Abstract
Background:
The aim of this study was to compare the biophysical profile parameters (BPP), especially amniotic fluid index (AFI), before and after administration of corticosteroids (dexamethasone and betamethasone) and these two groups with each other.
Materials and Methods:
This double-blind clinical trial study was done on 70 patients for 28-34 weeks women having at least one preterm labor in their previous pregnancy has been submitted in Al Zahra and Shahid Beheshti clinic in 2012-2013. 70 women were randomly allocated in two groups. The first group were received 8 mg each 12 h for 4 doses dexamethasone when the patient at risk of preterm labor admitted in clinic or hospitalized and the second group were received 12 mg betamethasone each 24 h similarly. Nonstress test (NST) and BPP and sonography AFI were done in all cases, and then NST was done in the before and 3 days after intervention. Data were analyzed by SPSS software version 20 using t-test, Chi-square, and Fisher's test.
Results:
There was no significant difference between the range of AFI before and after the drug intervention (P > 0.05). Two groups had no significant difference in AFI before and after administration of corticosteroids. No significant difference was seen in NST acceleration tests between the two groups before and after corticosteroids administration.
Conclusion:
Biophysical profile had a significant difference in dexamethasone group respect to that of betamethasone group. Furthermore, the biophysical profile had a significant difference before and after the administration of corticosteroids in two groups. It is noted that AFI had no role in this matter.
Keywords: Amniotic fluid index, betamethasone, biophysical profile, dexamethasone, preterm labor
INTRODUCTION
The term of preterm birth used to define the premature neonates considering pregnancies age of less than 34 weeks and corticosteroids are commonly prescribed to promote embryos’ lung maturity.[1] The amniotic fluid index (AFI) has been an integral component of fetal assessment during antepartum ultrasound examination for >20 years. Decreased amniotic fluid or oligohydramnios, is typically defined as an AFI below 5-cm, which represents the value below the first percentile. In term and near-term gestations, this 5-cm threshold has been associated with increased rates of complications, including small for gestational age neonate, nonreassuring fetal heart rate (FHR), stillbirth, and neonatal death. The amniotic fluid volume is most abundant in the early third trimester, subsequently decreasing until term. Before 34 weeks, the value of 8-cm is below the fifth percentile for gestational age.[2,3] AFI values between 5- and 8-cm have been termed “borderline.” Potential risks associated with borderline amniotic fluid in the preterm period are not fully understood.[2,3] Dexamethasone and betamethasone cause to produce surfactant in fetus lung and thereby it reduces the resistant between layers of airways and sacs to simply slide on each other and eventually easily breathing of neonate after birth preventing respiratory distress syndrome in neonate. Therefore, corticosteroids can be recommended in fetus at risk of preterm labor. The preferred gestational age for administration of dexamethasone and betamethasone is usually 28-34 weeks.[4] The usage dose of betamethasone is 0.17 mg/kg daily for 2 doses to reduce the risk of intraventricular hemorrhage, chronic lung diseases, necrotizing enterocolitis, and retinopathy of prematurity, sepsis, and Neonatal Intensive Care Unit administration. Considering the side effects of each drug, some studies have been done to evaluate the side effects of dexamethasone and betamethasone. Normal range of AFI in fetus is >8-23 cm and its average is 12.8-cm in Jackson study that measured the effect of corticosteroids on AFI which in about 72% of cases the AFI was decreased.[4,5]
Biophysical profile parameters (BPP) consists of five parameters including:
Fetal tone,
Fetal gross movement,
Fetal breathe,
AFI,
Nonstress test (NST) changes, each of these parameters is assigned number 0-2.
The normal score of BPP is 8-10 and 6-8 is unclear and below 6 considers as abnormal. BPP for the most of the fetus (95.95%) is normal.[6,7] In the Jackson study that measured BPP after administration of corticosteroids, fetal gross movement and AFI score were decreased in 44% and 87% of cases, respectively.[8]
To evaluate the effect of corticosteroids on NST, the parameters that change in NST are a short and long beat to beat which are decreased generally, and it can be neglect the corticosteroids effect on acceleration.[9] The difference between dexamethasone and betamethasone on NST, AFI, and BPP, dexamethasone has no clear effect on NST, AFI, and BPP but betamethasone usually decreases AFI in 63% of cases, short beat to beat in NST in all cases and fetal movement in 80% cases but other parameters have no changes.[9,10] Thus because of the importance of NST, AFI, and BPP on decision for the fetus, the changes after administration of dexamethasone and betamethasone on BPP, NST, and AFI and differences between their effects on the BPP, AFI, NST are important for us. Knowing of these changes prevents rash decision for patients.[9] In this study, we compared the effect of betamethasone versus dexamethasone on the AFI in the women at risk of preterm labor to the best decision may be made for each patient.
MATERIALS AND METHODS
This study is a double-blind clinical trial study registered in www.irct.ir with code of IRCT201212307513N2 and conducted on 70 patients for 28-34 weeks. Women having at least once preterm labor in their previous pregnancy have been submitted in Al Zahra Hospital and Shahid Beheshti clinic of obstetrics and gynecology in 2012-2013. In this study, neither the person who collected the data nor the mothers who received the drugs were aware of the prescribed medications. Inclusion criteria in our study were pregnancy, having at least once preterm labor or being at risk of preterm labor, no consumption of any drugs only ferrous sulfate or folic acid, having no background illness. The women with intrauterine growth retardation fetus, vaginal leak, gestational diabete mellitus, preeclampsia, consumption of any drugs, vaginal bleeding or if the neonate had anomaly after birth were excluded from the study. In our study, all cases with decrement of AFI, BPP fewer than 8 and nonreactive NST were hospitalized. Sample size was calculated with confidence level of (Z = 1− = 1/96) and the power of (2 = 1−B = 0.84).
These pregnant women were randomly allocated into two 35 populated groups. Seventy women were randomly allocated in two groups. The first group were received 8 mg each 12 h for 4 doses dexamethasone when the patient at risk of preterm labor admitted in clinic or hospitalized and the second group were received 12 mg betamethasone each 24 h similarly.
Nonstress test was measured in the before and 3 days after intervention. Accordingly only one sonographer measures the AFI of all 70 cases in this study.[8] Nevertheless, the measurement bias of that sonographer in different time of measurement could be about 5-cm. In order to avoid such bias, in this study, only more than 5-cm decrement in AFI and AFI under 8-cm is considered as a meaningful decrease. AFI sonography performed and repeated 3 days after administration of corticosteroids in each group and data were collected. NST has three parameters of variability in FHR:
Long beat to beat.
Short beat to beat.
Acceleration.
One observer performs NST after and before of administration of corticosteroids in each group and short beat to beat and a long beat to beat, and acceleration were evaluated. By the observer, each parameters of NST based on existence or absence were analyzed. One sonographer done sonography BPP before and after corticosteroids (3 days after taking corticosteroids) and AFI lower than 8-cm or any decrement more than 5-cm was registered. The BPP that consists of five components that each of them has a score 0 or 2. BPP score was NL (8-10), (6-8) BPP would be repeated, and (4-8) were hospitalized. BPP data combine two sources 1-ultrasound imaging and NST which has been previously explained above. If the NST is reactive, its score is 2 otherwise its score is 0. AFI which has been explained above is 0 if AFI <5 cm or a largest vertical pocket of amniotic fluid <2 cm otherwise is 2. The other parameter is fetal breathing movement score is 2 if ≥1 episode of rhythmic fetal breathing movement of ≥30 s within 30 min and otherwise is 0. Fetal movement is the other score and if ≥3 discrete body or limb movement within 30 min is 2 and otherwise is 0. Finally, fetal tones score, ≥1 episodes of a fetal extremity with return to flexion or opening or closing of a hand is 2 and otherwise is 0. All of the sonographic parts of BPP have been done by just one sonographist before administration of corticosteroids and 3 days after that and data collected.
Finally, the data entered to the computer and analyzed by IBM SPSS version 22 software. The Chi-square and Fisher's exact tests (for comparison of qualitative data between the two groups), Student's t-test (for comparison of quantitative data between the two groups) and repeated measures ANOVA test (for comparison of AFI changes in the before and after intervention between the two groups).
All mothers with nonreactive NST and AFI lower than 7 (AFI <7) and BPP lower than or equal to 8 (BPP ≤8) were admitted and observed until all these parameters became normal. All mothers included in our study sample size were closely observed until their delivery as well as all newborn in regards to their birth weight and their Apgar score within minute 1 and 5.
RESULTS
In our study, 70 women were chosen based on our inclusion criteria and were randomly divided into two groups. Betamethasone has been prescribed for the first group and dexamethasone for the second group. The average range of studied women age was 26.5 ± 6.2, and the mean of pregnancy age was 32 ± 1.9 other general information about each group population was shown in Table 1. Two groups were matched in gestational age, mother age, number of previous pregnancy; fetus expired after 20 weeks, number of living present children, and the number of abortion. Based on the Student's t-test, Chi-square and Fisher's tests, the distributions of variables between the two groups were not statistically significant between the two groups (P > 0.05). The average of AFI before and after giving the medication has been presented in Table 2. According to Student's t-test, no statistically difference between the two groups was seen in the before and after intervention (P > 0.05). Nevertheless, about 20% of cases had AFI decrement after betamethasone administration. There was no significant difference in AFI between these two groups before and after administration of corticosteroids but according to repeated measures ANOVA, time had statistical effect on AFI (P = 0.034) [Figure 1]. Furthermore, no statistically difference between NST acceleration tests was detected before and after corticosteroids administration in both groups.
Table 1.
Distribution of basic and general variables
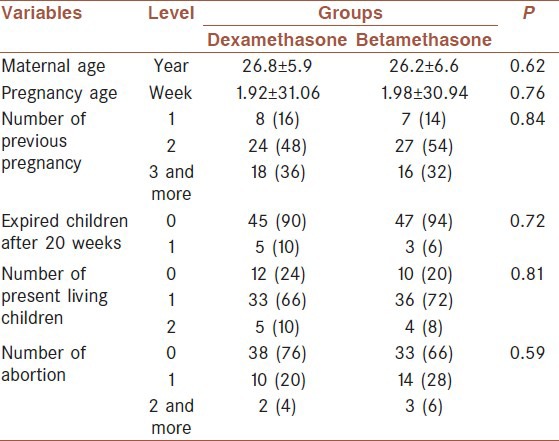
Table 2.
Distributions of NST and BPP variables
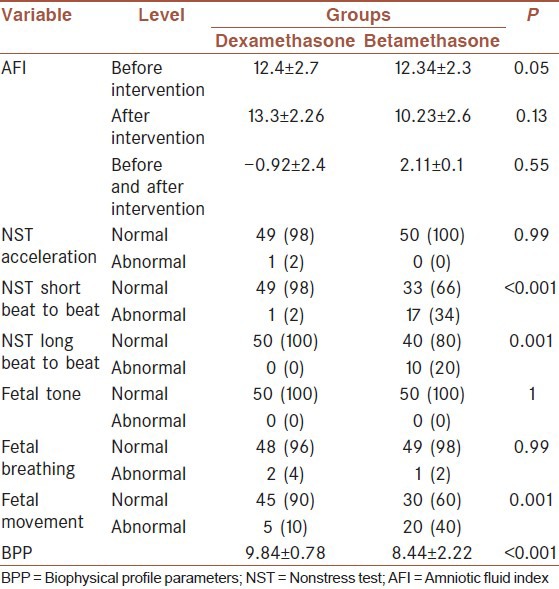
Figure 1.
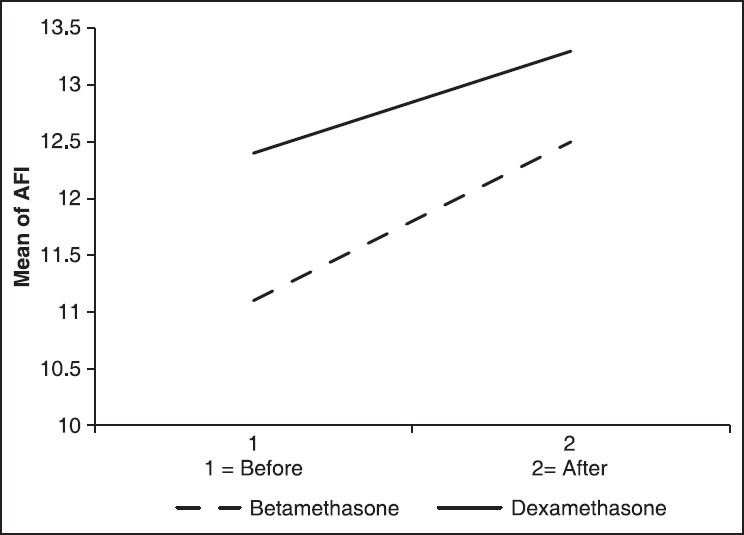
Amniotic fluid index changes in the before and after intervention between the two groups
The only statistically significant difference was found between the results of long beat to beat and short beat to beat test between the two groups. The results of BPP sonography have revealed no significant difference in fetal thoracic movement and fetal tone between the two groups but remarkably a great variance in fetal movement.
In the group who has received dexamethasone and in betamethasone receiver group there was normal fetal movement in 90% and 60% of the cases, respectively (P = 0.001). Furthermore, the average of BPP index between betamethasone and dexamethasone recipients was associated with a meaningful difference.
DISCUSSION
This study was performed to check, analyze, and compare the effect of betamethasone and dexamethasone on fetal index including AFI, BPP, NST in pregnant women with the pregnancy age of 28-34 weeks experiencing prematurity (premature delivery). Mothers were divided into two groups having no significant difference in their demographic information or history of past pregnancy. Thus, the changes in the results (alternance) could be due to the difference of the corticosteroids they were given at the last week of their pregnancy. According to our study results, although in some cases AFI was decreased, no significant influence on AFI was observed owning to corticosteroids injection, such that regarding to AFI, no remarkable variance was found between the two groups. Only it was observed that 3 days after corticosteroids injection, there was a 2.11 ± 0.1 cm decrease in AFI of betamethasone group and 0.9-cm in the dexamethasone group but as mentioned before there was no great variance. However, some other studies in contrary to ours have been related the AFI changes to corticosteroids injection. In Jackson study, the AFI has been decreased exactly after corticosteroids injection in about 72% of their sample size.[8] In Kazardoost et al. study which has been done in Yasooj Medical School in 2010, betamethasone injection was also considered as a reason for AFI decrease.[11]
The NST results showed no great variance in acceleration index between the recipients of dexamethasone and betamethasone with a significant difference between short beat to beat and a long beat to beat index in the mentioned groups. Short beat to beat index was normal in 98% of women in the dexamethasone group and 66% of betamethasone group. Long beat to beat in all of the dexamethasone group and 80% of betamethasone recipients was normal. Moreover, the results of BPP sonography have introduced a noticeable difference in fetal movement and thereby a biophysical profile showed difference between these two groups.
Furthermore, 90% of dexamethasone recipients and 60% of betamethasone ones had normal fetal movement. The same results were reported with BPP indicating that the dexamethasone group had greatly higher BPP. In Kazardoost et al. study, which was mentioned previously, all fetal movement had decreased after injection of betamethasone.[11] According to Mulder et al. study, only short and long beat to beat index were decreased due to corticosteroids injection, with no effect on acceleration.[12] Doyle et al. in a study concluded that the benefits of late dexamethasone may not outweigh actual or potential adverse effects but considering the evidence of both benefits and harms of treatment, and the limitations of the evidence at present, it appears prudent to reserve the use of late dexamethasone to infants who cannot be weaned from mechanical ventilation, and to minimize the dose and duration of any course of treatment.[4]
All studies taking into account corticosteroids influence on fetal indexes including AFI, BPP, and NST, reported no great changes in these indexes due to corticosteroids injection.[4] In Rotmensch et al. study on two groups of women exposed to (experiencing) preterm delivery prescribed with dexamethasone and betamethasone and the results were observed 48 h after injection. Fetal thoracic movement in 83% of betamethasone group and 90% of the dexamethasone group had been decreased. Fetal movement in 53.2% of dexamethasone recipients has been decreased. There was also a 48.6% decrease in fetal movement for betamethasone group, and there was a meaningful variance between these two groups.[13] In another study, the BPP status was assessed after corticosteroids injection. According to the study, for 44% of samples, gross fetal movement was decreased, and AFI and BPP index decrement has been reported for 87% of samples.[8] In our study, there were no differences in fetal tone and fetal thoracic movement between the two groups before and after of corticosteroids administration. Based on the results of this study and other similar works in this area, it can be concluded that dexamethasone has lower effect on AFI, BPP, and NST indexes respect to betamethasone and for this reason it is more preferred rather than betamethasone.
CONCLUSION
According to this study, AFI has no significant change before and after corticosteroids administration.
AUTHOR'S CONTRIBUTION
ZK and HGT have collected data and conducted the statistical analysis and prepared the first draft of manuscript. BK is the corresponding author of the manuscript and has participated in manuscript preparation. All authors have read and approved the content of the manuscript.
ACKNOWLEDGMENTS
We are appreciate to Dr. Sam Mirfendereski and Dr. Ali Foroutanfar for their help in sonographic data. Also we thanks Mr. Mehrabian for his help in data analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hass DM. Ante natal coritcosteroid for fetal lung development. BMJ Clin Evid. 2010;11:55–9. [Google Scholar]
- 2.Petrozella LN, Dashe JS, McIntire DD, Leveno KJ. Clinical significance of borderline amniotic fluid index and oligohydramnios in preterm pregnancy. Obstet Gynecol. 2011;117:338–42. doi: 10.1097/AOG.0b013e3182056766. [DOI] [PubMed] [Google Scholar]
- 3.Abbasalizadeh S, Pharabar ZN, Abbasalizadeh F, Ghojazadeh M, Goldust M. Efficacy of betamethasone on the fetal motion and biophysical profile and amniotic fluid index in preterm fetuses. Pak J Biol Sci. 2013;16:1569–73. doi: 10.3923/pjbs.2013.1569.1573. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: A systematic review. Neonatology. 2010;98:289–96. doi: 10.1159/000286212. [DOI] [PubMed] [Google Scholar]
- 5.Khandelwal M, Chang E, Hansen C, Hunter K, Milcarek B. Betamethasone dosing interval:12 or 24 hours apart? A randomized, noninferiority open trial. Am J Obstet Gynecol. 2012;206:201.e1–11. doi: 10.1016/j.ajog.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 6.NHS Evidence. Reduced Fetal Movements. Green-top Guideline 57; February. 2011. [Last accessed on 2014 Dec 15]. Available from: http://www.slog.org/pdf .
- 7.Frank AM. The Fetal Biophysical Profile. [Last accessed on 2014 Nov 16]. Available from: http://www.uptodate.com/contents/the-fetal-biophysical-profile .
- 8.Jackson JR, Kleeman S, Doerzbacher M, Lambers DS. The effect of glucocorticosteroid administration on fetal movements and biophysical profile scores in normal pregnancies. J Matern Fetal Neonatal Med. 2003;13:50–3. doi: 10.1080/jmf.13.1.50.53. [DOI] [PubMed] [Google Scholar]
- 9.Ville Y, Vincent Y, Tordjman N, Hue MV, Fernandez H, Frydman R. Effect of betamethasone on the fetal heart rate pattern assessed by computerized cardiotocography in normal twin pregnancies. Fetal Diagn Ther. 1995;10:301–6. doi: 10.1159/000264248. [DOI] [PubMed] [Google Scholar]
- 10.Subtil D, Tiberghien P, Devos P, Therby D, Leclerc G, Vaast P, et al. Immediate and delayed effects of antenatal corticosteroids on fetal heart rate: A randomized trial that compares betamethasone acetate and phosphate, betamethasone phosphate, and dexamethasone. Am J Obstet Gynecol. 2003;188:524–31. doi: 10.1067/mob.2003.136. [DOI] [PubMed] [Google Scholar]
- 11.Kazardoost S, Pooransari P, Mirzamoradi M, Abad Nazir HM. The effect of betamethasone on fetal movement, biophysical profile and fetal circulation in preterm fetuses. Life Sci J. 2012;9:354–6. [Google Scholar]
- 12.Mulder EJ, Derks JB, Visser GH. Effects of antenatal betamethasone administration on fetal heart rate and behavior in twin pregnancy. Pediatr Res. 2004;56:35–9. doi: 10.1203/01.PDR.0000130476.97700.2B. [DOI] [PubMed] [Google Scholar]
- 13.Rotmensch S, Liberati M, Celentano C, Efrat Z, Bar-Hava I, Kovo M, et al. The effect of betamethasone on fetal biophysical activities and Doppler velocimetry of umbilical and middle cerebral arteries. Acta Obstet Gynecol Scand. 1999;78:768–73. [PubMed] [Google Scholar]


