Abstract
Background:
Endometriosis is a chronic and progressive gynecological disorder and is manifest by dysmenorrhea and a major cause of infertility and chronic pelvic pain. The study was designed to compare the value of cervico-vaginal fluid of interleukin-1α (IL-1α) in patients with and without endometriosis.
Materials and Methods:
Fifty women were assessed in this case control study. The case group included 25 patients with endometriosis. The control group included 25 women without any evidence of endometriosis or any other genital disease. Endometriosis was confirmed by laparoscopy and histopathological examination. Cervico-vaginal fluid samples were obtained from patients during the follicular phase and preup surgery to assess the levels of IL-1α in cervico-vaginal fluid. The level of IL-1α was assessed using commercially available Avi Bionhuman Enzyme-Linked Immunosorbent Assay kits (FIN-01720, Vantaa, Finland) for IL-1α. Receiver-operator curve analysis was used to estimate the power of IL-1α to distinguish subjects with endometriosis from controls.
Results:
The cervico-vaginal fluid level of IL-1α in cases was 210.44 ± 40.11 pg/mL and in controls was 54.28 ± 25.73 pg/mL, the differences between two groups was statistically significant (P < 0.0001). The cut-off point for cervico-vaginal fluid IL-1 for endometriosis was 105 pg/mL, with a sensitivity of 100% (95% confidence interval [CI]: 86.2-100), and specificity of 100% (95% CI: 86.2-100).
Conclusion:
Results show a significant increase in the cervico-vaginal fluid levels of IL-1α, in women with endometriosis, that it can be a useful marker in the diagnosis of endometriosis.
Keywords: Cervico-vaginal fluid, cytokine, endometriosis, interleukin-1α, interleukins
INTRODUCTION
Endometriosis is a chronic and progressive gynecological disorder, which usually diagnosed in women of reproductive age, and defined as the growth of active endometrial glands and stroma outside the uterine cavity. It is manifest by dysmenorrhea and a major cause of infertility and chronic pelvic pain.[1,2]
In women with endometriosis, genetic and environmental factors, major hormonal and immune changes have been observed, particularly a chronic immune-inflammatory process, and the difficulty of diagnosis is the possible cause of variable incidence.[2,3,4] The incidence of endometriosis is unknown, and in the general population has cited percentages ranging from 1% to 10%.[5] The percentage in women with infertility is higher (30-40%).[6] Also, the incidence in women with pelvic pain is even higher, with an incidence of 82% in some reports.[7]
Only laparoscopy is the gold standard for the diagnosis of endometriosis, but it is invasive procedure with potential complications that requires general anesthesia and surgical skill.[8] Also multiple lines of evidence suggest that inflammation and immune responses play a pivotal role in the pathogenesis of endometriosis,[9,10,11] therefore many studies have turned to biomarkers, including tumor markers, tumor necrosis factor, and cytokines, especially the interleukins (IL) IL-1α, IL-6, and IL-8 to gain a better understanding of the pathogenic mechanisms underlying the disease.[12,13]
The cytokines are multifunctional proteins with a key role in hematopoiesis, immunity, infectious disease, homeostasis, tissue repair, and cellular development and growth.[14] The IL-1α plays a central role in the regulation of inflammation and immune responses. It is secreted mainly by activated monocytes and macrophages and also by T- and B-cells and NK cells.[15] It affects the activation of T-cells and the differentiation of B-cells. In patients with endometriosis, IL-1α has been isolated from the peritoneal fluid.[16,17]
The potential of using body fluid for the identification of diagnostic biomarkers has been recognized over the last 10 years.[18] Cervico-vaginal fluid has an important function in the homeostasis and immunity of the lower female genital tract, and its analysis may yield important information about the pathogenesis of numerous gynecological pathologies.[19] Also, the low cost and ease of sample collection, the possibility of observing high numbers of patients using multiple samples and avoidance of the risk associated with biopsies are the advantages of cervico-vaginal fluid as a source of biomarkers for these conditions.[20,21,22] So far, studies have evaluated the concentrations of selected cytokines in peritoneal fluid and/or peripheral blood samples and there is limited data on such correlations between concentrations of IL-1α in cervico-vaginal fluid and endometriosis. Therefore, the present study was aimed to investigate the IL-1α profile in the cervico-vaginal fluid of patients with endometriosis compared with healthy controls, and to test if it can provide a useful simple test for the diagnosis of endometriosis.
MATERIALS AND METHODS
From February to September 2013, in this case-control study, 50 women included 25 women with laparoscopically and histopathologically confirmed endometriosis and 25 women with any sign of endometriosis who had been referred to “Shahid Beheshti” and “Alzahra” hospitals in Isfahan, Iran, were enrolled in the study to assess the level of IL-1 in cervico-vaginal fluid. Women who referred with sing of endometriosis or who scheduled for tubal ligation, undergoing laparoscopy, and then, women with evidence of endometriosis were define as cases and those women with normal laparoscopy were define as controls. Women in both groups with mean age of 33.4 and 31.8 years old for cases and controls, respectively (age range between 20 and 40 years old) were eligible if they were in the follicular phase of the menstrual cycle at the time of laparoscopic examination, had regular menstrual cycles, normal blood counts at admission to the hospital, not used hormonal or immunomodulatory medications at least 3 months prior to enrollment in the study, no history of previous pelvic surgery or chronic systemic disease, and had not been pregnant at the time of study. Also, patients with acute infections or suspicion of malignancy located in genital or extra genital area were excluded from the study. This study was investigated and approved by the Ethics Committee of Isfahan University of Medical Sciences. After that participating subjects were explained about and informed of the purposes of the study written informed consent was obtained.
To collecting cervico-vaginal fluid samples, during the follicular phase and preopeartive surgery, patients were placed in the dorsal lithotomy position and 3 mL of sterile normal saline solution was inserted to posterior vaginal fornix compiled and is aspirated by the sterile syringe.
Power calculation for the study was based on the prevalence of endometriosis reported in the previous study.[23] The required sample size was calculated using estimating a single proportion formula. Assuming a power of 90% and a two-tailed P = 0.05 a population consisting of 25 patients with endometriosis and 25 controls was calculated.
Data collected were age (based on the date of birth and the date to be enrolled), body mass index (BMI, weight in kilograms divided by height in meters squared), parity (the number of times that she has given birth) and the level of IL-1α. To measure the level of IL-1α, cytokine concentrations, cervico-vaginal fluid samples were assessed using commercially available Avi Bionhuman Enzyme-Linked Immunosorbent Assay kits for IL-1α (FIN-01720, Vantaa, Finland) according to the instruction of the manufacturer.
The collected data were analyzed statistically with SPSS software version 20 (SPSS Inc., Chicago, IL, USA). Descriptive statistics is reported as mean ± standard deviation, median (inter quartile range) or number (%) as appropriate. Kolmogorov-Smirnov test was used to assess normality for level of IL-1α and results show that the distribution of level of IL-1α was normal in both groups. Independent sample t-test was used to compare age, BMI and the level of IL-1α between the two groups. Mann-Whitney U-test was applied to compare parity between the groups. Receiver-operator curve (ROC) analysis was used to estimate the power of IL-1α to distinguish subjects with endometriosis from controls and to choose optimal cut-off points for screening. All probability tests used were two-tailed, and alpha was set at 5%.
RESULTS
Thirty-six patients were reviewed to accessed 25 patients with endometriosis in the case group during the study period. Of 36 patients, eight not meet eligible criteria and three patients refused informed consent and did not enter to the study. Also, of 32 healthy subjects without endometriosis reviewed as a control group, seven subjects who did not meet eligible criteria or refused informed consent were excluded. Finally, 25 cases and 25 controls completed the study and analyzed. The recruitment process of studied population is shown in Figure 1.
Figure 1.
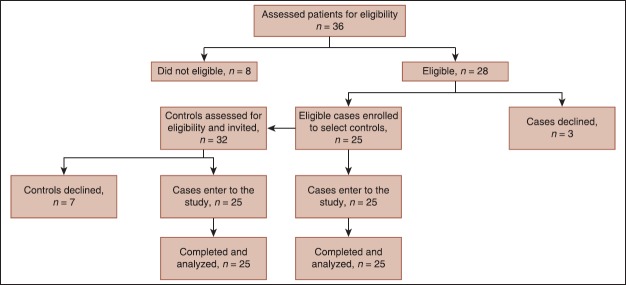
Study follow chart
The mean age of studied population was 32.3 + 3.2 years old. Table 1 shows a comparison of age, BMI, parity and mean of IL-1α between case and control groups. As shown mean of age (cases, 33.4 vs. control, 31.8 years), BMI (cases, 21.6 vs. control, 23), parity, between groups were similar and no significant differences were noted between groups (P > 0.05). Also, the cervico-vaginal fluid level of IL-1α in cases was significantly higher than controls (210.44 vs. 54.28 pg/mL, respectively, P < 0.0001).
Table 1.
Age, BMI, parity, and level of IL-1 between case and control groups
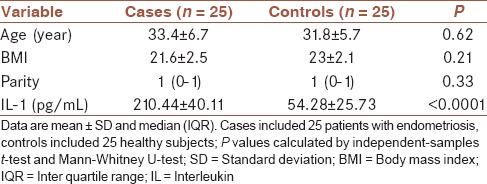
Figure 2 shows ROC curve analyzes that were used to evaluate the performance of the cervico-vaginal fluid IL-1α concentration as a biomarker for the prediction of endometriosis. Cervico-vaginal fluid IL-1α provided the best discriminative ability between subjects with endometriosis and the controls. The ROC curve analysis revealed a relatively high diagnostic value of cervico-vaginal fluid IL-1α, with an area under the curve of 1.00 (95% confidence interval [CI]: 0.928-1). Figure 3 shows the dot plots of IL-1 concentrations in patients with endometriosis and healthy group. The optimal cervico-vaginal fluid IL-1α threshold found using the ROC curve was 105 pg/mL, with a sensitivity of 100% (95% CI: 86.2-100), and specificity of 100% (95% CI: 86.2-100).
Figure 2.
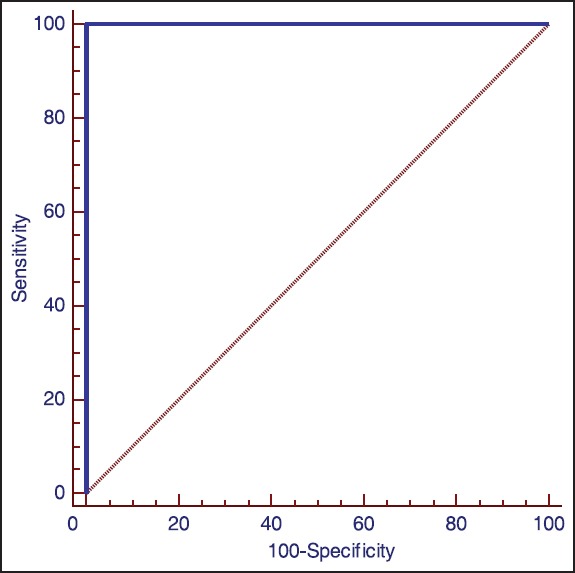
Receiver operating characteristic curves for maternal cervico-vaginal fluid interleukin-1 level for predicting endometriosis (area under the curve, 1.00; standard error, 0.0; P < 0.0001; sensitivity, 100 and specificity, 100)
Figure 3.
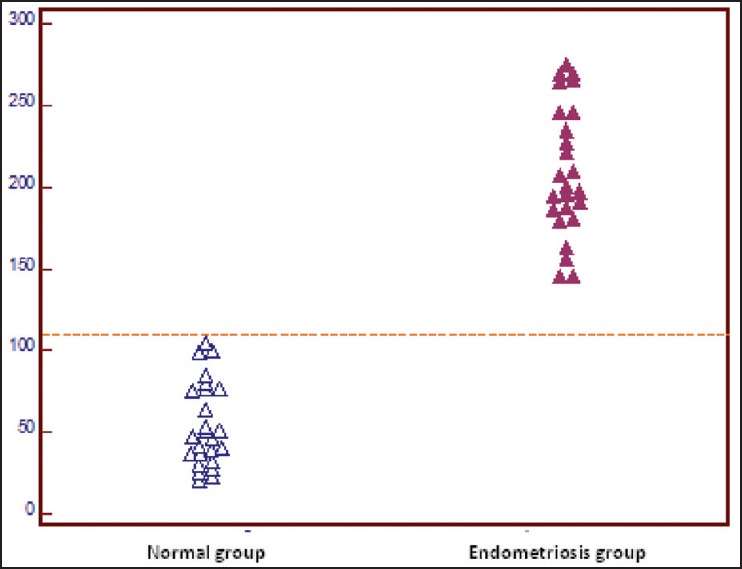
Dot plots of interleukin-1 (IL-1) concentrations in patients with endometriosis and healthy group. The respective cut-off value as determined by receiver-operated curves is shown by the dashed horizontal line. The level for IL-1 in cervico-vaginal fluid in the endometriosis group differed significantly from those in the control group (P < 0.0001)
DISCUSSION
However, because of nonspecific symptoms and late presentation, the diagnosis of endometriosis is difficult but a variety of abnormal immune functions are recognized in patients with endometriosis. The use of laparoscopy as gold standard method to endometriosis diagnosis is invasive procedure and is limited by available funding, human error, and the surgeon's experience.[24] Therefore, for screening patients at risk for endometriosis simple test as a marker would be developed to reduce the number of unnecessary interventions.
Interleukin-1α as a major cytokine which has been found to be elevated in endometriotic lesions, being linked to higher cyclooxygenase activity, prostaglandin production, adhesion protein synthesis, lymphocyte activation, and angiogenesis.[25] In the present study, we found that IL-1α was significantly increased in the cervico-vaginal fluid of women with endometriosis. Our findings revealed that cervico-vaginal fluid IL-1α provided a marker with high diagnostic value in 105 pg/mL as the best cut-off point (sensitivity of 100% and specificity of 100%) and odd ratios (2.39) in the prediction of endometriosis.
A major product of activated peritoneal macrophages is IL-1α[26] and recent studies suggest that in the generation of pain through interaction with nerve fibers macrophages may play a role.[27] Akoum et al.[28] reported a relationship between the cytokine's levels and pelvic pain in endometriosis in their study. Also, previous studies demonstrated that the soluble form of IL-1 receptor was significantly increased in the peritoneal fluid and in the eutopic endometrium of women with endometriosis.[29,30] Other studies reported increase in the level of IL-1 and IL-6, in the peritoneal fluid of women with endometriosis.[31,32] The usefulness of the serum cytokine evaluation in the discrimination between endometriosis and control patients is reported in previous studies. As reported, previous studies assess cytokines level in different body fluid such as serum, peritoneal fluid, and to the best of our knowledge cervico-vaginal fluid IL-1α has not been assessed in endometriosis patients, moreover, present study is the first to showing IL-1α measurement in women with endometriosis. And in similar with previous studies our results show an increase in the cervico-vaginal fluid of IL-1α in women with endometriosis compare to controls despite of different assessed body fluid. Findings in the present study have not been confirmed in any other study and could facilitate basic and clinical research on endometriosis.
We found that endometriosis is associated with increased concentrations of cervico-vaginal fluid IL-1α, and maybe this can be used as a probable clinical symptom in the diagnoses of women with endometriosis with the low cost and ease of sample collection and other advantages in compare to laparoscopy procedure as gold standard. It will be more important, whereas after laparoscopy procedure only one-third of women will diagnose with endometriosis, one-third will have no pelvic pathology, and other gynecological conditions will have diagnosed in the remaining one-third. And two-thirds of these women will be subjected to the potential risks as well as the cost associated with this procedure without actually having endometriosis.[33] One of the limitations of our study is that we did not collect data about the stage of endometriosis in studied patients, and we could not assess the association between stage of endometriosis and cervico-vaginal fluid IL-1α. Another limitation is that patients in our study were women with endometriosis base and other inflammatory disease such as sub-acute PID were not eligible, whereas, it is clear that IL-1α could be raise in these diseases, also, as a case control study another limitation is that controls were not selected by matching with cases; however, demographic characteristics were not significantly different between cases and controls. So, we believed that further studies are necessary to find more information about a useful marker to diagnose women with endometriosis more easily, assesse the relation between cervico-vaginal fluid level of IL-1α and severity of endometriosis, and also compare endometriosis patients with other inflammatory disease.
In summary, however small sample size is the main limitations of the present study which does not empower us to generalize the results to the entire female population. But our results revealed for the first time a significant increase in the cervico-vaginal fluid level of IL-1α, in women with endometriosis. And the cut-off point 105 pg/mL for cervico-vaginal fluid level of IL-1α may be a useful marker in the diagnosis of endometriosis with sensitivity of 100% and specificity of 100%. However, the data are limited and further studies are needed to be done to express the role of cervico-vaginal fluid level of IL-1α in women with endometriosis.
AUTHOR'S CONTRIBUTION
FM contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and ZShS contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript.
ACKNOWLEDGMENTS
Financial support was provided by the Isfahan University of Medical Sciences, Isfahan, Iran (No. 392324).
Footnotes
Source of Support: The Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Drosdzol-Cop A, Skrzypulec-Plinta V. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. J Obstet Gynaecol Res. 2012;38:1245–53. doi: 10.1111/j.1447-0756.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomassetti C, Meuleman C, Pexsters A, Mihalyi A, Kyama C, Simsa P, et al. Endometriosis, recurrent miscarriage and implantation failure: Is there an immunological link? Reprod Biomed Online. 2006;13:58–64. doi: 10.1016/s1472-6483(10)62016-0. [DOI] [PubMed] [Google Scholar]
- 4.Walter AJ, Hentz JG, Magtibay PM, Cornella JL, Magrina JF. Endometriosis: Correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol. 2001;184:1407–11. doi: 10.1067/mob.2001.115747. [DOI] [PubMed] [Google Scholar]
- 5.Othman Eel-D, Hornung D, Al-Hendy A. Biomarkers of endometriosis. Expert Opin Med Diagn. 2008;2:741–52. doi: 10.1517/17530059.2.7.741. [DOI] [PubMed] [Google Scholar]
- 6.D’Hooghe TM, Hill JA. Endometriosis. In: Berek JS, editor. Novak's Gynecology. 13th ed. Philadelphia, PA: Williams and Wilkins; 2002. pp. 931–72. [Google Scholar]
- 7.Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74:594–600. [PubMed] [Google Scholar]
- 8.Brosens IA, Brosens JJ. Is laparoscopy the gold standard for the diagnosis of endometriosis? Eur J Obstet Gynecol Reprod Biol. 2000;88:117–9. doi: 10.1016/s0301-2115(99)00184-0. [DOI] [PubMed] [Google Scholar]
- 9.Osuga Y, Koga K, Tsutsumi O, Igarashi T, Okagaki R, Takai Y, et al. Stem cell factor (SCF) concentrations in peritoneal fluid of women with or without endometriosis. Am J Reprod Immunol. 2000;44:231–5. doi: 10.1111/j.8755-8920.2000.440407.x. [DOI] [PubMed] [Google Scholar]
- 10.Nishida M, Nasu K, Ueda T, Fukuda J, Takai N, Miyakawa I. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: A possible mechanism involved in the pathogenesis of endometriosis. Mol Hum Reprod. 2005;11:29–34. doi: 10.1093/molehr/gah133. [DOI] [PubMed] [Google Scholar]
- 11.Bullimore DW. Endometriosis is sustained by tumour necrosis factor-alpha. Med Hypotheses. 2003;60:84–8. doi: 10.1016/s0306-9877(02)00336-5. [DOI] [PubMed] [Google Scholar]
- 12.Taketani Y, Kuo TM, Mizuno M. Comparison of cytokine levels and embryo toxicity in peritoneal fluid in infertile women with untreated or treated endometriosis. Am J Obstet Gynecol. 1992;167:265–70. doi: 10.1016/s0002-9378(11)91672-x. [DOI] [PubMed] [Google Scholar]
- 13.Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176:593–7. doi: 10.1016/s0002-9378(97)70553-2. [DOI] [PubMed] [Google Scholar]
- 14.Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–96. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 15.Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalu E, Sumar N, Giannopoulos T, Patel P, Croucher C, Sherriff E, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–5. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 17.Skrzypczak J, Szczepańska M, Puk E, Kamieniczna M, Kurpisz M. Peritoneal fluid cytokines and sICAM-1 in minimal endometriosis: Search for discriminating factors between infertility and/or endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;122:95–103. doi: 10.1016/j.ejogrb.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, et al. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zegels G, Van Raemdonck GA, Tjalma WA, Van Ostade XW. Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci. 2010;8:63. doi: 10.1186/1477-5956-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good DM, Thongboonkerd V, Novak J, Bascands JL, Schanstra JP, Coon JJ, et al. Body fluid proteomics for biomarker discovery: Lessons from the past hold the key to success in the future. J Proteome Res. 2007;6:4549–55. doi: 10.1021/pr070529w. [DOI] [PubMed] [Google Scholar]
- 21.Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–53. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veenstra TD, Conrads TP, Hood BL, Avellino AM, Ellenbogen RG, Morrison RS. Biomarkers: Mining the biofluid proteome. Mol Cell Proteomics. 2005;4:409–18. doi: 10.1074/mcp.M500006-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Paul Dmowski W, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:245–63. doi: 10.1016/j.bpobgyn.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Socolov R, Butureanu S, Angioni S, Sindilar A, Boiculese L, Cozma L, et al. The value of serological markers in the diagnosis and prognosis of endometriosis: A prospective case-control study. Eur J Obstet Gynecol Reprod Biol. 2011;154:215–7. doi: 10.1016/j.ejogrb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Mihalyi A, Mutinda K, Simsa P, Debrock S, Mwenda JM, D’Hooghe TM. Role of immunologic and inflammatory factors in the development of endometriosis: Indications for treatment strategies. Therapy. 2005;2:623–39. [Google Scholar]
- 26.Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K, Tamaya T. Expression of interleukin-1 (IL-1) beta messenger ribonucleic acid (mRNA) and IL-1 receptor antagonist MRNA in peritoneal macrophages from patients with endometriosis. Fertil Steril. 1992;57:535–42. doi: 10.1016/s0015-0282(16)54896-1. [DOI] [PubMed] [Google Scholar]
- 27.Tran LV, Tokushige N, Berbic M, Markham R, Fraser IS. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod. 2009;24:835–41. doi: 10.1093/humrep/den483. [DOI] [PubMed] [Google Scholar]
- 28.Akoum A, Al-Akoum M, Lemay A, Maheux R, Leboeuf M. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril. 2008;89:1618–24. doi: 10.1016/j.fertnstert.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Guay S, Michaud N, Bourcier N, Leboeuf M, Lemyre M, Mailloux J, et al. Distinct expression of the soluble and the membrane-bound forms of interleukin-1 receptor accessory protein in the endometrium of women with endometriosis. Fertil Steril. 2011;95:1284–90. doi: 10.1016/j.fertnstert.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 30.Michaud N, Al-Akoum M, Gagnon G, Girard K, Blanchet P, Rousseau JA, et al. Decreased concentrations of soluble interleukin-1 receptor accessory protein levels in the peritoneal fluid of women with endometriosis. J Reprod Immunol. 2011;92:68–73. doi: 10.1016/j.jri.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Barcz E, Milewski L, Dziunycz P, Kaminski P, Ploski R, Malejczyk J. Peritoneal cytokines and adhesion formation in endometriosis: An inverse association with vascular endothelial growth factor concentration. Fertil Steril. 2012;97:1380–6.e1. doi: 10.1016/j.fertnstert.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 32.Velasco I, Acién P, Campos A, Acién MI, Ruiz-Maciá E. Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J Reprod Immunol. 2010;84:199–205. doi: 10.1016/j.jri.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Garry R. Diagnosis of endometriosis and pelvic pain. Fertil Steril. 2006;86:1307–9. doi: 10.1016/j.fertnstert.2006.06.045. [DOI] [PubMed] [Google Scholar]


