Abstract
The formation of epithelial tissues allows organisms to specialise and form tissues with diverse functions and compartmentalised environments. The tight controls on cell growth and migration required to maintain epithelia can present problems such as the development and spread of cancer when normal pathways are disrupted. By attaining a deeper understanding of how cell migration is suppressed to maintain the epithelial organisation and how it is reactivated when epithelial tissues become mesenchymal, new insights into both cancer and development can be gained. Here we discuss recent developments in our understanding of epithelial and mesenchymal regulation of the actin cytoskeleton in normal and cancerous tissue, with a focus on the pancreas and intestinal tract.
Keywords: epithelial, mesenchymal, invasion, migration, pancreas, intestine, colorectal, actin
Epithelia are highly organised sheets of cells that serve to form a barrier between external and internal spaces in tissues. They are important for the formation of tubes and the creation of a luminal space where the internal environment can be rendered distinct from the outside world. Epithelial specialisation arose when eukaryotic organisms committed to being multicellular and having functionally specialised tissues, rather than just growing as colonies of more or less identical clonal cells. Epithelial cells are polarised with respect to top and bottom, as well as within the plane of the tissue. Epithelial cells form junctions with their neighbours, involving specialised cytoskeletal protein assemblies. While metazoans have the clearest commitment to epithelial specialisation, it is interesting that epithelial-associated junctional proteins have been found in more ancient organisms and structures resembling epithelia have been described for example in the social amoeba Dictyostelium discoideum (Dickinson et al, 2011). Cancers arising in epithelial tissues are known as carcinomas and much effort has been devoted to unravelling the molecular programmes that occur during formation and progression of carcinomas. One of the most well-studied features of carcinomas, associated with increased aggressiveness and metastatic spread, is the loss of epithelial integrity and specialisation, called epithelial to mesenchymal transition or EMT.
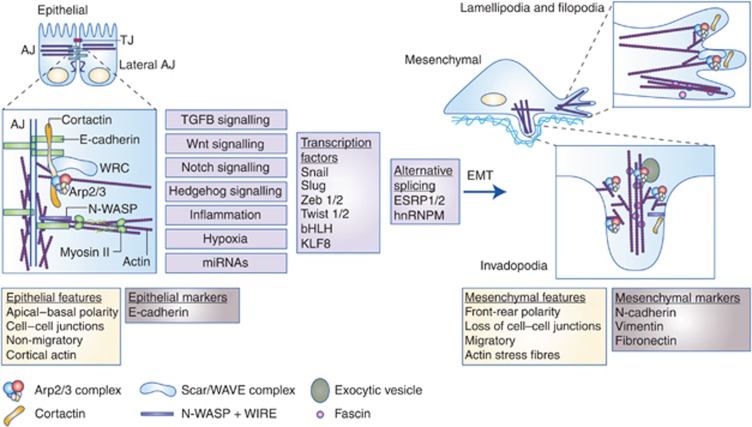
EMT results in loss of features characteristic of epithelial cells – cell–cell adhesions, polarity and amotility and acquisition of a mesenchymal phenotype – spindled shape, motility and ability to invade. These phenotypic changes are accompanied by a loss of epithelial cell markers such as E-cadherin and increased expression of mesenchymal markers such as N-cadherin, vimentin and fibronectin (Figure 1).
Figure 1.
Involvement of actin cytoskeletal reorganisation in EMT. Epithelial cells make tight junctions (TJ, red) that act as a permeability barrier between the outside world and the tissue and adherens juncions (AJ) that provide mechanical stability and strength by connections with the actin cytoskeleton and the transmembrane cadherin proteins. E-cadherins (green) are major scaffold proteins controlling adherens junction integrity and direct links between E-cadherins and actin nucleating proteins, such as WRC (light blue) and Arp2/3 complex (multicoloured) provide a basis for sequestering the actin nucleation machinery when cells are non-motile and also for harnessing actin nucleation to provide actin cytoskeletal scaffolding for tissue integrity. N-WASP (purple) acts with WIRE (not shown) to stabilise and bundle cortical actin filaments (Wu et al, 2014). Cortactin (orange) is a scaffold, binding both N-WASP and E-cadherin to recruit ARP2/3 and WRC to adherens junctions. EMT can be driven by a number of signalling pathways (purple boxes, see text for references) that result in the activation of transcriptional programmes and alternative splicing. In mesenchymal cells, E-cadherin is lost and the actin cytoskeleton undergoes a number of changes, resulting in a shift of actin and its regulatory proteins and complexes from the cortex towards the leading edge of migrating cells, where they form lamellipodia. A specialised mesenchymal actin bundling protein, fascin, organises actin filaments at sites of filopodia and is also recruited to invadopodia, the invasive protrusions of cancer cells, along with Arp2/3 complex, cortactin and N-WASP. These changes are accompanied by the expression of mesenchymal markers such as N-cadherin, vimentin and fibronectin and a change in cell polarity (from the apico–basal polarity in epithelial cells to the front–back polarity in mesenchymal cells).
EMT was first described in the early 1980s (Greenburg and Hay, 1982) but was termed as epithelial–mesenchymal transformation. This was later amended to epithelial–mesenchymal transition, to reflect the fact that the changes are reversible by mesenchymal–epithelial transition (MET). EMT is crucial to many of the normal developmental processes of metazoa. For example, in mammals, the early embryo forms as a ball of epithelial-like cells and must undergo EMT to invade and grow in the uterus (a process known as implantation). There has been much debate about how to define EMT; for example, should cells that have developed some mesenchymal features but still retain some epithelial ones be classed as having undergone EMT, or does this represent ‘partial' EMT? This remains a subject of continuing controversy, which we will not touch on here. In 2008, three types of EMT were defined at a meeting of experts at Cold Spring Harbor Laboratory (Kalluri and Weinberg, 2009; Zeisberg and Neilson, 2009). Although this was by no means the end of the ongoing discussions about how to define EMT, we believe that it forms a useful basis for discussion for this review and a starting point for gaining a deeper understanding of EMT in development and cancer:
Type 1 EMT: this is the ‘normal' process of transition of epithelial cells to a mesenchymal state during implantation and embryonic development, as part of the processes of gastrulation and neural crest formation.
Type 2 EMT: this EMT programme occurs in response to inflammation and is integral to the processes of tissue repair, regeneration and in cases where the inflammatory response is prolonged, fibrosis. This type can also be induced in cancer.
Type 3 EMT: this EMT programme is observed in neoplastic tumour-forming cells as a part of tumour dedifferentiation. Once having undergone EMT, tumour cells are able to invade and migrate to distant sites where they may establish metastases – by definition, conferring properties of malignancy on these cells. This can be accompanied by MET whereby the metastatic tumour nodule once again takes on epithelial characteristics.
For transitions between epithelial and mesenchymal states, cells need to tightly control their motility programmes. Control happens at multiple levels, including gene expression, post-translational modifications and reorganisation of the actin cytoskeleton. Here we discuss EMT during development and in cancer of the pancreas and intestinal tract and we highlight some of the actin cytoskeletal changes that occur during EMT and our emerging understanding of how the cytoskeleton and motility are regulated during these processes.
Reprogramming during EMT
All types of EMT are driven by a combination of intrinsic programming of cells and environmental factors, such as signals from the stroma. Two of the most important signalling pathways driving EMT are the transforming growth factor beta (TGFβ) and Wnt pathways (Tam and Weinberg, 2013). Other pathways include receptor tyrosine kinase, Notch and Hedgehog signalling pathways. These signalling pathways trigger a reprogramming of gene expression patterns via various methods, including transcriptional changes, alternative splicing pathways and altered expression of micro-RNA (miR). Transcriptional changes are largely thought to be governed by a handful of so-called EMT Transcription factors or EMT Tfs (Figure 1) that include the Snail superfamily (e.g., Snail, Slug, Zeb1/2, Twist 1/2, bHLH and KLF8; reviewed in Diaz-Lopez et al, 2014). These transcription factors repress the epithelial programme of gene expression (e.g., E-cadherin) and enhance mesenchymal expression (e.g., upregulate N-cadherin and vimentin). Alternative splicing occurs in EMT and changes the expression pattern of many proteins via factors such as ESRP1/2 and hnRNPM (Ishii et al, 2014; Xu et al, 2014). MiRs are small non-coding RNA that interact with mRNA and cause silencing or regulation of transcription (Diaz-Lopez et al, 2014). miRs are also major players in the EMT process and are a subject of much research for pancreatic and intestinal cancers, for example, miR-200 and miR34 (Figure 1 and Diaz-Lopez et al, 2014). Together these transcriptional and post-transcriptional regulators, driven by signalling pathways from the microenvironment, regulate the various programmes that have been described collectively as EMT, but although there are common threads, different tissues and environmental conexts can trigger quite diverse changes associated with loss of epithelial status and gain of mesenchymal functions.
Downstream of transcriptional changes, alternative splicing and miRNA regulation, many cytoskeletal proteins are altered in their expression, localisation or activity. In addition to the downregulation of E-cadherin, cells gain the intermediate filament protein vimentin and change their expression patterns of several other adhesion molecules such as integrins and cell surface glycoproteins. They may upregulate other cadherin isoforms, such as N-cadherin. The actin bundling protein fascin is specifically expressed in response to the EMT programme in colorectal and pancreatic cancers (Li et al, 2014) but less is known about its role in developmental delamination. Cells undergoing EMT also modulate production of extracellular matrix proteins such as fibronectin (Chen et al, 2008; Medici et al, 2008). At least in breast cancer, alternative splicing controls proteins such as the actin-binding protein Mena, which switches to an invasion promoting form (Mena-inv) and the membrane receptor CD44 (Goswami et al, 2009; Pignatelli et al, 2014; Xu et al, 2014). The organisation of the actin cytoskeleton is tightly linked to cell–cell junctions in the epithelia and this changes dramatically when cells delaminate and lose their adherens and tight junctions (Figure 1). Many of the actin filament nucleating proteins are not transcriptionally regulated, but are differently localised or regulated. For example, the actin nucleation proteins Scar/WAVE complex, N-WASP, Arp2/3 complex and cortactin localise to cell–cell junctions in epithelial cells, but are released from junctions and redirected to cell-leading edges when cells become mesenchymal (Figure 1). Many of these changes are controlled by the activity of the Rho-family small GTPases, including Rac1 and Cdc42, which have both been implicated in regulating the dynamics of epithelial cell junctions (Woodham and Machesky, 2014).
EMT and cell migration during development of the pancreas and intestinal tract
EMT first occurs very early in the development of the intestinal tract. Under the influence of Wnt signalling (Liu et al, 1999) and downstream mediators belonging to the TGFβ superfamily (Andersson et al, 2007), epiblast cells in the primitive streak of the embryo undergo EMT, migrating internally to produce the mesoderm and endoderm. The mechanisms by which this is orchestrated are reviewed in Chuai et al (2012). The epiblast cells that do not undergo EMT remain on the surface, forming the ectoderm (Acloque et al, 2009) and subsequently cells of the endoderm form an epithelial tube extending for the length of the embryo. This tube differentiates to form three different sections – the foregut (gives rise to the pharynx, oesophagus and stomach), midgut (gives rise to the small intestine and proximal large intestine) and hindgut (gives rise to the mid and distal large intestine). At midgestation, the endoderm undergoes further differentiation in response to signals from the mesoderm and eventually the intestinal epithelium specialises to form villi and crypts containing specialised cell types.
In contrast to the intestinal lining, which retains its epithelial status from the time of formation of the endoderm, some components of the pancreas and liver require the cells to undergo a further round of EMT and MET. For example, pancreatic bud cells undergo EMT and migrate away from the epithelium to form the endocrine cells of the Islets of Langerhans (Johansson and Grapin-Botton, 2002). E-cadherin expression is repressed in a subset of cells, termed neurogenin3+-expressing insulin-producing cells, leading to migration and clustering to form islets. The EMT transcription factor Slug (also called Snail2) inversely correlates with E-cadherin expression in the developing pancreas and has been implicated in delamination and migration of the neurogenin3+ endocrine progenitor cells during islet formation (Rukstalis and Habener, 2007).
The Rho-family GTPase Cdc42 and its downstream target N-WASP are key players in pancreatic islet formation, as the expression of constitutively active Cdc42 prevents delamination, disassembly of actin at cell–cell junctions and migration (Kesavan et al, 2014). N-WASP depletion can partially rescue this phenotype, suggesting that N-WASP-mediated stabilisation of junctional actin needs to be repressed for the delamination process to complete (Kesavan et al, 2009; Kesavan et al, 2014). Cdc42 is also implicated in the formation of tubules in the developing pancreas as it has a central role in apical polarisation and thus lumen formation (Kesavan et al, 2009). In contrast, Rac1 was implicated in the mobilisation of E-cadherin junctions in the developing islets, as expression of a dominant negative Rac1 prevented migration (Greiner et al, 2009).
Under the control of the Rho GTPases, the actin cytoskeleton has an important role in epithelial cell–cell junctions, providing connectivity and strength and serving as a platform for signalling and membrane trafficking. Although next to nothing is known of the specific roles of the actin nucleation proteins in the intestinal tract, the key actin organisers N-WASP, cortactin and Arp2/3 complex have all been implicated in actin dynamics at cell–cell junctions in epithelia in tissue culture systems. Rather than stimulating new actin polymerisation for protrusion and migration, as it does in mesenchymal migrating cells, N-WASP functions together with its binding partner WIRE to stabilise and bundle actin filaments at cell–cell junctions to allow for generation of tension by myosin-II (Wu et al, 2014; Figure 1). This junctional tension can regulate whether cells are integrated or excluded from the epithelial monolayers, so is an interesting potential contributor to delamination and extrusion from the epithelium. Ras-transformed cells undergo abnormal extrusion from epithelial tissues (Hogan et al, 2009) and this may contribute to cancer cells breaking away from primary tumours and thus gaining access to other tissues in the body. The actin nucleation-promoting protein cortactin is implicated as a major scaffold in epithelial cell junctions, with direct interactions between cortactin, N-WASP and E-cadherin having a role in recruitment of Arp2/3 complex and the Scar/WAVE complex, to promote actin nucleation at adherens junctions (Han et al, 2014; Figure 1). In contrast, N-WASP, Scar/WAVE proteins, cortactin and Arp2/3 complex localise to leading edge protrusions of migrating cells, where they contribute to membrane dynamics and protrusion (Figure 1). Clearly, ancient proteins involved in motility, such as WASP-family proteins, have evolved features that allow them to promote epithelial cell organisation when they need to be restricted from nucleating leading edge actin assembly and we propose that gaining a deeper understanding of these features would make a valuable contribution to our understanding of EMT and cancer spread.
EMT in cancers of the intestinal tract and pancreas
EMT has been widely proposed as a mechanism used by carcinoma cells to regain developmental motile and invasive properties and disseminate throughout the body. Epithelial tumours usually initiate by gradually increasing degrees of dysplasia. For example, colorectal adenocarcinomas usually develop through several benign stages before becoming malignant. First, there is formation of aberrant crypt foci (ACFs, abnormal clusters of crypt cells), some of which may be dysplastic. Continued growth of ACFs results in formation of benign protrusions of epithelium (polyps). Some polyps are simply hyperplastic and generally do not advance beyond this stage. Those containing dysplasia are called adenomas; if they continue to grow and accumulate additional genetic abnormalities, they may progress through low-to-high grade dysplasia and finally to malignant adenocarcinomas (Fearon and Vogelstein, 1990). How well differentiated a cancer is (how much it histologically resembles the normal epithelial tissue) has clinical relevance in terms of prognosis and is expressed in terms of the ‘grade' of cancer (Grades 1–3 for colorectal cancer). Likewise, pancreatic ductal adenocarcinoma is thought to arise by gradual increases in the dysplasia, which is graded as Pancreatic Intraepithelial Neoplasia stages 1–3 (Distler et al, 2014). The role of EMT transcription factors and EMT in these changes is only partially understood and most of the pancreatic precancerous lesions in a mouse model of PDAC retained E-cadherin junctions even though at later stages they expressed the EMT transcription factor Slug (Li et al, 2014). Human intestinal adenomas contain hallmarks of some aspects of EMT (Chen et al, 2008).
There is a large body of evidence to suggest that EMT associated changes in gene expression and cell morphology occur in carcinomas and that they contribute to the aggressiveness, invasiveness and spread (Tam and Weinberg, 2013). Studies investigating the prognostic significance of EMT markers in human cancers are summarised in Table 1. Two of the most heavily implicated pathways in cancer EMT are the Wnt and TGFβ signalling pathways. Greater than 80% of all sporadic colorectal carcinomas harbour mutations in the Wnt signalling pathway, such as loss of adenomatous polyposis coli (APC) leading to the constitutive hyperactivation of Wnt signalling. Low E-cadherin correlates with poor survival in multiple clinical studies and is an independent prognostic indicator in at least five studies (Table 1). However, APC loss alone, although it triggers hyperproliferation and benign tumour formation, is insufficient to drive the development of cancer; increasing genetic instability is thought to be another major contributing factor (Bogaert and Prenen, 2014) as is signalling from TGFβ and Wnt signalling from the stroma. EMT is often only apparent at the tumour–stroma interface in colorectal cancers, because there seems to be a threshold of signalling necessary to sustain nuclear β-catenin and to drive invasive behaviour (Brabletz et al, 2001). The cells at the leading tumour edges frequently show elevation of Zeb1 transcription factor (reviewed in Schmalhofer et al (2009)) and Zeb1 is a Wnt target in colorectal cancer (Sanchez-Tillo et al, 2011) that correlates with poor survival (Table 1). Zeb1 promotes EMT changes partly by repression of miR-200 family members ((Burk et al, 2008) and Table 1). Several miRs have been implicated as correlating with poor survival in colorectal cancer (Table 1) and low miR-212 is an independent prognostic indicator of poor outcome (Table 1). Other EMT transcription factors, Snail1/2 (Slug) and Twist also have been correlated with poor survival in GI cancers (Table 1) as have hallmarks of EMT such as increased vimentin and fibronectin (Table 1).
Table 1. Summary of cancer studies implicating EMT markers in prognosis and outcomes for several epithelial cancers of the gastrointestinal tract.
| Marker | Authors | Year | Journal | Site | Method | No. of cases | KMC LRT P-value | CoxPH HR | HR P-value | Outcome | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
E-cadherin − | |||||||||||
| Kroepli et al | 2013 | BMC Cancer | Colorectal | TMA IHC | 250 | 0.87 | NT | OS | |||
| Bellovin et al | 2005 | Cancer Res | Colorectal | TMA IHC | 557 | 0.0127 | Not shown | NS | OS | ||
| Knösel et al | 2012 | Int J Colorectal Dis | Colorectal (high grade) | TMA IHC | 402 | 0.083 | NT | OS | |||
| Yun et al | 2014 | Oncology | Colorectal | TMA IHC | 409 | 0.009 | 1.984 (0.539–7.296) | 0.303 | OS | ||
| Yun et al | 2014 | Oncology | Colorectal | TMA IHC | 409 | 0.003 | 5.098 (1.801–14.430) | 0.002 | DFS | ||
| Jie et al | 2013 | Dig Dis Sci | Colorectal | WTB IHC | 108 | <0.01 | NT | OS | |||
| Shioiri et al | 2006 | Br J Cancer | Colorectal | WTB IHC | 138 | 0.0066 | 2.249 (1.164–4.343) | 0.0158 | OS | ||
| Fujikawa et al | 2012 | J Gastroenterol | Colorectal | qPCR | 102 | <0.01 | Not shown | NS | OS | ||
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.018 | 2.09 (1.11–4.27) | 0.0208 | OS | ||
| Chen et al | 2014 | Tumour Biol | Gallbladder | WTB IHC | 93 | NT | 1.856 (1.034–2.976) | 0.026 | OS | ||
| Hou et al | 2012 | Med Oncol | Gastric | WTB IHC | 158 | <0.001 | 0.574 (0.371–0.886) | 0.012 | OS | HR for E-cad positivity | |
| |
Kim et al |
2009 |
Histopathol |
Gastric |
TMA IHC |
598 |
0.0006 |
Not shown |
NS |
OS |
|
|
Summary: 9/10 significant differences in OS, 1/1 significant difference in DFS, 4/8 independent prognostic variable OS, 1/1 independent prognostic variable of DFS | |||||||||||
|
Snail + | |||||||||||
| Kroepli et al | 2013 | BMC Cancer | Colorectal | TMA IHC | 251 | 0.57 | NT | OS | |||
| Franci et al | 2009 | PLoS One | Colorectal | TMA IHC | 162 | 0.011 | NT | OS | |||
| Kim et al | 2014 | Oncol Rep | Colorectal | qPCR | 109 | 0.014 | 2.11 (1.03–4.33) | 0.041 | OS | ||
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.3413 | NT | OS | |||
| Shin et al | 2012 | BMC Cancer | Gastric | TMA IHC | 314 | 0.023 | 0.590 (0.363–0.958) | 0.033 | OS | HR for Snail negativity | |
| |
Kim et al |
2009 |
Histopathol |
Gastric |
TMA IHC |
598 |
<0.0001 |
1.31 |
0.041 |
OS |
|
|
Summary: 4/6 significant differences in OS, 3/3 independent prognostic variable of OS | |||||||||||
|
Slug + | |||||||||||
| Shioiri et al | 2006 | Br J Cancer | Colorectal | WTB IHC | 138 | <0.0001 | 2.212 (1.127–4.342) | 0.021 | OS | ||
| |
Nitta et al |
2014 |
BJC |
Bile duct |
TMA IHC |
117 |
0.6143 |
NT |
|
OS |
|
|
Summary: 1/2 significant difference in OS, 1/1 independent prognostic variable of OS | |||||||||||
|
Twist + | |||||||||||
| Twist 1 | Gomez et al | 2011 | PLoS One | Colorectal | qPCR | 151 | 0.001 | 2.73 (1.5–4.84) | 0.001 | OS | |
| Gomez et al | 2011 | PLoS One | Colorectal | qPCR | 151 | 0.16 (0.02 for Stage 1 only) | 1.99 (1.05–3.82) | 0.036 | DFS | ||
| Kim et al | 2014 | Oncol Rep | Colorectal | qPCR | 109 | 0.002 | 2.29 (1.04–5.00) | 0.039 | OS | ||
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.5203 | NT | OS | |||
| Ru et al | 2010 | Path and Oncol Res | Gastric (Stages 1–3) | TMA IHC | 436 | <0.05 | Not shown | <0.001 | OS | ||
| Twist 2 | Yu et al | 2013 | World J Gastroenterol | Colorectal | WTB IHC | 93 | 0.015 | 5.744 (1.347–24.298) | 0.018 | OS | |
| |
Yu et al |
2013 |
World J Gastroenterol |
Colorectal |
WTB IHC |
93 |
0.012 |
3.264 (1.455–7.375) |
0.004 |
DFS |
|
|
Summary: 4/5 significant differences in OS, 2/2 signficant difference in DFS, 4/4 independent prognostic variable OS, 2/2 significance as independent prognostic variable of DFS | |||||||||||
|
Zeb + | |||||||||||
| Zeb 1 | Liu et al | 2012 | Cancer Sci | Colorectal | WTB IHC | 203 | <0.05 | NT | OS | ||
| Zheng et al | 2013 | Oncol Lett | Colorectal | qPCR | 92 | 0.01 | 2.237 (1.008–4.968) | 0.048 | OS | ||
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.379 | NT | OS | |||
| Bronsert et al | 2014 | Surgery | Pancreas (Tumour) | WTB IHC | 112 | 0.043 | Not shown | NS | OS | ||
| Bronsert et al | 2014 | Surgery | Pancreas (Stroma) | WTB IHC | 112 | 0.032 | 1.772 (1.033–3.041) | 0.038 | OS | ||
| Zeb 2 | Kahlert | 2011 | Cancer Sci | Colorectal (invasive front) | IHC | 175 | <0.0001 | 2.48 (1.16–5.27) | 0.02 | CSS | |
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.938 | NT | OS | |||
| |
Dai et al |
2012 |
Dig Dis Sci |
Gastric |
WTB IHC |
76 |
<0.05 |
NS |
|
OS |
|
|
Summary: 5/7 significant differences in OS, 1/1 signficant difference in CSS, 2/3 independent prognostic variable of OS, 1/1 showed significance as independent prognostic variable of CSS | |||||||||||
|
Vimentin + | |||||||||||
| Yun et al | 2014 | Oncology | Colorectal | TMA IHC | 409 | NT | 0.769 (0.419–1.413) | 0.398 | OS | ||
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.0193 | 1.21 (0.61–2.25) | 0.5662 | OS | ||
| Chen et al | 2014 | Tumour Biol | Gallbladder | WTB IHC | 93 | NT | 1.645 (0.956–2.756) | 0.043 | OS | ||
| Kim et al | 2009 | Histopathol | Gastric | TMA IHC | 598 | 0.0008 | Not shown | NS | OS | ||
| Hou et al | 2012 | Med Oncol | Gastric | WTB IHC | 158 | 0.029 | 1.444 (0.910–2.291) | 0.119 | OS | ||
| |
Otsuki et al |
2011 |
Oncol Rep |
Gastric |
qPCR |
106 |
0.019 |
2.1 (1–4.4) |
0.036 |
DFS |
|
|
Summary: 3/3 significant differences in OS, 1/1 signficant difference in DFS, 1/5 independent prognostic variable of OS, 1/1 independent prognostic variable of DFS | |||||||||||
|
Fibronectin + | |||||||||||
| Yun et al | 2014 | Oncology | Colorectal | TMA IHC | 409 | NT | 0.802 (0.437–1.474) | 0.478 | OS | ||
| |
Nitta et al |
2014 |
BJC |
Bile duct |
TMA IHC |
117 |
0.0092 |
1.08 (0.64–1.79) |
0.9093 |
OS |
|
|
Summary: 1/1 significant difference in OS, 0/2 signficance as independent variable of OS | |||||||||||
|
alpha-SMA + | |||||||||||
| Yun et al | 2014 | Oncology | Colorectal | TMA IHC | 409 | NT | 0.997 (0.611–1.627) | 0.991 | OS | ||
| |
Nitta et al |
2014 |
BJC |
Bile duct |
TMA IHC |
117 |
0.5216 |
NT |
|
OS |
|
|
Summary: 0/1 significant difference in OS, 0/1 independent variable of OS | |||||||||||
|
N-cadherin + | |||||||||||
| Jie et al | 2013 | Dig Dis Sci | Colorectal | WTB IHC | 108 | 0.41 | NT | OS | |||
| Nitta et al | 2014 | BJC | Bile duct | TMA IHC | 117 | 0.0004 | 2.53 (1.36–4.54) | 0.0038 | OS | ||
| |
Kim et al |
2009 |
Histopathol |
Gastric |
TMA IHC |
598 |
0.002 |
Not shown |
NS |
OS |
|
|
Summary: 2/3 significant differences in OS, 1/1 independent variable of OS | |||||||||||
|
TGF-Beta + | |||||||||||
| |
Calon et al |
2012 |
Cancer Cell |
Colorectal |
qPCR |
335 |
Not shown |
100 |
<0.0001 |
DFS |
|
|
Summary: 1/1 signficance as independent variable of OS | |||||||||||
|
miR | |||||||||||
| miR-132 (low) | Zheng et al | 2014 | World J Gastroenterol | Colorectal | qPCR | 62 | <0.001 | NT | DFS | ||
| miRNA-19b (high) | Kahlert | 2011 | Cancer Sci | Colorectal liver mets | qPCR | 30 | 0.04 | NT | OS | ||
| miRNA-19b (high) | Kahlert | 2011 | Cancer Sci | Colorectal liver mets | qPCR | 30 | 0.002 | NT | DFS | ||
| miR-194 (high) | Kahlert | 2011 | Cancer Sci | Colorectal liver mets | qPCR | 30 | 0.003 | NT | OS | ||
| miR-194 (high) | Kahlert | 2011 | Cancer Sci | Colorectal liver mets | qPCR | 30 | 0.008 | NT | DFS | ||
| miR-212 (low) | Meng et al | 2013 | Gastroenterology | Colorectal | qPCR | 180 | 0.0015 | 0.403 (0.195–0.829) | 0.014 | OS | HR for high miR-212 |
| miR-212 (low) | Meng et al | 2013 | Gastroenterology | Colorectal | qPCR | 180 | 0.0045 | NT | DFS | ||
| miR-30a (low) |
Liu et al |
2014 |
Febs Letters |
Hepatocellular |
qPCR |
63 |
0.015 |
3.2 (1.5–6.8) |
0.002 |
DFS |
|
|
Summary: 3/3 significant differences in OS, 5/5 significant difference in DFS, 1/1 signficance as independent variable of OS, 1/1 significance as independent variable of DFS | |||||||||||
|
Combination (“mesenchymal phenotype”) | |||||||||||
| Vim: E-cad ratio >1.24 | Mashita et al | 2014 | J Surg Oncol | Colorectal | qPCR | 150 | 0.0085 | 1.48 (0.47–4.35) | 0.485 | DFS | |
| Snail1+, Vimentin +, E-cad −, CD44 + | Ryu et al | 2012 | Hum Pathol | Gastric | TMA IHC | 276 | <0.001 | 2.072 (1.077–3.986) | 0.29 | DFS | |
| Snail1+, Vimentin +, E-cad -, CD44 + | Ryu et al | 2012 | Hum Pathol | Gastric | TMA IHC | 276 | <0.001 | 1.930 (0.993–3.752) | 1.052 | OS | |
| Low E-cad, vimentin + | Lahat et al | 2014 | Ann Surg Oncol | Pancreas (IPMN) | WTB IHC | 33 | 0.007 | 1.93 (1.4–3.77) | 0.05 | OS | |
| Twist +, Bmi-1 + |
Ishikawa et al |
2014 |
J Gastroenterol Hepatol |
Pancreatic (IPMN) |
WTB IHC |
35 |
<0.05 |
NT |
|
DFS |
|
| Summary: 2/2 significant differences in OS, 3/3 significant difference in DFS, 1/2 signficance as independent variable of OS, 0/2 significance as independent variable of DFS | |||||||||||
Abbreviations: CoxPH=Cox proportional hazards multivariate analysis; CSS=cancer-specific survival; DFS=disease-free survival; HR=hazard ratio; IHC=immunohistochemistry; IPMN=intraductal papillary mucinous neoplasm; KMC LRT=Kaplan–Meier survival curve log-rank test; NS=not significant; NT=not tested; OS=overall survival; qPCR=quantitative PCR; TMA=tissue microarray; WTB=whole tissue blocks.
Recent studies showing the usefulness of various markers of EMT, such as transcription factors, cytoskeletal markers and micro-RNAs are summarised.
In addition to transcriptional changes, EMT in cancer promotes similar changes to the cytoskeleton as developmental EMT, with cell–cell adherens junctions becoming more labile and cell migration increasing. Although we know almost nothing about how this works in pancreatic and intestinal cancers, studies from other cell types might inform future research. For example, in A431 human squamous carcinoma cells, E-cadherin mobility and turnover at junctions increases in invading tumours (Serrels et al, 2009). In many cell types, collective invasion, where cells move together in strands, but maintain some junctional contacts with neighbours, can be mediated by the loss of E-cadherin and full or partial replacement with N-cadherin (reviewed recently in Etienne-Manneville (2014)). N-cadherin promotes mobility and has recently been found to treadmill along the adjacent side interfaces between migrating astrocytes to promote collective migration (Peglion et al, 2014). N-cadherin has been implicated in EMT changes in colorectal cancer (Hu et al, 2014), so these mechanisms may be relevant for tumour invasion. In addition to the breakdown of cell–cell adhesions, proteins such as N-WASP, Scar/WAVE complex, cortactin and Arp2/3 complex mobilise away from junctions and towards the leading edges of cells where they actively induce protrusions that can interact with and remodel the surrounding stroma (Figure 1 and reviewed in McNiven (2013)). Actin polymerisation driven by these protein assemblies drives cell protrusion and migration away from the tumour site. Cells assemble matrix-degrading structures termed invadopodia that contain major actin nucleation proteins and that interface with adhesion and matrix metalloprotease secretion machinery (for recent reviews, see McNiven(2013); Beaty and Condeelis (2014)). The actin bundling protein fascin is also a major target of cancer EMT and is thought to promote invasiveness, migration and metastatic potential in multiple cancer types, including pancreatic (Li et al, 2014) and colorectal (Hashimoto et al, 2006). Secretion of matrix metalloproteases increases during EMT (Ota et al, 2009) and cells gain the ability to migrate through three-dimensional (3D) extracellular matrix and to breach tight barriers such as the basement membranes that surround epithelial organs.
The appearance of tumour buds, or clusters of invaded cells surrounding a tumour, is a feature particularly associated with metastasis and poor prognosis in cancers of the gastrointestinal tract, including colorectal and pancreatic cancer (Park et al, 2005; Karamitopoulou et al, 2013). These budding cells have many features that support the hypothesis that they have undergone EMT, including decrease or loss of E-cadherin, expression of mesenchymal markers and activation of the Wnt signalling pathway (Lugli et al, 2012). A recent study of invasive cancers used 3D reconstruction of serial sections of tumour margins to demonstrate that human pancreatic, lung, breast and colorectal cancers invade almost exclusively as collective strands rather than as individual cells (Bronsert et al, 2014). Tumour buds were visualised in 3D reconstructions as strands of cells still attached to the primary tumour that had altered E-cadherin staining, increased expression of Zeb1 and altered polarity features. It would be interesting to know how EMT changes in tumour buds correlate with actin cytoskeletal mobilisation and reorganisation, but this awaits more advanced imaging methods and cancer models.
We have mostly discussed the role of the actin machinery in migration of cells away from the primary tumour, but metastasis involves many steps, including also seeding of escaped tumour cells in distant sites. Two recent studies highlight the actin cytoskeletal and integrin-dependent pathways that contribute to seeding of cancer cells in the lungs and formation of early metastatic nodules (Shibue et al, 2012; Shibue et al, 2013). The authors identify actin-rich filopod-like protrusions (FLP) that contain integrin and allow cells to attach to matrix and activate their prosurvival and growth pathways via focal adhesion kinase. These FLP structures are enhanced by the actin nucleation formin protein mDia2 and regulated by the small GTPase Rif (Shibue et al, 2012). In addition, FLP are enhanced by expression of the integrin:actin linker protein β-parvin (Shibue et al, 2013). It is not clear yet whether this pathway is controlled by cancer associated EMT, but expression of the EMT transcription factors Twist or Snail or knockdown of E-cadherin enhanced the FLP pathway, suggesting a potential connection (Shibue et al, 2013).
Summary
The formation of epithelia by multicellular organisms has required that cells evolve mechanisms to tightly control protein expression, activation status and localisation. Most epithelial tissues have some plasticity in their differentiation status and can convert between epithelial and mesenchymal if the right signals are given. During cancer, the EMT programme becomes unregulated or misregulated to produce changes that resemble type-1 developmental EMT, but that also have significant differences. Many different signals can provoke EMT-like changes in cancer that lead to breakdown or mobilisation of epithelial junctions and enhance the progression and spread of the cancer. There is a wealth of evidence from the clinical literature suggesting a positive correlation between EMT signalling and transcriptional changes and poor outcome in many cancers, including those of the pancreas and intestinal tract.
Very little is known about how the motility machinery reorganises during EMT. Although the loss of E-cadherin junctions is the most prominent feature of most EMT transitions, many other changes occur and the actin nucleation-promoting proteins such as N-WASP, Scar/WAVE and cortactin have specific roles both in epithelial and mesenchymal cells. Rho-family GTPases participate in regulation of the actin cytoskeleton in both epithelial and mesenchymal cells and seem to have important roles in developmental and cancer-related EMT.
Cancer EMT is clearly very different from developmental EMT, but parallels exist and EMT-related changes in cancer correlate strongly with progression and poor outcome. Cancer EMT can be partial and both solid tumours and circulating tumour cells may co-express epithelial and mesenchymal markers (Armstrong et al, 2011). Furthermore, the mesenchymal status is not sufficient in all cases to confer metastasis, as there are some benign tumours (which by definition, do not usually metastasise) that typically show aggressive local invasion, for example, giant cell tumour of the bone (Fletcher et al, 2002) and ameloblastoma (Barnes, 2005). The importance of EMT in cancer has been challenged (Tarin et al, 2005) and it appears that many tumours that histologically are ‘epithelial' can be aggressively metastatic. Many questions remain about which aspects of EMT promote metastatic dissemination and how cancers hijack developmental EMT to progress. Likewise, the precise regulation of key actin motility proteins during EMT and MET is only beginning to be understood and may provide insight that will be clinically useful.
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119 (6:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, Bertolino P, Ibanez CF. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol. 2007;311 (2:500–511. doi: 10.1016/j.ydbio.2007.08.060. [DOI] [PubMed] [Google Scholar]
- Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9 (8:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. Pathology and Genetics of Head and Neck Tumours. IARC Press: Lyon; 2005. [Google Scholar]
- Beaty BT, Condeelis J. Digging a little deeper: The stages of invadopodium formation and maturation. Eur J Cell Biol. 2014;93 (10-12:438–444. doi: 10.1016/j.ejcb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014;27 (1:9–14. [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98 (18:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J, Hopt U, Keck T, Stickeler E, Passlick B, Schilling O, Reiss C, Vashist Y, Brabletz T, Berger J, Lotz J, Olesch J, Werner M, Wellner U. Cancer cell invasion and EMT marker expression - a three-dimensional study of the human cancer-host interface. J Pathol. 2014;234 (3:410–422. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9 (6:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Halberg RB, Burch RP, Dove WF. Intestinal adenomagenesis involves core molecular signatures of the epithelial-mesenchymal transition. J Mol Histol. 2008;39 (3:283–294. doi: 10.1007/s10735-008-9164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuai M, Hughes D, Weijer CJ. Collective epithelial and mesenchymal cell migration during gastrulation. Curr Genomics. 2012;13 (4:267–277. doi: 10.2174/138920212800793357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Lopez A, Moreno-Bueno G, Cano A. Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives. Cancer Manag Res. 2014;6:205–216. doi: 10.2147/CMAR.S38156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Nelson WJ, Weis WI. A polarized epithelium organized by beta- and alpha-catenin predates cadherin and metazoan origins. Science. 2011;331 (6022:1336–1339. doi: 10.1126/science.1199633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler M, Aust D, Weitz J, Pilarsky C, Grutzmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed Res Int. 2014;2014:474905. doi: 10.1155/2014/474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. Neighborly relations during collective migration. Curr Opin Cell Biol. 2014;30C:51–59. doi: 10.1016/j.ceb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61 (5:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fletcher CDM, Unni KK, Mertens F. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France; 2002. [Google Scholar]
- Goswami S, Philippar U, Sun D, Patsialou A, Avraham J, Wang W, Di Modugno F, Nistico P, Gertler FB, Condeelis JS. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastas. 2009;26 (2:153–159. doi: 10.1007/s10585-008-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95 (1:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner TU, Kesavan G, Stahlberg A, Semb H. Rac1 regulates pancreatic islet morphogenesis. BMC Dev Biol. 2009;9:2. doi: 10.1186/1471-213X-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SP, Gambin Y, Gomez GA, Verma S, Giles N, Michael M, Wu SK, Guo Z, Johnston W, Sierecki E, Parton RG, Alexandrov K, Yap AS. Cortactin scaffolds Arp2/3 and WAVE2 at the epithelial zonula adherens. J Biol Chem. 2014;289 (11:7764–7775. doi: 10.1074/jbc.M113.544478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer. 2006;6:241. doi: 10.1186/1471-2407-6-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C, Dupre-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-Lopez LA, Vincent JP, Itoh Y, Hosoya H, Pichaud F, Fujita Y. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol. 2009;11 (4:460–467. doi: 10.1038/ncb1853. [DOI] [PubMed] [Google Scholar]
- Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo H, Tian T, Ruan ZP, Kang XM, Wang J, Wang SH, Nan KJ. SDF-1/CXCR4 promotes epithelial-mesenchymal transition and progression of colorectal cancer by activation of the Wnt/beta-catenin signaling pathway. Cancer Lett. 2014;354 (2:417–426. doi: 10.1016/j.canlet.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Ishii H, Saitoh M, Sakamoto K, Kondo T, Katoh R, Tanaka S, Motizuki M, Masuyama K, Miyazawa K. Epithelial splicing regulatory proteins 1 (ESRP1) and 2 (ESRP2) suppress cancer cell motility via different mechanisms. J Biol Chem. 2014;289 (40:27386–27399. doi: 10.1074/jbc.M114.589432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson KA, Grapin-Botton A. Development and diseases of the pancreas. Clin Genet. 2002;62 (1:14–23. doi: 10.1034/j.1399-0004.2002.620102.x. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119 (6:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitopoulou E, Zlobec I, Born D, Kondi-Pafiti A, Lykoudis P, Mellou A, Gennatas K, Gloor B, Lugli A. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer. 2013;49 (5:1032–1039. doi: 10.1016/j.ejca.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Kesavan G, Lieven O, Mamidi A, Ohlin ZL, Johansson JK, Li WC, Lommel S, Greiner TU, Semb H. Cdc42/N-WASP signaling links actin dynamics to pancreatic beta cell delamination and differentiation. Development. 2014;141 (3:685–696. doi: 10.1242/dev.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan G, Sand FW, Greiner TU, Johansson JK, Kobberup S, Wu X, Brakebusch C, Semb H. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139 (4:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A, Jamieson NB, MacKay CJ, Carter CR, Leung HY, Yamashiro S, Blyth K, Sansom OJ, Machesky LM. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology. 2014;146 (5:1386–96 e1-17. doi: 10.1053/j.gastro.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22 (4:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer. 2012;106 (11:1713–1717. doi: 10.1038/bjc.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA. Breaking away: matrix remodeling from the leading edge. Trends Cell Biol. 2013;23 (1:16–21. doi: 10.1016/j.tcb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19 (11:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci USA. 2009;106 (48:20318–20323. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum. 2005;48 (8:1597–1602. doi: 10.1007/s10350-005-0060-6. [DOI] [PubMed] [Google Scholar]
- Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol. 2014;16 (7:639–651. doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- Pignatelli J, Goswami S, Jones JG, Rohan TE, Pieri E, Chen X, Adler E, Cox D, Maleki S, Bresnick A, Gertler FB, Condeelis JS, Oktay MH. Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Sci Signal. 2014;7 (353:ra112. doi: 10.1126/scisignal.2005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis JM, Habener JF. Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr Patterns. 2007;7 (4:471–479. doi: 10.1016/j.modgep.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA. 2011;108 (48:19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer metast Rev. 2009;28 (1-2:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- Serrels A, Timpson P, Canel M, Schwarz JP, Carragher NO, Frame MC, Brunton VG, Anderson KI. Real-time study of E-cadherin and membrane dynamics in living animals: implications for disease modeling and drug development. Cancer Res. 2009;69 (7:2714–2719. doi: 10.1158/0008-5472.CAN-08-4308. [DOI] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012;2 (8:706–721. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Weinberg RA. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell. 2013;24 (4:481–498. doi: 10.1016/j.ccr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19 (11:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF.2005The fallacy of epithelial mesenchymal transition in neoplasia Cancer Res 65(145996–6000.; discussion 6000-1. [DOI] [PubMed] [Google Scholar]
- Woodham EF, Machesky LM. Polarised cell migration: intrinsic and extrinsic drivers. Curr Opin Cell Biol. 2014;30C:25–32. doi: 10.1016/j.ceb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Wu SK, Gomez GA, Michael M, Verma S, Cox HL, Lefevre JG, Parton RG, Hamilton NA, Neufeld Z, Yap AS. Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol. 2014;16 (2:167–178. doi: 10.1038/ncb2900. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gao XD, Lee JH, Huang H, Tan H, Ahn J, Reinke LM, Peter ME, Feng Y, Gius D, Siziopikou KP, Peng J, Xiao X, Cheng C. Cell type-restricted activity of hnRNPM promotes breast cancer metastasis via regulating alternative splicing. Genes Dev. 2014;28 (11:1191–1203. doi: 10.1101/gad.241968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119 (6:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]