. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med 167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 28.Marriott HM, Dockrell DH. 2006. Streptococcus pneumoniae: The role of apoptosis in host defense and pathogenesis. Int J Biochem Cell Biol 38:1848–1854. doi: 10.1016/j.biocel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Marriott HM, Dockrell DH. 2007. The role of the macrophage in lung disease mediated by bacteria. Exp Lung Res 33:493–505. doi: 10.1080/01902140701756562. [DOI] [PubMed] [Google Scholar]
- 30.Yuste J, Botto M, Paton JC, Holden DW, Brown JS. 2005. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J Immunol 175:1813–1819. doi: 10.4049/jimmunol.175.3.1813. [DOI] [PubMed] [Google Scholar]
- 31.Kerr A, Paterson G. 2005. Innate immune defense against pneumococcal pneumonia requires pulmonary complement component C3. Infect Immun 73:4245–4252. doi: 10.1128/IAI.73.7.4245-4252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith AM, McCullers JA, Adler FR. 2011. Mathematical model of a three-stage innate immune response to a pneumococcal lung infection. J Theor Biol 276:106–116. doi: 10.1016/j.jtbi.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nugent KM, Pesanti EL. 1982. Nonphagocytic clearance of Staphylococcus aureus from murine lungs. Infect Immun 36:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyams C, Yuste J, Bax K, Camberlein E, Weiser J, Brown J. 2010. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infect Immun 78:716–725. doi: 10.1128/IAI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. 2012. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 67:540–545. doi: 10.1136/thoraxjnl-2011-201092. [DOI] [PubMed] [Google Scholar]