Abstract
Background:
High-temperature-required protein A2 (HtrA2), a protein relating with apoptosis in a caspases-dependent and non-dependent manner, has been reported to be associated with chemosensitivity in several human cancers.
Methods:
Tissue microarrays made from 142 patients with high-grade serous ovarian adenocarcinoma were evaluated to assess whether HtrA2 expression was related with several clinical parameters.
Results:
Negative HtrA2 expression was observed in 36 cases (25%) of the patients, and related with significantly lower response rates of primary chemotherapy than those with positive HtrA2 expression (56% vs 83%, P<0.01). In addition, negative HtrA2 expression was identified as an independent worse prognostic factor for progression-free survival and overall survival by multivariate analyses. Furthermore, HtrA2 downregulation modulated sensitivity to platinum in serous ovarian cancer cells in vitro.
Conclusions:
HtrA2 expression was a predictor for sensitivity to chemotherapy, and could be a candidate of molecular target in the treatment of high-grade serous ovarian cancers.
Keywords: ovarian cancer, high-grade serous adenocarcinoma, platinum sensitivity, high-temperature-required protein A2 (HtrA2)
Ovarian cancer is one of the lethal gynaecological malignancies despite aggressive debulking surgery and primary chemotherapy, which is explained by a high rate of recurrence after the standard therapy (Cannistra, 2004). Among several histological subtypes, the serous subtype showed a relatively higher response rate to platinum-based chemotherapy in comparison with type I cancers such as clear-cell and the mucinous subtype (Shimada et al, 2009; Takano et al, 2012). Platinum agents are key drugs for primary chemotherapy for ovarian cancers; however, clinical biomarkers for prediction of sensitivity to the therapy have not been established.
High-temperature-required protein A (HtrA) family of serine protease was well conserved from bacteria to humans (Lipinska et al, 1990), and was associated with several important conditions such as inflammation, apoptosis, and protection against stress stimuli (Clausen et al, 2002). Until now, four human HtrAs have been identified: HtrA1 (Hu et al, 1998), HtrA2 (Faccio et al, 2000), HtrA3 (Nie et al, 2003), and HtrA4 (Inagaki et al, 2012). Among them, it has been suggested that HtrA1 and HtrA2 proteins were associated with chemosensitivity in ovarian cancer cell lines (Yang et al, 2005; Chien et al, 2006). HtrA2, a mitochondrial pro-apoptotic serine protease, was one of the X-chromosome-linked inhibitor of apoptosis (XIAP) inhibitors. It indirectly induced permeabilisation of the outer mitochondrial membrane and subsequent cytochrome c-dependent caspases activation (Suzuki et al, 2004). Also, in response to cellular stress, HtrA2 releases from mitochondria to cytosol where it functions as one of apoptotic proteins (Izeradjene et al, 2004). Once released, HtrA2 promotes caspases activation by direct binding to XIAP and attenuates XIAP inhibition of caspases (Suzuki et al, 2004). Therefore, HtrA2 is a protein to enhance caspases activation through multiple pathways. However, in ovarian cancers, the relationship between HtrA2 protein expression and clinical parameters has not been fully understood.
In the present study, the association between HtrA2 expression and clinicopathological features was evaluated using tissue microarrays made from 142 cases with high-grade serous ovarian cancers. Also, the experiment to determine whether downregulation of HtrA2 could modulate sensitivity to cisplatin was performed using an in vitro model. The usefulness of HtrA2 as a biomarker of high-grade serous ovarian cancers has been analysed in the present study.
Materials and methods
Patients and tissue microarray
Tissue blocks from 142 patients with pure high-grade serous ovarian cancer who received surgery at the National Defense Medical College Hospital between 1984 and 2008 were used. All specimens were reviewed, and patients with pure high-grade serous ovarian cancers were enrolled in the study. Patients receiving neoadjuvant chemotherapy and those with histology including other types were excluded. The mean age of all patients was 54.5 years. The stage distributions of all patients according to the International Federation of Obstetrics and Gynecology (FIGO) classification were 16 in stage I, 11 in stage II, 79 in stage III, and 36 in stage IV. Twenty-eight patients had no residual tumours (RTs) after a primary debulking surgery, 23 had 1 cm< RTs, and 91 had 1 cm> RTs. Sixty-six patients underwent chemotherapy using regimens containing taxane and platinum (paclitaxel plus carboplatin (n=57) and docetaxel plus cisplatin (n=9)). Seventy-six patients received conventional platinum-based regimens (cyclophosphamide (C), adriamycin (A), and cisplatin (P) (n=65), or C plus P (n=11)).
Two core specimens, 1.5 mm in diameter, for each case were taken from cancer blocks and transferred to recipient blocks using a Tissue Microarrayer (Beecher Instrument, Silver Spring, MD, USA). All specimens were cut into 4-μm-thick slices to make sections for immunohistochemical (IHC) staining. Satisfactory IHC staining was obtained in all cases. The research project was approved by the Institutional Ethical Review Board Committee of the National Defense Medical College, Tokorozawa, Japan.
IHC staining and interpretation
For IHC staining, we used a rabbit monoclonal antibody for HtrA2 (EPR22; dilution 1 : 100 Abcam, Cambridge, MA, USA). Tissue microarray slides were deparaffinised in xylene and hydrated with alcohol, boiled in an autoclave at 121 °C for 15 min in 0.01 mol l−1 citrate buffer (pH 6.0), and then allowed to cool at room temperature. Endogenous peroxidase activity was blocked by 0.3% H2O2/methanol. The slides were incubated at 4 °C overnight with primary antibodies and reacted with the DAKO EnVision+ system-horseradish peroxidase (HRP)-labelled polymer (Dako Denmark A/S, Glostrup, Denmark) as a secondary antibody for 30 min at room temperature. Specific antigen–antibody reactions were visualised with 0.2% diaminobenzine tetrahydrochloride and hydrogen peroxide, and counterstained with Mayer haematoxylin. Negative control studies were performed using an isotype-matched antibody (EPR25A, Abcam), and confirmation of no significant staining in the negative control sections was carried out. As positive control, formalin-fixed paraffin-embedded human colon tissues were stained.
All of the immunostained sections were evaluated, and more than 1000 cells from five high-power fields in each specimen were selected randomly to determine the score. Immunoreactivity was scored according to the staining intensity as follows: weak (0), moderate (1+), or strong (2+) in parts of more than 50% of immunoreactive components. According to the scoring, the patients were divided into two groups: the negative HtrA2 group (score 0), and the positive HtrA2 group (score 1 and score 2). Two observers independently evaluated and interpreted the results of IHC staining without knowledge of the clinical data of each patient. During the course of interpretation of immunohistochemistry, a multiviewer microscope was not provided and any discrepancies between the two observers were resolved by discussion.
Cell lines and culture conditions
Ovarian cancer cell lines, KF28 (Kikuchi et al, 1986), KFr13 (Yamamoto et al, 2000), and MH (Sasa et al, 1993) were used. These were grown as monolayer cultures in RPMI-1640+GlutaMAX (Invitrogen Japan KK, Tokyo, Japan) medium supplemented with 10% fetal bovine serum (Invitrogen Japan KK), 100 U penicillin per ml, and 100 mg streptomycin per ml (Invitrogen Japan KK) in a humidified atmosphere of 5% CO2 at 37 °C, and routinely tested for mycoplasma infection. Protein concentrations were determined using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA).
Transient transfection
Non-specific control siRNA and HtrA2-specific siRNA were purchased from Cell Signaling Technology (Danvers, MA, USA). KF28 cells cultured in 3.5-cm plates were transfected with 300 nM of HtrA2-specific siRNA I or II and control siRNA using Lipofectamine 2000 (Invitrogen Japan KK) according to the manufacturer's specifications. KF28 cells transfected with non-specific control siRNA (KF28-C), and HtrA2-specific siRNA (KF28-H) were further analysed. HtrA2 knockdown was confirmed by western blot analysis in all the experiments.
Cell proliferation and cytotoxicity assay
Cisplatin was obtained from Bristol Meier's Squib Oncology (Tokyo, Japan). KF28, KF28-C, KF28-H, KFr13, and MH were seeded onto 96-well plates at ∼1 × 104 or 4 × 104 cells per cm2 for cytotoxicity assays. These cells were treated with cisplatin after the monolayer was formed. IC50 was measured after 5 days from the treatment of cisplatin at different concentrations for 24 h. Cell viability was determined by the MTT method using Tetra Color ONE (Seikagaku Corporation, Tokyo, Japan) according to the manufacturer's instructions. For the evaluation of IC50 of KF28-C and KF28-H, cells were transfected with siRNA 5 days before they were treated with different concentrations of cisplatin for 24 h, and IC50 was determined immediately after cisplatin exposure.
Preparation of cell lysate for western blot analysis
Protein lysates of all cells were extracted with RIPA buffer (Wako Pure Chemical Industries Ltd, Osaka, Japan) including Complete Protease Inhibitor Cocktail and PhosSTOP Phosphatase Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Ten μg of the protein lysates were loaded onto Mini-PROTEIN TGXTM gel (Bio-Rad Laboratories). After electrophoresis, proteins were transferred to PVDF membranes using the Trans-Blot TurboTM Transfer System Transfer Pack (Bio-Rad Laboratories). Subsequently, the membranes were blocked for 1 h in 4% BSA in TBS with 0.5% Tween-20 (PBS-T) and incubated overnight at 4 °C with primary antibody in TBS-T with 4% BSA. Antibodies concentrations in this study were 1/2500 for rabbit anti-HtrA2 (Cell Signaling Technology) and 1/5000 for HRP-conjugated β-actin (Cell Signaling Technology). After washing three times with TBS-T, membranes were incubated using HRP-conjugated anti-rabbit secondary antibody (Cell Signaling Technology) for 1 h at room temperature. After washing three times with TBS-T, they were visualised using the ECL detection system (GE Healthcare UK Ltd, England, UK). Pictures were taken using a LAS-3000 (Fujifilm, Minato, Tokyo). Protein expression was determined densitometrically and normalised against β-actin expression using the Multi Gauge version 3.1 software (Fujifilm).
Statistical analysis
The Stat View software version 5.0 (SAS Institution Inc., Cary, NC, USA) was used for statistical analysis. Progression-free survival (PFS) was defined as the interval between first treatment and death or the date of disease progression. Overall survival (OS) was defined as the interval between first treatment and death. The stage of cancer was determined according to the FIGO system. Performance status (PS) was evaluated by ECOG (Eastern Cooperative Oncology Group) criteria. Response rate was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) criteria (Therasse et al, 2000). The χ2-test, Fisher's exact test and Mann–Whitney U-test were used to evaluate differences in the correlation between expression of HtrA2 and clinicopathological parameters. PFS and OS curves were generated using the method of Kaplan–Meier. The comparison of the survival distributions was made using a log-rank test. Cox's proportional hazards model was used for multivariate analysis of PFS and OS. All experiments were repeated independently at least three times. All values were presented as mean±s.d. Statistical significance between two groups was determined using a two-tailed t-test. Statistical significance was defined as a P-value of <0.05.
Results
IHC analysis and the association of clinicopathological features and prognosis according to the levels of HtrA2
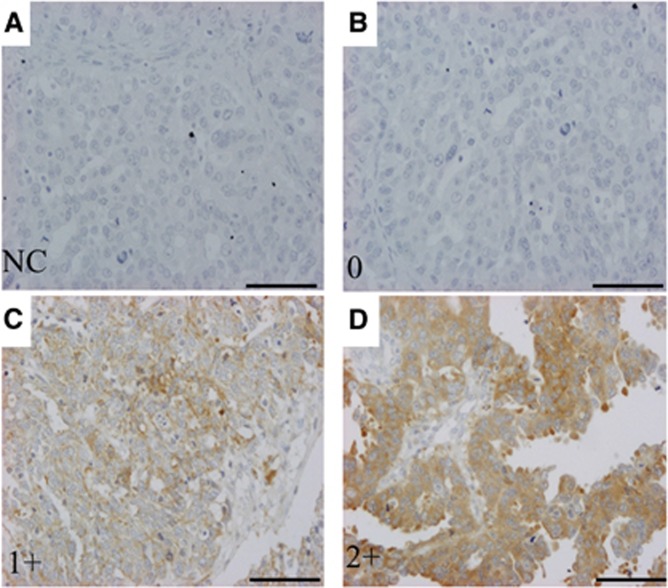
A total of 142 patients with high-grade serous ovarian cancers were identified. Among them, 36 cases (25%) had score 0, 89 cases (63%) had score 1+, and 17 patients (12%) had score 2+ (Figure 1). Overall, negative HtrA2 was observed in 36 (25%) patients, and positive HtrA2 was detected in 106 (75%) cases.
Figure 1.
Immunochemical stains of HtrA2 in tissue microarray-based samples of ovarian serous cell carcinomas ( × 20). Immunohistochemical stains by a rabbit monoclonal antibody for HtrA2 (EPR22; dilution 1 : 100, Abcam) was evaluated. Immunoreactivity was scored according to the staining intensity as follows: weak (0), moderate (1+), and strong (2+). (A) Negative control (NC). (B) Score 0. (C) Score 1+. (D) Score 2+. Scale bar, 100 μm.
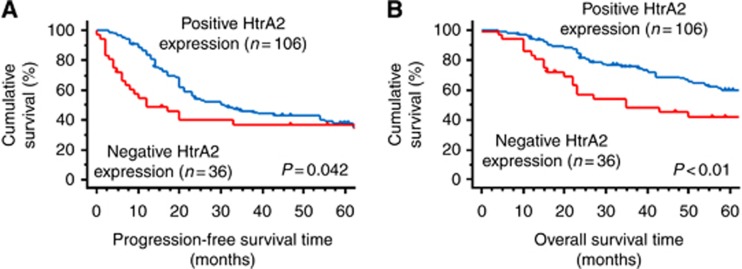
There were no significant differences in age, PS, FIGO stage, and RT diameter according to HtrA2 expression (Table 1). The cases with negative HtrA2 expression showed a lower response rate compared with those with positive HtrA2 expression (56% vs 85%, P<0.01, Table 2). Multivariate analysis for PFS and OS revealed that negative HtrA2 expression was an independent prognostic factor for PFS (hazard ratio (HR)=1.87; 95% confidence interval (CI), 1.16–3.03; P=0.01) and OS (HR=2.00; 95% CI, 1.15–3.42; P=0.01, Table 3). Furthermore, the cases with negative HtrA2 expression had worse PFS and OS than those with positive HtrA2 expression (Figure 2A: PFS, P=0.042; Figure 2B: OS, P<0.01).
Table 1. Characteristics of the patients with high-grade serous adenocarcinoma (n=142).
| Clinicopathological variables | HtrA2-negative expression (n=36) (%) | HtrA2-positive expression (n=106) (%) | P-value |
|---|---|---|---|
|
Age (years) | |||
| <55 | 20 (56) | 57 (54) | 0.85 |
| ⩾55 |
16 (44) |
49 (46) |
|
|
ECOG PS | |||
| 0 | 33 (92) | 102 (96) | 0.37 |
| 1/2 |
3 (8) |
4 (4) |
|
|
FIGO stage | |||
| I | 4 (11) | 12 (11) | 0.70 |
| II | 4 (11) | 7 (7) | |
| III | 21 (58) | 58 (55) | |
| IV |
7 (20) |
29 (27) |
|
|
Residual tumour | |||
| None | 9 (25) | 19 (18) | 0.48 |
| <1 cm | 4 (11) | 19 (18) | |
| >1 cm |
23 (64) |
68 (64) |
|
|
Adjuvant chemotherapy | |||
| Conventional platinum-based chemotherapy | 34 (94) | 42 (40) | <0.01 |
| Taxane+platinum | 2 (6) | 64 (60) | |
Abbreviations: ECOG=Eastern Cooperative Oncology Group; FIGO=The International Federation of Gynecology and Obstetrics; PS=performance status.
Table 2. Response of primary chemotherapy in the patients with evaluable diseases (n=114).
| HtrA2-negative expression (n=27) (%) | HtrA2-positive expression (n=87) (%) | P-value | |
|---|---|---|---|
|
Response | |||
| CR | 11 (41) | 62 (71) | <0.01 |
| PR | 4 (15) | 12 (14) | |
| SD | 3 (11) | 3 (3) | |
| PD |
9 (33) |
10 (12) |
|
| Response rate | 56% | 85% | |
Abbreviations: CR=complete response; PD=progressive disease; PR=partial response; SD=stable disease.
Response rate (%)=CR+PR/all cases.
Table 3. Multivariate analyses for progression-free survival and overall survival in the patients with ovarian high-grade serous adenocarcinomas.
|
Progression-free survivals |
Overall survivals |
|||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
Age (years) | ||||||
| >55 vs ⩽55 |
0.76 |
(0.50–1.16) |
0.20 |
0.75 |
(0.46–1.21) |
0.23 |
|
ECOG PS | ||||||
| 1/2 vs 0 |
2.09 |
(0.94–4.63) |
0.07 |
3.73 |
(1.55–9.01) |
<0.01 |
|
FIGO stage | ||||||
| III/IV vs I/II |
1.80 |
(0.77–4.23) |
0.17 |
0.99 |
(0.36–2.73) |
0.98 |
|
Residual tumour | ||||||
| Present vs absent |
5.68 |
(2.13–15.1) |
<0.01 |
5.29 |
(1.71–16.1) |
<0.01 |
|
Chemotherapy regimen | ||||||
| Conventional platinum based vs taxane+platinum |
1.06 |
(0.68–1.65) |
0.81 |
1.63 |
(0.97–2.75) |
0.07 |
|
HtrA2 expression | ||||||
| Negative vs positive | 1.87 | (1.16–3.03) | 0.01 | 2.00 | (1.15–3.45) | 0.01 |
Abbreviations: CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; FIGO=The International Federation of Gynecology and Obstetrics; HR=hazard ratio; PS=performance status.
Figure 2.
Progression-frees survival (PFS) and overall survival (OS) curves of the 142 patients with high-grade serous adenocarcinomas according to expression levels of HtrA2. (A) PFS of the patients with negative HtrA2 expression was significantly worse than that with positive HtrA2 expression (P=0.042). (B) OS of the patients with negative HtrA2 expression was significantly worse than that with positive HtrA2 expression (P<0.01).
The association between cisplatin sensitivities and HtrA2 protein expressions in serous ovarian cancer cells
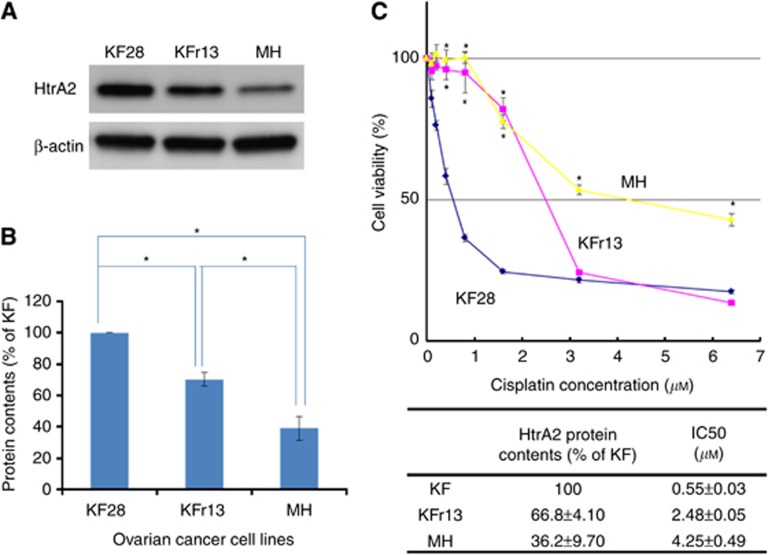
Endogenous HtrA2 protein levels and IC50 of cisplatin were evaluated for KF28, KFr13, and MH. HtrA2 expression was significantly lower in KFr13 and MH, compared with that of KF28 (Figure 3A and B; P<0.01 and P<0.01). IC50 of cisplatin was 0.55±0.03 in KF28, 2.48±0.05 in KFr13, and 4.25±0.49 μM in MH cells, respectively (Figure 3C).
Figure 3.
HtrA2 protein expression and cisplatin sensitivity. (A) HtrA2 protein concentrations of KF28, KFr13, and MH were analysed by western blotting. (B) HtrA2 protein expression level that was densitometrically normalised against β-actin expression is shown. Blots were scanned and quantified by densitometry. HtrA2 protein concentration was significantly lower in KFr13 and MH compared with that of KF28. MH had significantly lower HtrA2 protein expression in comparison with KFr13. *P<0.01. (C) Cell viability of KF28, KFr13, and MH at the fifth day from cisplatin treatment was evaluated using the MTT assay. IC50 of KFr13 was higher than KF28 (P<0.01), and IC50 of MH was higher than that of KFr13 (P=0.01). The data represented the mean±s.d. of at least three experiments. *P<0.01.
HtrA2 downregulation was associated with chemoresistance in ovarian serous cell lines
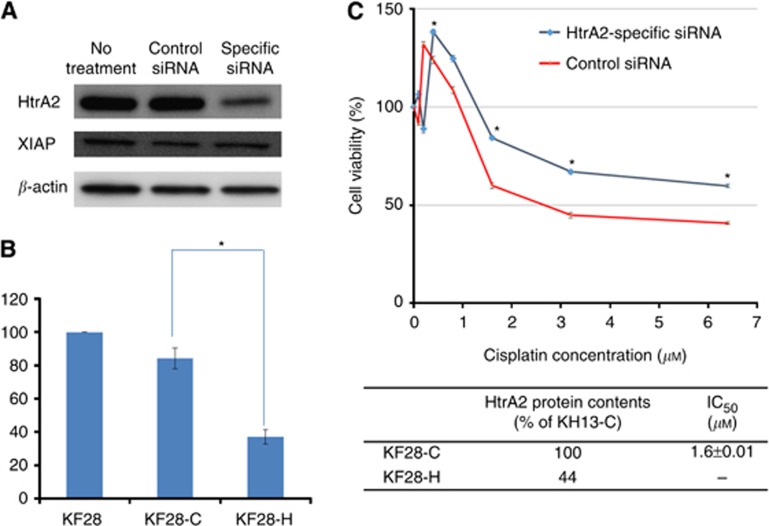
Further, an experiment to evalute whether HtrA2 downregulation by HtrA2-specific siRNA decreased sensitivity to cisplatin was performed in a cisplatin-sensitive cancer cell line, KF28. HtrA2 expression was significantly lower in KF28-H compared with that of KF28-C (Figure 4A and B, P<0.01). However, XIAP expression was not modulated by HtrA2 downregulation by HtrA2-specific siRNA. Five days after transfection, sensitivity to cisplatin was measured by 24-h cisplatin treatment, and KF28-H showed resistance to cisplatin treatment (Figure 4C).
Figure 4.
Downregulation of HtrA2 by a specific siRNA decreased the sensitivity to cisplatin in cisplatin-sensitive serous ovarian cancer cells. (A) Downregulation of HtrA2 by a specific siRNA was observed in KF28 cells by western blotting analysis. (B) HtrA2 protein expression level of KF28 cells transfected with non-specific control siRNA (KF28-C), and HtrA2-specific siRNA (KF28-H) were analysed. Blots were scanned and quantified by densitometry. HtrA2 expression level of KF28-H was significantly lower than that of KF28-C. *P<0.01 (C) Cell viability was evaluated by the MTT assay. Five days after transfection with siRNA, sensitivity to cisplatin was measured by 24-h cisplatin treatment, and KF28-H showed resistance to cisplatin treatment. The data represented the mean±s.d. of at least three experiments. *P<0.01.
Discussion
In the present study, IHC analysis demonstrated that 36 (25%) cases with high-grade serous ovarian cancer had negative HtrA2 expression. The cases with negative HtrA2 expression were associated with significantly lower response rates of primary chemotherapy. Previous reports demonstrated the mRNA levels of HtrA1 and HtrA3, and the protein expression of HtrA3 were significantly lower in ovarian cancer tissues compared with those in normal ovarian tissues (Chien et al, 2004); Narkiewicz et al, 2008). However, some reports did not show statistically significant differences of mRNA and protein expressions of HtrA2 between ovarian cancer and normal ovary tissues (Narkiewicz et al, 2008; Singh et al, 2013). It might be possible that the difference was derived from inclusion of several histological subtypes, or a small sample size.
Furthermore, negative HtrA2 expression was an independent factor of PFS and OS in addition to RT diameters. To our knowledge, it is the first report to demonstrate the association between the HtrA2 expression and response for the platinum-based chemotherapy, in addition to prognosis. HtrA2 protein expressions could be a biomarker candidate for response to primary chemotherapy in patients with SAC. HtrA2 is involved in a series of biological functions as an inducer of apoptosis (Zurawa-Janicka et al, 2013). A report indicated that inhibition of autophagy enhanced the apoptosis-inducing effects of cisplatin, and activated cell death by releasing HtrA2 from mitochondria (Kaminskyy et al, 2012). In addition, HtrA2 was released from mitochondria to cytosol, and caused annexin A2 cleavage and then cell cycle inhibition or apoptosis (Wang et al, 2009). Several reports confirmed that HtrA2 could affect progression of cancer cells via modulating apoptosis signalling pathway. It is possible that HtrA2 could enhance the apoptosis signalling pathway and alter sensitivity to platinum agents, especially in ovarian cancers.
Previous investigation using ovarian cancer cells suggested that cytosolic HtrA2 level was an important marker in cisplatin-induced apoptosis and was partly regulated by XIAP in vitro (Yang et al, 2005). First, three ovarian serous cancer cell lines tested in the present study clearly showed an inverse correlation of HtrA2 expression and sensitivity to cisplatin. Next, an experiment to determine whether downregulation of HtrA2 could lead to resistance to cisplatin was conducted. As a result, HtrA2 downregulation by siRNA was related with lower sensitivity to cisplatin in ovarian cancer cell lines; however, XIAP expression was not altered by HtrA2 downregulation. It was suggested that sensitivity to platinum was regulated by the XIAP-independent pathway in our system.
In conclusion, HtrA2 protein expression was related with a response rate to cisplatin-based chemotherapy, and prognosis of the patients with high-grade serous ovarian cancer. HtrA2 knockdown was related with resistance to platinum in vitro. HtrA2 expression could be not only a biomarker for response to chemotherapy, but also for prognoses of ovarian cancer patients.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- Chien J, Aletti G, Baldi A, Catalano V, Muretto P, Keeney GL, Kalli KR, Staub J, Ehrmann M, Cliby WA, Lee YK, Bible KC, Hartmann LC, Kaufmann SH, Shridhar V. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest. 2006;116:1994–2004. doi: 10.1172/JCI27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien J, Staub J, Hu SI, Erickson-Johnson MR, Couch FJ, Smith DI, Crowl RM, Kaufmann SH, Shridhar V. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene. 2004;23:1636–1644. doi: 10.1038/sj.onc.1207271. [DOI] [PubMed] [Google Scholar]
- Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- Faccio L, Fusco C, Chen A, Martinotti S, Bonventre JV, Zervos AS. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J Biol Chem. 2000;275:2581–2588. doi: 10.1074/jbc.275.4.2581. [DOI] [PubMed] [Google Scholar]
- Hu SI, Carozza M, Klein M, Nantermet P, Luk D, Crowl RM. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J Biol Chem. 1998;273:34406–34412. doi: 10.1074/jbc.273.51.34406. [DOI] [PubMed] [Google Scholar]
- Inagaki A, Nishizawa H, Ota S, Suzuki M, Inuzuka H, Miyamura H, Sekiya T, Kurahashi H, Udagawa Y. Upregulation of HtrA4 in the placentas of patients with severe pre-eclampsia. Placenta. 2012;33:919–926. doi: 10.1016/j.placenta.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Izeradjene K, Douglas L, Delaney AB, Houghton JA. Casein kinase I attenuates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by regulating the recruitment of fas-associated death domain and procaspase-8 to the death-inducing signaling complex. Cancer Res. 2004;64:8036–8044. doi: 10.1158/0008-5472.CAN-04-0762. [DOI] [PubMed] [Google Scholar]
- Kaminskyy VO, Piskunova T, Zborovskaya IB, Tchevkina EM, Zhivotovsky B. Suppression of basal autophagy reduces lung cancer cell proliferation and enhances caspase-dependent and -independent apoptosis by stimulating ROS formation. Autophagy. 2012;8:1032–1044. doi: 10.4161/auto.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Miyauchi M, Kizawa I, Oomori K, Katoh K. Establishment of a cisplatin-resistant human ovarian cancer cell line. J Natl Cancer Inst. 1986;77:1181–1185. [PubMed] [Google Scholar]
- Lipinska B, Zylicz M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz J, Klasa-Mazurkiewicz D, Zurawa-Janicka D, Skorko-Glonek J, Emerich J, Lipinska B. Changes in mRNA and protein levels of human HtrA1, HtrA2 and HtrA3 in ovarian cancer. Clin Biochem. 2008;41:561–569. doi: 10.1016/j.clinbiochem.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Nie GY, Hampton A, Li Y, Findlay JK, Salamonsen LA. Identification and cloning of two isoforms of human high-temperature requirement factor A3 (HtrA3), characterization of its genomic structure and comparison of its tissue distribution with HtrA1 and HtrA2. Biochem J. 2003;371:39–48. doi: 10.1042/BJ20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasa H, Hisano A, Ishii K, Hirata J, Kikuchi Y, Nagata I, Sendoo A, Aida S, Tamai S, Sugita M. Establishment of a cisplatin-resistant human on\varian cancer cell line. Human Cell. 1993;6:106–113. [PubMed] [Google Scholar]
- Shimada M, Kigawa J, Ohishi Y, Yasuda M, Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T, Kaku T. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009;113:331–334. doi: 10.1016/j.ygyno.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Singh H, Li Y, Fuller PJ, Harrison C, Rao J, Stephens AN, Nie G. HtrA3 is downregulated in cancer cell lines and significantly reduced in primary serous and granulosa cell ovarian tumors. J Cancer. 2013;4 (2:152–164. doi: 10.7150/jca.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Takahashi-Niki K, Akagi T, Hashikawa T, Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ. 2004;11:208–216. doi: 10.1038/sj.cdd.4401343. [DOI] [PubMed] [Google Scholar]
- Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: is there a role of histology-specific treatment. J Exp Clin Cancer Res. 2012;31:53. doi: 10.1186/1756-9966-31-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Wang CY, Lin YS, Su WC, Chen CL, Lin CF. Glycogen synthase kinase-3 and Omi/HtrA2 induce annexin A2 cleavage followed by cell cycle inhibition and apoptosis. Mol Biol Cell. 2009;20:4153–4161. doi: 10.1091/mbc.E09-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Kikuchi Y, Kudoh K, Nagata I. Modulation of cisplatin sensitivity by taxol in cisplatin-sensitive and -resistant human ovarian carcinoma cell lines. J Cancer Res Clin Oncol. 2000;126:168–172. doi: 10.1007/s004320050027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xing H, Gao Q, Chen G, Lu Y, Wang S, Ma D. Regulation of HtrA2 by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cells. Gynecol Oncol. 2005;97:413–421. doi: 10.1016/j.ygyno.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Zurawa-Janicka D, Jarzab M, Polit A, Skorko-Glonek J, Lesner A, Gitlin A, Gieldon A, Ciarkowski J, Glaza P, Lubomska A, Lipinska B. Temperature-induced changes of HtrA2(Omi) protease activity and structure. Cell Stress Chaperones. 2013;18:35–51. doi: 10.1007/s12192-012-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]