Abstract
Background:
Several clinical trials have compared chemotherapy alone and chemoradiotherapy (CRT) for locally advanced pancreatic cancer (LAPC) treatment. However, predictive biomarkers for optimal therapy of LAPC remain to be identified.
We retrospectively estimated amplification of the ACTN4 gene to determine its usefulness as a predictive biomarker for LAPC.
Methods:
The copy number of ACTN4 in 91 biopsy specimens of LAPC before treatment was evaluated using fluorescence in situ hybridisation (FISH).
Results:
There were no statistically significant differences in overall survival (OS) or progression-free survival (PFS) of LAPC between patients treated with chemotherapy alone or with CRT. In a subgroup analysis of patients treated with CRT, patients with a copy number increase (CNI) of ACTN4 had a worse prognosis of OS than those with a normal copy number (NCN) of ACTN4 (P=0.0005, log-rank test). However, OS in the subgroup treated with chemotherapy alone was not significantly different between patients with a CNI and a NCN of ACTN4. In the patients with a NCN of ACTN4, the median survival time of PFS in CRT-treated patients was longer than that of patients treated with chemotherapy alone (P=0.049).
Conclusions:
The copy number of ACTN4 is a predictive biomarker for CRT of LAPC.
Keywords: copy number increase of ACTN4, locally advanced pancreatic cancer, chemotherapy, chemoradiotherapy, predictive biomarker
Despite progress in clinical cancer medicine in the fields of imaging technology, surgical management, therapeutic modalities and molecular-targeted therapy, the prognosis of pancreatic cancer has remained dismal. Every year in Japan, ∼27 000 patients are diagnosed with pancreatic cancer, with almost the same number dying from this disease (Mayahara et al, 2012). Indeed, the 5-year overall survival (OS) rate of patients with pancreatic cancer is ⩽5% (Johung et al, 2012).
Locally advanced pancreatic cancer (LPAC) is defined as a surgically unresectable disease without detectable metastasis. Effective therapy for patients with LAPC is not only crucial for any hope of long-term survival, but also necessary for symptom management. Because survival rates for patients with LAPC are generally low, treatment recommendations often involve aggressive multimodal therapies (Savir et al, 2013). A multidisciplinary approach involving surgical oncologists, medical oncologists and radiation oncologists is strongly recommended for balanced discussion of management options (Pawlik et al, 2008; Katz et al, 2013; Mian et al, 2014).
At present, treatment options for LAPC include chemotherapy alone, induction chemotherapy followed by chemoradiotherapy (CRT) or definitive CRT. Numerous randomised trials have been performed to compare the survival benefit between chemotherapy alone and CRT for LAPC (Chauffert et al, 2008; Loehrer et al, 2011). Nevertheless, as there are some contradictory results, the most effective treatment has not yet been defined for patients with LAPC (Savir et al, 2013; Mian et al, 2014). Radiotherapy focussed on the primary site does not have a direct impact on distant metastatic lesions and radiotherapy should therefore be limited to patients without metastases (Berger et al, 2008). If pancreatic cancer oncologists can accurately evaluate the occult distant metastasis before deciding the therapeutic strategy, they should be able to choose the optimal therapy for individual patients with LAPC. However, it is not yet possible to accurately detect micrometastatic lesions using imaging technology. Therefore, elucidation of biomarkers that can accurately evaluate metastatic potential from biopsy samples obtained from patients with LAPC is very important for deciding the best personalised therapeutic strategy from multimodal therapies.
In 1998, we identified actinin-4 (gene name ACTN4) as an actin-binding protein that is closely associated with cancer invasion and metastasis (Honda et al, 1998; Hayashida et al, 2005). Immunohistochemical analysis (IHC) showed that overexpression of the actinin-4 protein was significantly correlated with a poor prognosis for breast (Honda et al, 1998), pancreas (Kikuchi et al, 2008), ovary (Yamamoto et al, 2007, 2009, 2012) and lung cancer (Miyanaga et al, 2013; Noro et al, 2013).
We subsequently found that gene amplification of ACTN4, which is the gene name of the actinin-4 protein, is responsible for overexpression of the actinin-4 protein in a number of pancreatic cancer patients (Kikuchi et al, 2008). Using fluorescence in situ hybridisation (FISH), we then reported that gene amplification of ACTN4 is a good biomarker for identification of patients with a poor prognosis for ovarian cancer (Yamamoto et al, 2009), salivary gland carcinoma (Watabe et al, 2014) and stage-I adenocarcinoma of the lung (Noro et al, 2013).
In this study, we retrospectively investigated the status of actinin-4 protein expression and ACTN4 copy number in biopsy samples of LAPC patients. We confirmed the possibility that ACTN4 copy number is useful as a predictive and prognostic biomarker of CRT for LAPC.
Materials and methods
Patients
A total of 91 patients who were diagnosed as having LAPC from May 2001 until December 2003 underwent chemotherapy alone or CRT at the National Cancer Center Central Hospital (Tokyo, Japan). All patients were diagnosed as adenocarcinoma of the pancreas by fine needle biopsy. This study was reviewed and approved by the institutional ethical committee and informed consent was obtained from the patients for this study.
At first diagnosis, multidetector computed tomography (CT) involving the chest and abdomen was performed for assessment of the local extension of the primary tumour, and for exclusion of distant metastasis. The CT-based criteria regarding tumour unresectability included enhancement or occlusion of the coeliac trunk, common hepatic artery, superior mesenteric artery or aorta (Ikeda et al, 2007; Mayahara et al, 2012).
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) pathology blocks, which were made to diagnose the biopsy specimens, were cut into 4 μm-thick sections.
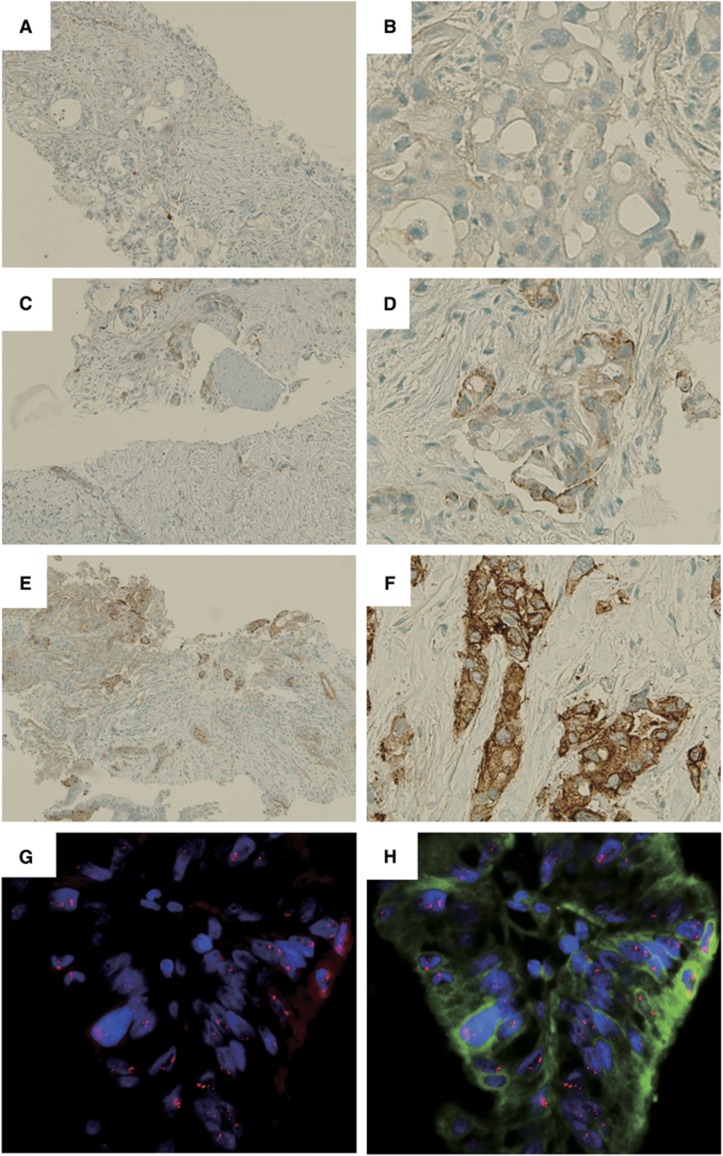
An anti-actinin-4 monoclonal antibody was established by our group (Miyanaga et al, 2013; Noro et al, 2013) (Abnova, Taipei, Taiwan). Immunostaining of actinin-4 was performed using the Ventana DABMap detection kit and an automated slide stainer (Discovery XT; Ventana Medical System, Tucson, AZ, USA) (Watabe et al, 2014). The immunohistochemical staining of actinin-4 was classified into two groups: positive and negative. Positive was defined as strong protein expression of actinin-4 in the cytoplasm and cell membranes of cancer cells. Negative was defined as no detection of actinin-4 protein in cancer cells or weak expression of actinin-4 in the cytoplasm or cell membrane of cancer cells (Figure 1). Two independent investigators (TW and YW) who had no clinical information about these cases evaluated the staining pattern.
Figure 1.
Immunohistochemical (IHC) and fluorescence in situ hybridisation (FISH) analyses of representative actinin-4 protein expression and ACTN4 copy number, respectively, in LAPC biopsy specimens. (A–F) Immunohistochemical analysis of actinin-4 protein expression. Representative cases of no expression (A, B), weak expression (C, D) and strong expression (E, F) of actinin-4 protein in LAPC cells. (A), (C) and (E) are low-magnitude images. (B), (D) and (F) are high-magnitude images of regions of (A), (C) and (E), respectively. (G, H) Fluorescence in situ hybridisation analysis of representative cases with a copy number increase (CNI) in ACTN4.
Fluorescence in situ hybridisation
The FISH probes of the bacterial artificial chromosome (BAC) clone containing ACTN4 were prepared by our group (Noro et al, 2013) (Abnova). The labelled BAC clone DNA was subjected to FISH as previously described. Sections that were cut from an FFPE biopsy block (4 μm thick) were hybridised with FISH probes at 37 °C for 48 h. The nuclei were counterstained with 4,6-duamidino-2-phenylindone. The number of fluorescence signals corresponding to the copy number of ACTN4 in the nuclei of 20 interphase tumour cells was counted (TW and YW) (Watabe et al, 2014).
The FISH patterns were defined as described previously. Briefly, the biopsy samples were grouped as normal copy number (NCN) (two or fewer ACTN4 signals in >90% of cells) and copy number increase (CNI) (four or more ACTN4 signals in >10% of the tumour cells) (Figure 1) (Watabe et al, 2014).
Statistical analysis
Significant correlations were detected by using Fisher's exact test. Overall survival and progression-free survival (PFS) were measured as the period from first diagnosis to the event or last follow-up and were estimated by Kaplan–Meier analysis. Significant differences between curves of OS or PFS were assessed using the log-rank test. Univariate and multivariate analyses for death were performed using the Cox regression model. Data were analysed using the StatFlex statistical software package (version 6.0; Artiteck, Osaka, Japan) or the R-project (http://www.r-project.org/) (Honda et al, 2005, 2012; Noro et al, 2013).
Results
Patient characteristics and survival benefit comparison between chemotherapy alone and CRT
In all, 34 patients with LAPC underwent chemotherapy alone. The regimens of chemotherapy alone comprised gemcitabine (GEM) alone (n=29), combination of GEM and erlotinib (n=1), combination of GEM and S-1 (n=3) or S-1 alone (n=1). A total of 57 patients with LAPC underwent CRT. The regimens of CRT comprised radiotherapy (RT) and 5-fluorouracil (5-FU) (n=39), RT and GEM (n=10) and RT and S-1 (n=8). The median age of patients and tumour size for all of the cases was 63.0 years and 37.4 mm, respectively. Statistical significances of patient characteristics with respect to age, gender, Eastern Cooperative Oncology Group Performance Status (PS), tumour size, lymph node metastasis and location of tumours were calculated. No statistically significant differences were observed between any of these factors and chemotherapy alone or CRT (Table 1).
Table 1. Baseline patient characteristics.
| |
Total |
CRT |
CT |
|
|||
|---|---|---|---|---|---|---|---|
| Characteristic | Number | % | Number | % | Number | % | P-value* |
| Median age, years (63.0) |
|
|
|
|
|
|
0.0501 |
| <63.0 | 45 | 49.5 | 32 | 56.1 | 13 | 38.2 | |
| ⩾63.0 |
46 |
50.5 |
25 |
43.9 |
21 |
61.8 |
|
| Gender |
|
|
|
|
|
|
1 |
| Male | 53 | 58.2 | 33 | 57.9 | 20 | 58.8 | |
| Female |
38 |
41.8 |
24 |
42.1 |
14 |
41.2 |
|
| PS |
|
|
|
|
|
|
0.2681 |
| 0 | 26 | 28.6 | 17 | 29.8 | 9 | 26.5 | |
| 1 | 63 | 69.2 | 40 | 70.2 | 23 | 67.6 | |
| 2 |
2 |
2.2 |
0 |
0.0 |
2 |
5.9 |
|
| Median tumour size, mm (37.4) |
|
|
|
|
|
|
0.3862 |
| <37.4 mm | 44 | 48.4 | 14 | 41.2 | 30 | 52.6 | |
| ⩾37.4 mm |
47 |
51.6 |
20 |
58.8 |
27 |
47.4 |
|
| Lymph node metastasis |
|
|
|
|
|
|
1 |
| Negative | 64 | 70.3 | 40 | 70.2 | 24 | 70.6 | |
| Positive |
27 |
29.7 |
17 |
29.8 |
10 |
29.4 |
|
| Location of the tumour |
|
|
|
|
|
|
0.0501 |
| Head of pancreas |
43 |
47.3 |
22 |
38.6 |
21 |
61.8 |
|
| Body or tail of pancreas |
48 |
52.7 |
35 |
61.4 |
13 |
38.2 |
|
| CA19-9 |
|
|
|
|
|
|
|
| <1000 U ml−1 | 62 | 68.1 | 39 | 68.4 | 23 | 67.6 | 1 |
| ⩾1000 U ml−1 | 29 | 31.9 | 18 | 31.6 | 11 | 32.4 | |
Abbreviations: CRT=chemoradiotherapy; CT=chemotherapy; PS=Eastern Cooperative Oncology Group Performance Status.
*P-value: Fisher's exact test (two sided).
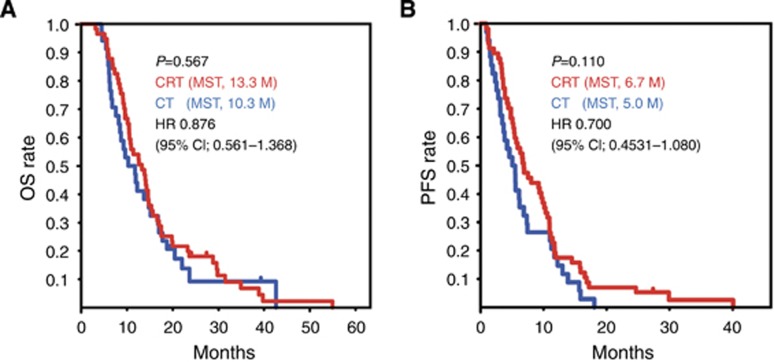
The statistical significance of differences between the benefit of chemotherapy alone and that of CRT for OS and PFS was also calculated. In the absence of biomarker selection, no statistically significant differences in survival benefits in terms of OS and PFS were found between patients treated with chemotherapy alone and those treated with CRT (Figure 2).
Figure 2.
Kaplan–Meier analyses of overall survival (OS) and progression-free survival (PFS) in all locally advanced pancreatic cancer (LAPC) cases. The survival curves of all LAPC patients treated with chemotherapy alone (CT, blue lines) or with chemoradiotherapy (CRT, red lines) are shown. Statistically significant differences in OS (A) and PFS (B) were calculated using a log-rank test. Median survival time (MST) is shown in months (M). The clinical benefit of CT vs CRT was calculated by univariate Cox regression analysis (hazard ratio (HR) and 95% confidence interval (95% CI)). The y axis is the rate of OS or PFS, and the x axis is the time after first diagnosis (months).
Prognostic impact of protein expression of actinin-4 in patients with LAPC
We previously showed that protein overexpression of actinin-4 is a prognostic biomarker for resectable invasive ductal adenocarcinoma of the pancreas (Kikuchi et al, 2007). We investigated the protein expression level of actinin-4 in LAPC by using IHC. The 91 patients with LAPC were classified into one of two groups based on actinin-4 protein expression; positive (66 patients, 72.5%) and negative (25 patients, 27.5%). We investigated correlations between protein expression of actinin-4 and the following patient characteristics: age, gender, PS, size of tumour, lymph node metastasis and treatment strategy (chemotherapy alone or CRT). Protein expression of actinin-4 was statistically correlated with tumour location (P=0.0379; Table 2).
Table 2. Association of protein expression of actinin-4 and copy number increase in ACTN4 with clinicopathological characteristics of locally advanced pancreatic cancer.
| |
Actinin-4 IHC |
ACTN4
FISH |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Positive | % | Negative | % | P-value* | Positive | % | Negative | % | P-value* |
| Median age, years (63.0) |
|
|
|
|
0.159 |
|
|
|
|
0.41 |
| <63.0 | 36 | 54.5 | 9 | 36.0 | 9 | 60.0 | 36 | 47.4 | ||
| ⩾63.0 |
30 |
45.5 |
16 |
64.0 |
|
6 |
40.0 |
40 |
52.6 |
|
| Gender |
|
|
|
|
0.6348 |
|
|
|
|
0.02 |
| Male | 37 | 56.1 | 16 | 64.0 | 13 | 86.7 | 40 | 52.6 | ||
| Female |
29 |
43.9 |
9 |
36.0 |
|
2 |
13.3 |
36 |
47.4 |
|
| PS |
|
|
|
|
0.3506 |
|
|
|
|
0.679 |
| 0 | 21 | 31.8 | 5 | 20.0 | 3 | 20.0 | 23 | 30.3 | ||
| 1 | 44 | 66.7 | 19 | 76.0 | 12 | 80.0 | 51 | 67.1 | ||
| 2 |
1 |
1.5 |
1 |
4.0 |
|
0 |
0.0 |
2 |
2.6 |
|
| Tumour size |
|
|
|
|
0.8647 |
|
|
|
|
1 |
| <37.4 mm | 33 | 50.0 | 12 | 48.0 | 7 | 46.7 | 38 | 50.0 | ||
| ⩾37.4 mm |
33 |
50.0 |
13 |
52.0 |
|
8 |
53.3 |
38 |
50.0 |
|
| Lymph node metastasis |
|
|
|
|
0.4478 |
|
|
|
|
0.059 |
| Negative | 48 | 72.7 | 16 | 64.0 | 7 | 46.7 | 57 | 75.0 | ||
| Positive |
18 |
27.3 |
9 |
36.0 |
|
8 |
53.3 |
19 |
25.0 |
|
| Location of the tumour |
|
|
|
|
0.0379 |
|
|
|
|
0.156 |
| Head of pancreas | 31 | 47.0 | 18 | 72.0 | 10 | 66.7 | 33 | 43.4 | ||
| Body or tail of pancreas |
35 |
53.0 |
7 |
28.0 |
|
5 |
33.3 |
43 |
56.6 |
|
| CA19-9 |
|
|
|
|
0.451 |
|
|
|
|
0.227 |
| <1000 U ml−1 | 43 | 65.2 | 19 | 76.0 | 8 | 53.3 | 54 | 71.1 | ||
| ⩾1000 U ml−1 |
23 |
34.8 |
6 |
24.0 |
|
7 |
46.7 |
22 |
28.9 |
|
| Therapy |
|
|
|
|
1 |
|
|
|
|
1 |
| CT | 25 | 37.9 | 9 | 36.0 | 6 | 40.0 | 28 | 36.8 | ||
| CRT | 41 | 62.1 | 16 | 64.0 | 9 | 60.0 | 48 | 63.2 | ||
Abbreviations: ACTN4=actinin-4; CRT=chemoradiotherapy; CT=chemotherapy; FISH=fluorescence in situ hybridisation; IHC=immunohistochemistry; PS=Eastern Cooperative Oncology Group Performance Status.
*P-value: Fisher's exact test (two sided). Bold entries indicate statistically significance.
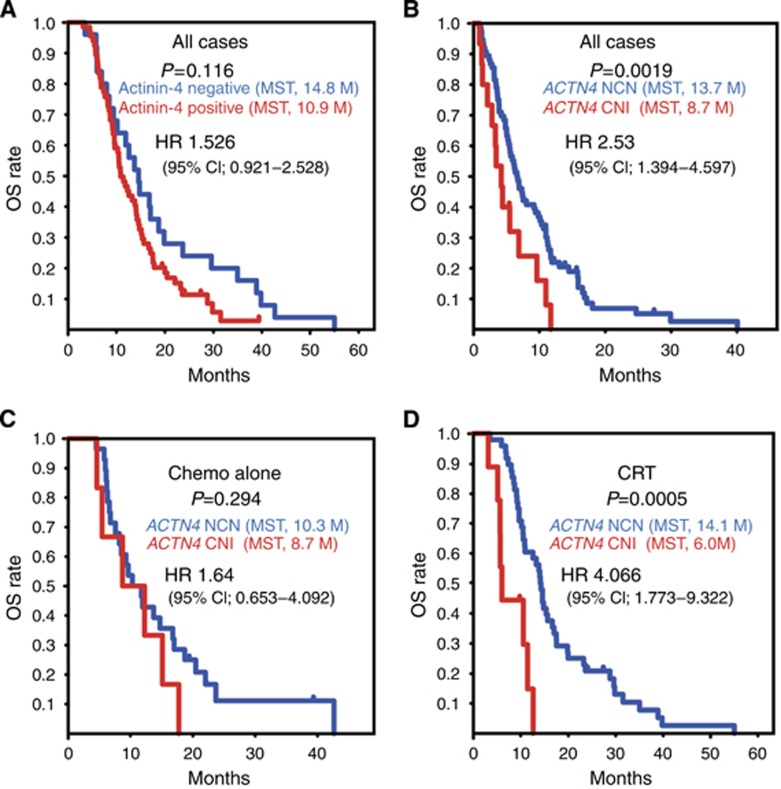
We determined whether protein expression of actinin-4 provided benefit for OS to patients with LAPC by comparing the OS of cases of LAPC with and without actinin-4 expression (total, n=91). No statistically significant difference in OS between actinin-4 protein-positive and -negative cases was found (P=0.116, log-rank test; Figure 3A). However, although a statistical significance was not found by Kaplan–Meier analysis, the median survival time (MST) of OS of cases positive for acitinin-4 protein was 10.9 months, which was shorter than the MST of the negative cases (14.8 months) by 3.9 months (Figure 3A).
Figure 3.
Kaplan–Meier analyses of survival relative to protein expression of actinin-4 and copy number of ACTN4. (A) Overall survival (OS) curves based on protein expression of actinin-4. The blue line represents patients with negative expression of actinin-4. The red line represents patients with positive expression of actinin-4. (B–D) The OS curves based on ACTN4 copy number status in all cases (n=91) (B), in the subgroup treated with chemotherapy alone (Chemo alone, n=34) (C) and in the chemoradiotherapy (CRT)-treated subgroup (n=57) (D). The blue lines represent patients who were evaluated as normal copy number (NCN) of ACTN4. The red lines represent patients who were evaluated as copy number increase (CNI) of ACTN4. Statistical parameters were calculated as described for Figure 2. The y axis is the rate of OS, and the x axis is the time after first diagnosis (months).
Determination of the copy number of ACTN4 by FISH, and prognostic impact of copy number of ACTN4 for LAPC
It is known that gene amplification of ACTN4 is responsible for overexpression of actinin-4 protein in a number of patients with pancreatic cancer. In addition, gene amplification of ACTN4 predicts a poorer prognosis than protein overexpression of actinin-4 in ovarian (Yamamoto et al, 2009), lung (Noro et al, 2013) and salivary gland cancer (Watabe et al, 2014). To evaluate the significance of ACTN4 as a prognostic factor for LAPC, we determined the copy number of ACTN4 in patients with LAPC by FISH. Of the 91 LAPC patients whom we examined, 76 patients were classified as NCN (83.5%) and 15 patients were classified as CNI (16.5%). Although only 1 of the 25 cases who were negative for actinin-4 protein (4.0%) had a CNI of ACTN4, 14 of the 66 cases who were actinin-4 protein positive (21.2%) had a CNI of ACTN4 (Table 3). We also analysed association of the ACTN4 copy number, as assessed by FISH analysis, with clinicopathological characteristics. There were statistically significant differences between gender and copy number of ACTN4 (P=0.02; Table 2).
Table 3. Statistical analysis of the association between the status of protein expression of actinin-4 and the copy number of ACTN4.
| |
Copy number status of
ACTN4 |
|
|
|
|---|---|---|---|---|
| Status of actinin-4 with IHC | NCN (%) | CNI (%) | Total | P-value* |
| Negative |
24 (96.0) |
1 (4.0) |
25 |
0.042 |
| Positive |
52 (78.8) |
14 (21.2) |
66 |
|
| Total | 76 (83.5) | 15 (16.5) | 91 | |
Abbreviations: ACTN4=actinin-4; CNI=copy number increase; IHC=immunohistochemistry; NCN=normal copy number.
*P-value: Fisher's exact test (one sided). Bold entry indicates statistically significance.
When all cases of LAPC were considered, the difference in OS between cases with a CNI and those with NCN of ACTN4 was statistically significant (P=0.0019, log-rank test). The MST of OS in the cases with a CNI of ACTN4 (8.7 months) was also significantly shorter than the MST of NCN cases (13.7 months) by 5 months (P=0.0019; Figure 3B).
Prognostic impact of the serum level of CA19-9 in patients with LAPC
The serum level of CA19-9 has been reported to be a prognostic factor for patients with LAPC (Berger et al, 2008; Mayahara et al, 2012; Yang et al, 2013). We confirmed the usefulness of the serum level of CA19-9 as a prognostic factor for patients with LAPC in our study. The LAPC patients were classified into one of two groups: CA19-9 high expression (⩾1000 U ml−1) and CA19-9 low–intermediate expression (<1000 U ml−1), as previously described (Mayahara et al, 2012). There was a statistically significant difference in OS between the CA19-9 high-expression group and the CA19-9 low–intermediate-expression group (P=0.0003, log-rank test; Supplementary Figure 1). The MST of the CA19-9 high-expression group was 9.3 months, which was shorter than the MST of the CA19-9 low–intermediate-expression group (14.6 months) by 5.3 months.
Univariate analysis indicated that the risk factors for death of LAPC patients were: lymph node metastasis, serum level of CA19-9 (cutoff value; 1000 U ml−1) and copy number status of ACTN4. The hazard ratios (HRs) for the death of patients with LAPC of lymph node metastasis, CA19-9 and a CNI of ACTN4 were: 1.606 (95% confidence interval (CI); 1.008–2.560, P=0.0463), 2.354 (95% CI; 1.479–3.761, P=0.0003) and 2.531 (95% CI; 1.394–4.597, P=0.0023), respectively. By multivariate analysis, the serum level of CA19-9 (HR; 2.325, 95% CI; 1.416–3.818, P=0.0009) and a CNI of ACTN4 (HR; 2.645, 95% CI; 1.439–4.861, P=0.0017) were independent risk factors for the death of patients with LAPC. The HR of CNI of ACTN4 (HR; 2.531) was slightly higher than that of the serum level of CA19-9 (HR; 2.354; Table 4).
Table 4. Univariate and multivariate Cox proportional hazard models to predict survival of patients with locally advanced pancreatic cancer receiving chemotherapy or chemoradiotherapy.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
|
Median age, years (63.0) | ||||||
| <63.0/⩾63.0 |
0.959 |
0.624–1.474 |
0.8498 |
|
|
|
|
Gender | ||||||
| Male/female |
0.802 |
0.519–1.249 |
0.334 |
|
|
|
|
PS | ||||||
| 0/1 and 2 |
1.126 |
0.697–1.819 |
0.6270 |
|
|
|
|
Median tumour size, mm (37.4) | ||||||
| <37.4 mm/⩾37.4 mm |
1.066 |
0.665–1.709 |
0.7902 |
|
|
|
|
Lymph node metastasis | ||||||
| Negative/positive |
1.606 |
1.008–2.560 |
0.0463 |
1.199 |
0.7654–1.978 |
0.0740 |
|
Location of the tumour | ||||||
| Head/body or tail of pancreas |
0.764 |
0.492–1.185 |
0.2294 |
|
|
|
|
CA19-9 | ||||||
| <1000/⩾1000 U ml−1 |
2.354 |
1.479–3.761 |
0.0003 |
2.325 |
1.416–3.818 |
0.0009 |
|
Actinin-4 IHC | ||||||
| Negative/positive |
1.526 |
0.922–2.528 |
0.1004 |
|
|
|
|
ACTN4 FISH | ||||||
| NCN/CNI | 2.531 | 1.394–4.597 | 0.0023 | 2.645 | 1.439–4.861 | 0.0017 |
Abbreviations: ACTN4=actinin-4; 95% CI=95% confidence interval; CNI=copy number increase; FISH=fluorescence in situ hybridisation; HR=hazard ratio; IHC=immunohistochemistry; NCN=normal copy number; PS=Eastern Cooperative Oncology Group Performance Status. Bold entries indicate statistically significance.
Evaluation of OS in subgroup analyses of treatment strategy with copy number of ACTN4
A biomarker that can evaluate the potential for metastatic activity in tumour cells has the possibility of use as a predictive biomarker of CRT. It is known that ACTN4 is an oncogene that is associated with cancer metastasis and cell invasion. In order to evaluate the benefit for OS based on the copy number status of ACTN4 for each treatment strategy, the patients with LAPC were classified into one of two subgroups on the basis of treatment strategies: a chemotherapy-alone group and a CRT group. We then analysed the impact of the copy number status of ACTN4 on OS of each subgroup. No statistical significance was observed between OS of patients with a NCN and with a CNI of ACTN4 in the chemotherapy-alone subgroup (P=0.294, log-rank test). The MST of CNI and NCN of ACTN4 patients was almost the same at 8.7 and 10.3 months, respectively (Figure 3C). Univariate Cox regression analysis indicated that the HR for death of CNI patients compared with NCN patients was 1.64 (95% CI; 0.653–4.092) in the chemotherapy-alone subgroup, and no statistically significant difference was found between CNI and NCN patients in the chemotherapy-alone subgroup (P=0.291).
In contrast, in the subgroup who underwent CRT, the OS of CNI of ACTN4 patients was significantly worse than that of patients with a NCN (P=0.0005, log-rank test), and the MST of CNI of ACTN4 patients (6.0 months) was definitely shorter than that of NCN of ACTN4 patients (14.1 months; Figure 3D). Univariate Cox regression analysis of the CRT groups indicated that the HR for death of CNI patients compared with that for NCN patients was 4.066 (95% CI; 1.773–9.322), and the difference between CNI and NCN groups was statistically significant (P=0.0009). The HR for death in the comparison between CNI and NCN of ACTN4 (4.066) patients in the CRT subgroup was higher than that of the HR in the comparison between the CNI and NCN of ACTN4 patients in all 91 cases (HR; 2.531; Table 4).
We also calculated the prognostic impact of the serum level of CA19-9 in each subgroup of therapeutic strategy on OS. The OS of patients with high expression of CA19-9 was significantly worse than that of patients with low–intermediate expression of CA19-9 in both subgroups of the chemotherapy-alone group (P=0.00218, log-rank test; Supplementary Figure 2) and the CRT group (P=0.0095; Supplementary Figure 3).
The benefit for PFS of CRT-treated patients who were selected by copy number status of ACTN4
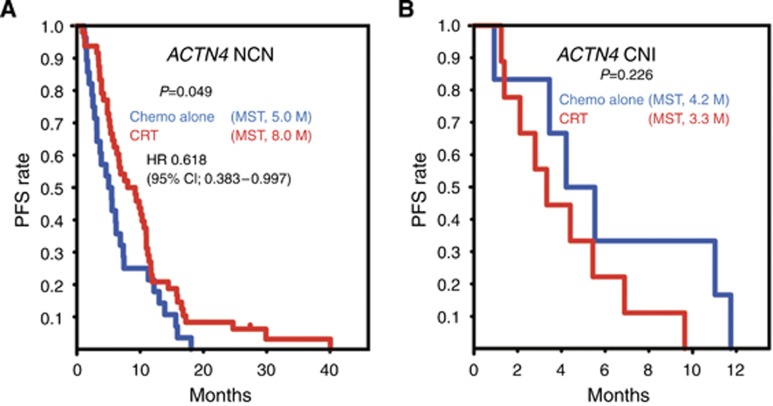
We further examined the ability of ACTN4 copy number to function as a predictive biomarker for CRT using subgroup analysis of the copy number status of ACTN4. We classified the patients into CNI and NCI subgroups of ACTN4 and compared PFS in these CNI and NCI subgroups of ACTN4 patients between the two arms of chemotherapy alone and CRT. Kaplan–Meier analysis indicated a statistically significant difference in the PFS of NCN patients in the chemotherapy-alone group compared with that of the CRT subgroup (P=0.049; Figure 4A). The median PFS of the patients who were evaluated as NCN of ACTN4 in the CRT subgroup was 8.0 months, whereas that for NCN of ACTN4 patients in the chemotherapy-alone subgroup was 5.0 months. Thus, the median PFS of patients with NCN of ACTN4 in the CRT subgroup was longer than that of patients with NCN of ACTN4 in the chemotherapy-alone subgroup by 3 months. The HR for tumour progression of patients with NCN of ACTN4 in the CRT subgroup compared with the chemotherapy-alone subgroup was 0.618 (95% CI; 0.383–0.997). No statistically significant difference in the PFS of patients with a CNI of ACTN4 was noted between the chemotherapy-alone and the CRT subgroups (P=0.226; Figure 4B). However, the MST of PFS in patients with a CNI of ACTN4 in the chemotherapy-alone subgroup (4.2 months) was slightly longer (0.9 month longer) than that of patients with a CNI of ACTN4 in the CRT subgroup (3.3 months). For both cohorts, there were no statistically significant differences in OS between the chemotherapy-alone and the CRT subgroups (data not shown).
Figure 4.
Kaplan–Meier analyses of progression-free survival (PFS) in CNI and NCN subgroups of ACTN4. The PFS curves of patients with a NCN of ACTN4 (A) or a CNI of ACTN4 (B), treated with chemotherapy alone (chemo alone, blue line) or with chemoradiotherapy (CRT, red line). The y axis is the rate of PFS and the x axis is the time after first diagnosis (months). Statistical parameters were calculated as described for Figure 2.
Discussion
In this study, we demonstrated that CNI of ACTN4 is a predictive biomarker for the therapeutic strategy of LAPC. Although there have been a large number of studies and trials regarding the best chemotherapeutic strategy for extension of survival of patients with LAPC (Colucci et al, 2002; Huguet et al, 2007; Moore et al, 2007; Chauffert et al, 2008; Loehrer et al, 2011), the optimal therapy for patients with LAPC has not yet been decided upon. Clinical trials have reported contradictory results. Thus, the ECOG E4201, FFCD/SFRO and LAP07 phase III trials reported that the MST of OS in patients who received CRT was improved (Loehrer et al, 2011), decreased (Chauffert et al, 2008) or showed no statistically significant survival benefit compared with patients who received chemotherapy alone. The results of these studies suggest that there is a potential benefit to selecting appropriate patients for intensified treatment.
In order to select either chemotherapy or CRT as a treatment strategy, the metastatic potential of the tumour itself needs to be accurately evaluated. This is because radiotherapy can only exert a direct physicochemical effect on the tumour at the primary tumour site that is exposed to radiation, whereas chemotherapy can access both the primary tumour and distant metastasis. Therefore, patients with latent metastatic lesions, including lesions that cannot be detected using modern technology, should receive only strong chemotherapy, whereas patients who definitely have no distant metastatic lesions before initial treatment should receive CRT in order to exert sufficient physicochemical impact on the primary tumour site. Our finding that ACTN4 copy number is a predictive marker for selection of therapy for LAPC should therefore prove valuable for optimisation of treatment strategy and help to provide the maximum personalised medicine for individual patients. Other predictive markers for treatment selection strategy have been suggested. Smad4 (Dpc4) is a tumour-suppressor gene involved in cell motility that is inactivated in 53% of pancreatic cancers. Prospective validation of smad4 expression in cytological specimens suggested that smad4 may be a predictive biomarker, and that analysis of smad4 levels may lead to personalised treatment strategies for patients with LAPC (Crane et al, 2011).
In the present paper we could not find any statistically significant difference in OS or PFS between LAPC patients who were treated with either chemotherapy alone or with CRT (Figure 2), again suggesting the need for a predictive marker for selection of patients for specific treatment. The potential predictive marker we considered was gene amplification of ACTN4.
The ACTN4 gene encodes the actinin-4 protein, an actin-bundling protein that was isolated by our group in 1998 (Honda et al, 1998). Its protein overexpression is closely associated with cancer invasion and cell motility. Actinin-4 has one actin-binding domain at the N-terminus, and actinin-4 monomers can form a homodimer by binding in the opposite direction to form a dumbbell-shaped structure (Otey and Carpen, 2004). The actinin-4 homodimer can strongly bind F-actin and subsequently form bundling F-actin. Moreover, the bundling F-actin formed by actinin-4 makes strong contact with the cell membrane, following which cellular protrusions that are associated with cell motility are formed on the cell membrane (Welsch et al, 2009). The protein overexpression of actinin-4 in cancer cells stimulates dynamic remodelling of the actin cytoskeleton, and it is for this reason that actinin-4-overexpressing cancer cells have metastatic potential (Hayashida et al, 2005). Indeed, there are some reports that patients with cancers showing protein overexpression of actinin-4 have significantly worse OS than patients with cancers who are negative for actinin-4 (Honda et al, 1998; Yamamoto et al, 2007; Noro et al, 2013). Moreover, Kikuchi et al (2008) reported that protein overexpression of actinin-4 was a poor prognostic factor for invasive ductal adenocarcinoma of the pancreas. However, in the present study we could not find a statistically significant positive correlation between actinin-4 protein overexpression and poor prognosis. One difference between our present study and the previous study of Kikuchi et al (2008) was that in the latter study protein expression of actinin-4 was immunohistochemically evaluated using whole pathological sections that were obtained from surgical samples, whereas in the present study protein expression of actinin-4 was immunohistochemically evaluated using biopsy specimens of LAPC. In the study of Kikuchi et al (2008), the staining pattern of endothelial cells as an internal control was used to accurately evaluate the protein expression level of actinin-4 in tumour cells. However, accurate evaluation of the protein expression level of actinin-4 from biopsy specimens was more difficult than from whole pathological sections because the biopsy specimens did not always include endothelial cells. These technical problems may therefore explain the difference in the results of the two studies. One cause of protein overexpression of actinin-4 in cancer cells is amplification of the ACTN4 gene (Kikuchi et al, 2008) and it has been reported that the CNI of ACTN4 is a better prognostic predictor than protein expression of actinin-4 (Yamamoto et al, 2009; Noro et al, 2013; Watabe et al, 2014). We found a statistically significant difference in OS between patients with a CNI and those with a NCN, and patients with a CNI had a worse prognosis in terms of OS than NCN patients (Figure 3B). Furthermore, multivariate Cox regression analysis indicated that a CNI of ACTN4 and high serum CA19-9 levels were independent prognostic factors for the death of patients, and that the HR of CNI of ACTN4 was higher than that of high CA19-9 levels (Table 4). These data confirmed the usefulness of CA19-9 as a prognostic factor for LAPC and further suggested that ACTN4 might be a prognostic factor for LAPC.
Subgroup analyses of CNI and NCN patients who were treated with chemotherapy alone or with CRT using FISH to calculate ACTN4 copy number showed that whereas the copy number of ACTN4 may be a predictive biomarker for CRT of LAPC, CA19-9 was not a predictive biomarker for either chemotherapy alone or CRT. Thus, there was no statistically significant difference in OS between CNI and NCN patients in the subgroup who were treated with chemotherapy alone (Figure 3C). However, in the subgroup of patients who were treated with CRT, the CNI patients with an MST of 14.1 months had a significantly longer survival time than NCN patients who had an MST of 6.0 months (Figure 3D). In contrast, serum CA19-9 levels showed statistically significant differences in terms of OS for both subgroups (Supplementary Figures 1–3).
Our data further confirmed the usefulness of ACTN4 as a predictive biomarker for CRT in the study of the PFS of patients with LAPC who were classified into CNI and NCN of ACTN4 groups and were then further classified into subgroups based on therapeutic strategies. We found a statistically significant difference in good prognosis of PFS between the NCN group treated with CRT (MST of PFS of 8.0 months) compared with the NCN group treated with chemotherapy alone (5.0 months; Figure 4A). Interestingly, although no statistically significant difference in PFS was found between the subgroups of CNI of ACTN4 who were treated with chemotherapy alone or with CRT, the MST of PFS was the reverse of that seen in the NCN group, with the MST of chemotherapy alone being 4.2 months and that of CRT being shorter at 3.3 months. These data suggest that, when considering therapy for LAPC patients, patients with a NCN of ACTN4 should at least undergo CRT (Figure 4B). However, no statistically significant difference in benefit in OS was noted in subgroup analysis of CNI and NCN of ACTN4 groups. It was considered that the number of patients in the subgroup of ACTN4 was too small to statistically prove the clinical benefit of chemotherapy alone in the subgroup with CNI of ACTN4.
In conclusion, we showed that the copy number of ACTN4 is not only a prognostic biomarker, but also a candidate predictive biomarker for the decision regarding effective treatment strategy. Although this was a retrospective study, it suggested that patients without gene amplification of ACTN4 should undergo CRT. Although it was concluded that ACTN4 is a biomarker of potential metastasis, this does not necessarily contraindicate a potential function for ACTN4 copy number as a predictive biomarker for CRT of LAPC. More detailed analyses, including a prospective study, should be carried out to prove this possibility.
Acknowledgments
This work was supported by a Grant-in Aid for Scientific Research (B) and a Challenging Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology (METX) of Japan (to KH) and the National Cancer Center Research and Development Fund (23-A-38, and 23-A-11; to KH).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Berger AC, Garcia M, Jr., Hoffman JP, Regine WF, Abrams RA, Safran H, Konski A, Benson AB, 3rd, MacDonald J, Willett CG. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26 (36:5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, Bosset JF, Aparicio T, Mineur L, Azzedine A, Hammel P, Butel J, Stremsdoerfer N, Maingon P, Bedenne L. Phase III trial comparing intensive induction chemoradiotherapy (60Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19 (9:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer. 2002;94 (4:902–910. [PubMed] [Google Scholar]
- Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, Haque W, Hobbs BD, Krishnan S, Fleming JB, Das P, Lee JE, Abbruzzese JL, Wolff RA. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29 (22:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Honda K, Idogawa M, Ino Y, Ono M, Tsuchida A, Aoki T, Hirohashi S, Yamada T. E-cadherin regulates the association between beta-catenin and actinin-4. Cancer Res. 2005;65 (19:8836–8845. doi: 10.1158/0008-5472.CAN-05-0718. [DOI] [PubMed] [Google Scholar]
- Honda K, Okusaka T, Felix K, Nakamori S, Sata N, Nagai H, Ioka T, Tsuchida A, Shimahara T, Shimahara M, Yasunami Y, Kuwabara H, Sakuma T, Otsuka Y, Ota N, Shitashige M, Kosuge T, Buchler MW, Yamada T. Altered plasma apolipoprotein modifications in patients with pancreatic cancer: protein characterization and multi-institutional validation. PLoS One. 2012;7 (10:e46908. doi: 10.1371/journal.pone.0046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140 (6:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yamada T, Hayashida Y, Idogawa M, Sato S, Hasegawa F, Ino Y, Ono M, Hirohashi S. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128 (1:51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, de Gramont A, Louvet C. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25 (3:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Okusaka T, Ito Y, Ueno H, Morizane C, Furuse J, Ishii H, Kawashima M, Kagami Y, Ikeda H. A phase I trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer. Br J Cancer. 2007;96 (11:1650–1655. doi: 10.1038/sj.bjc.6603788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johung K, Saif MW, Chang BW. Treatment of locally advanced pancreatic cancer: the role of radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82 (2:508–518. doi: 10.1016/j.ijrobp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, Pisters PW, Posner MC, Berlin JD, Ahmad SA. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20 (8:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Honda K, Handa Y, Kato H, Yamashita K, Umaki T, Shitashige M, Ono M, Tsuchida A, Aoki T, Hirohashi S, Yamada T. Serum albumin-associated peptides of patients with uterine endometrial cancer. Cancer Sci. 2007;98 (6:822–829. doi: 10.1111/j.1349-7006.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Honda K, Tsuda H, Hiraoka N, Imoto I, Kosuge T, Umaki T, Onozato K, Shitashige M, Yamaguchi U, Ono M, Tsuchida A, Aoki T, Inazawa J, Hirohashi S, Yamada T. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clin Cancer Res. 2008;14 (17:5348–5356. doi: 10.1158/1078-0432.CCR-08-0075. [DOI] [PubMed] [Google Scholar]
- Loehrer PJ, Sr., Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, Benson AB., 3rd Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29 (31:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayahara H, Ito Y, Morizane C, Ueno H, Okusaka T, Kondo S, Murakami N, Morota M, Sumi M, Itami J. Salvage chemoradiotherapy after primary chemotherapy for locally advanced pancreatic cancer: a single-institution retrospective analysis. BMC Cancer. 2012;12:609. doi: 10.1186/1471-2407-12-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OY, Ram AN, Tuli R, Herman JM. Management options in locally advanced pancreatic cancer. Curr Oncol Rep. 2014;16 (6:388. doi: 10.1007/s11912-014-0388-y. [DOI] [PubMed] [Google Scholar]
- Miyanaga A, Honda K, Tsuta K, Masuda M, Yamaguchi U, Fujii G, Miyamoto A, Shinagawa S, Miura N, Tsuda H, Sakuma T, Asamura H, Gemma A, Yamada T. Diagnostic and prognostic significance of the alternatively spliced ACTN4 variant in high-grade neuroendocrine pulmonary tumours. Ann Oncol. 2013;24 (1:84–90. doi: 10.1093/annonc/mds215. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials G Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25 (15:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Noro R, Honda K, Tsuta K, Ishii G, Maeshima AM, Miura N, Furuta K, Shibata T, Tsuda H, Ochiai A, Sakuma T, Nishijima N, Gemma A, Asamura H, Nagai K, Yamada T. Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell motility gene amplification. Ann Oncol. 2013;24 (10:2594–2600. doi: 10.1093/annonc/mdt293. [DOI] [PubMed] [Google Scholar]
- Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton. 2004;58 (2:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, Ali S, Fishman EK, Schulick RD, Herman JM, Johns Hopkins Multidisciplinary Pancreas Clinic T Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15 (8:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savir G, Huber KE, Saif MW. Locally advanced pancreatic cancer. Looking beyond traditional chemotherapy and radiation. JOP. 2013;14 (4:337–339. doi: 10.6092/1590-8577/1677. [DOI] [PubMed] [Google Scholar]
- Watabe Y, Mori T, Yoshimoto S, Nomura T, Shibahara T, Yamada T, Honda K. Copy number increase of ACTN4 is a prognostic indicator in salivary gland carcinoma. Cancer Med. 2014;3 (3:613–622. doi: 10.1002/cam4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch T, Keleg S, Bergmann F, Bauer S, Hinz U, Schmidt J. Actinin-4 expression in primary and metastasized pancreatic ductal adenocarcinoma. Pancreas. 2009;38 (8:968–976. doi: 10.1097/MPA.0b013e3181b28d6f. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Honda K, Kita T, Takano M, Tamai S, Inazawa J, Yamada T, Matsubara O. Actinin-4 expression in ovarian cancer: a novel prognostic indicator independent of clinical stage and histological type. Mod Pathol. 2007;20 (12:1278–1285. doi: 10.1038/modpathol.3800966. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Honda K, Onozato K, Takano M, Tamai S, Imoto I, Inazawa J, Yamada T, Matsubara O. Actinin-4 gene amplification in ovarian cancer: a candidate oncogene associated with poor patient prognosis and tumor chemoresistance. Mod Pathol. 2009;22 (4:499–507. doi: 10.1038/modpathol.2008.234. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsuda H, Honda K, Takano M, Tamai S, Imoto I, Inazawa J, Yamada T, Matsubara O. ACTN4 gene amplification and actinin-4 protein overexpression drive tumour development and histological progression in a high-grade subset of ovarian clear-cell adenocarcinomas. Histopathology. 2012;60 (7:1073–1083. doi: 10.1111/j.1365-2559.2011.04163.x. [DOI] [PubMed] [Google Scholar]
- Yang GY, Malik NK, Chandrasekhar R, Ma WW, Flaherty L, Iyer R, Kuvshinoff B, Gibbs J, Wilding G, Warren G, May KS. Change in CA 19-9 levels after chemoradiotherapy predicts survival in patients with locally advanced unresectable pancreatic cancer. J Gastrointest Oncol. 2013;4 (4:361–369. doi: 10.3978/j.issn.2078-6891.2013.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.