Abstract
Background:
To evaluate the visual and refractive outcomes of cataract surgery with toric intraocular lens (IOL) implantation at a teaching hospital of the United Kingdom.
Design:
Prospective interventional case series.
Materials and Methods:
This study compared the outcome of 3 groups of patients: Group 1 included 25 eyes with cataract and more than 2.5 diopters (D) of corneal astigmatism receiving a toric monofocal IOL; Group 2 had 18 patients with cataract and more than 2.5 D of astigmatism but receiving a non-toric monofocal IOL; while Group 3 had 25 patients with cataract and less than 1.5 D of astigmatism and receiving a non-toric monofocal IOL. Data collected included uncorrected (UDVA) and corrected (CDVA) distance visual acuities, refraction and corneal keratometry. Postoperative examinations were scheduled at 1 and 6 weeks.
Results:
Postoperatively the mean UDVA was LogMAR 0.27 ± 0.20 (equivalent snellen acuity of 20/37) in Group 1, 0.54 ± 0.22 (20/69) in Group 2 and 0.16 ± 0.20 (20/29) in Group 3. The mean CDVA was LogMAR 0.08 ± 0.13 (20/24) in Group 1, 0.23 ± 0.16 (20/34) in Group 2 and 0.04 ± 0.13 in Group 3 (20/22). The mean preoperative keratometric cylinder was 3.78 ± 1.0 D in Group 1, 3.41 ± 1.47 D in Group 2 and 0.97 ± 0.43D in Group 3; the mean postoperative subjective cylinder was 1.2 ± 0.68 D in Group 1, 3.23 ± 1.41 D in Group 2 and 0.95 ± 0.58 D in Group 3. The difference was statistically significant for the postoperative refractive cylinder values when comparing Group 1 to Group 2 (P = <0.0001) but the difference was insignificant between Group 1 and Group 3 (P = 0.23).
Conclusion:
Toric IOL implantation is an effective option to manage corneal astigmatism at the time of cataract surgery and to optimise visual outcomes for astigmatic patients when comparing to outcomes for their non-astigmatic counterparts.
Keywords: Astigmatism, cataract, toric intraocular lens
Introduction
Outcomes of cataract surgery have improved greatly in recent years, and patients’ demands and expectations are increasing all the while. As such, surgeons are increasingly considering the refractive result of surgery when in the planning stages. While myopes and hyperopes have reaped the benefit of the correction of their spherical errors when having cataract surgery, astigmats have not generally been as fortunate. Traditional options to address astigmatism at the time of cataract surgery include placing the surgical incision “on axis” placing an additional opposite clear corneal incision, and other incisional interventions such as limbal relaxing incision. However, these techniques offer variable results and may not have been adopted by the majority of cataract surgeons. Reasons may include factors such as the need for pre-operative topography and other aspects of pre-operative planning that may not be available to the majority of cataract surgeons; the need for specialised equipment during surgery such as a micrometer diamond knife and pachymeter which also may not be available to the majority of cataract surgeons; and other factors specific to individual surgeons and clinical settings. The option of utilising a toric IOL may appeal to a greater proportion of cataract surgeons in a broader range of clinical settings, and is the focus of this paper. While non-toric IOLs are successful in achieving good unaided visual acuity, these implants only correct the spherical part of the refractive error and do not address the corneal astigmatic error.[1] In this day and age, patients not only expect restoration of vision but also to gain spectacle independence, especially for distance.
In a recently published paper,[2] the prevalence of corneal astigmatism in our population was studied to evaluate the need for toric IOL implantation. It showed that 40.41% of the patients presenting for cataract surgery had more than one dioptre (D) of astigmatism, 20.5% had 1.5 D or more and 4.61% had more than 2.5 D of corneal astigmatism.[2] There are various options[2] available to address corneal astigmatism at the time of surgery and the use of a toric IOL is gaining popularity among corneal surgeons. We performed this study to evaluate the visual and refractive results of our first 25 consecutive patients receiving the same platform of toric IOL.
Materials and Methods
This study contained three patient groups. Group 1 included 25 consecutive patients undergoing cataract surgery with implantation of the Rayner TFlex toric IOL (Rayner Intraocular Lenses Ltd., East Sussex, England) and having pre-existing keratometric astigmatism of 2.5 D or more. Four surgeons in our Department participated in the protocol to implant toric IOLs and all eligible patients were placed on surgical lists for one of these surgeons. Before starting to use a toric IOL at our hospital, we analyzed the prevalence of keratometric astigmatism at our unit.[2] Our hospital designed a protocol according to which all patients with pre-existing keratometric astigmatism of 3 D or more and an age-related cataract would be entitled to receive a toric IOL. Patients with astigmatism between 2.5 and 3 D could receive a toric IOL at the discretion of the Consultant (only two out of these 25) especially if there was a significant risk of manifesting astigmatism postoperatively (patients with minimal refractive cylinder but high keratometric cylinder). Data collection for the toric IOL group was done prospectively. We also prospectively collected data for 25 patients who had keratometric astigmatism of less than 1.5 D and had cataract surgery with a non-toric IOL (AcrySof SA60AT, Alcon Laboratories, Inc, Fort Worth, Texas)-these patients formed Group 3. A further 18 patients formed Group 2 consisting of patients who had more than 2.5 D of keratometric astigmatism and who received a non-toric IOL (AcrySof SA60AT). These patients had retrospective data collection as they were operated on 6 months prior to the implementation of the toric IOL protocol in our hospital. In our unit, patients with astigmatism between 1.5D and 2.5 D had on axis incision usually combined with an opposite clear corneal incision (OCCI) and these patients had to be excluded for the purpose of this study.
Preoperatively, all patients had a full ophthalmic examination including visual acuity, Goldmann applanation tonometry, slit-lamp bio-microscopy with dilated ophthalmoscopy, subjective refraction or auto refraction if not already supplied by the referring optometrist, and partial coherence interferometry (IOL Master, Carl Zeiss Meditec, Jena, Germany). All patients presented with age-related cataracts and patients with stable and mild ocular morbidities such as glaucoma, dry macular degeneration and diabetic retinopathy were included. This study followed protocols according to the local institutional health board review of the hospital.
Intraocular lens model selection
All patients receiving a toric IOL had implantation of a T-Flex 623 T one piece hydrophilic acrylic IOL (Rayner Intraocular Lenses Ltd. East Sussex, England) and all patients receiving a non-toric IOL had AcrySof SA60AT IOL (Alcon Laboratories Inc). An experienced surgeon selected the appropriate IOL for all patients and in case of toric IOL, Rayner's online IOL calculator (Raytrace) was used to determine the IOL power and IOL alignment. Surgically induced astigmatism of 0.25 D was assumed in all cases.
Surgical technique
Preoperatively, corneal reference marks were placed at 0 degree and 180 degrees with the patient sitting upright to correct for cyclotorsion. With the patient in the surgical area and draped for surgery, axis marks were placed on the cornea before the eye was entered. This was achieved using a Mendez degree gauge and Bores axis marker. Intraoperatively, the implantation axis was determined using the corneal reference marks and the alignment axis of the IOL was indicated by marks on the IOL placed during the manufacturing process. The incisions were limbal and made superiorly with a width of either 2.2 mm or 2.6 mm depending on the surgeon's preference. This was followed by a central capsulorrhexis of approximately 5.0 mm. Cataract extraction techniques varied and included divide and conquer as well as immediate chop. After the foldable toric IOL was inserted, it was rotated to within 10 degrees of the final position, the ophthalmic viscosurgical device was removed and the IOL was rotated into final position by exact alignment of the reference marks on the toric IOL with the implantation axis marks on the cornea. Postoperatively, all patients were prescribed a fixed-combination eye drop of neomycin 3 mg/mL and betamethasone 1 mg/mL for 4 weeks.
Follow up
All patients were seen at 1 day, 1 week, 6 weeks and 12 weeks postoperatively. Visual and refractive data were collected at the 6 or 12 weeks visit. At the 1st day post-op visit an uncorrected distance visual acuity of 20/60 or better was accepted as satisfactory and the patient was scheduled for a further clinic visit. If the visual acuity at the 1st visit was less than 20/60, and could not be explained by ocular co-morbidity, the IOL axis was assessed against the intended position. Dilated pupil retro-illumination was also undertaken for assessing misalignment of the IOL though it has not been analyzed for the purpose of this study. None of the 25 patients with Toric IOL had significant misalignment (more than 10°) or needed to have re-positioning of the IOL.
Statistical analysis
The Student t-test was used to compare preoperative and postoperative refractive and keratometric data. All statistical analyses were two-sided and P values less than 0.05 were considered statistically significant. All data were entered in the Microsoft Office Excel program 2007 (Microsoft Corp.) and statistical analysis performed using Graph Pad Prism software (Graph Pad Software version 5, Inc San Diego, CA).
Results
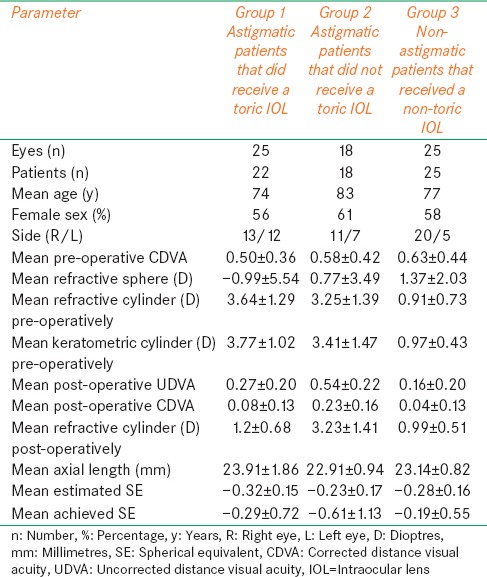
This study included three groups of patients. Group 1 included 25 eyes of 22 consecutive patients in the toric IOL group. Group 2 had 18 astigmatic eyes of 18 patients that did not receive a toric IOL, and Group 3 had 25 non-astigmatic eyes of 25 patients and these received a non-toric IOL. Table 1 shows preoperative and patient demographic data in all three groups.
Table 1.
Patient demographics and operative data in all three groups
Visual acuity
Table 1 shows the mean preoperative and postoperative visual acuities which improved in all the three groups. The mean CDVA improved from LogMAR 0.50 (20/63) to LogMAR 0.08 (20/24) (P = <0.0001) in the toric IOL group, from LogMAR 0.58 (20/76) to 0.23 (20/34) (P = 0.003) in Group 2 and from LogMAR 0.63 (20/85) to 0.04 (20/22) (P= <0.0001) in Group 3. The difference between the mean preoperative CDVA and postoperative UDVA was statistically significant in Group 1 (the toric IOL group) (P = 0.012) as well as in group 3 (P= <0.0001) but not in group 2 (P = 0.72). The difference between postoperative UDVA and postoperative CDVA was statistically significant in all the three groups (P = 0.0003 for Group 1, P = 0.02 for Group 2, P=<0.0001 for Group 3). Postoperative UDVA of LogMAR 0.3 (20/40) or better was achieved in 76% of eyes in the toric IOL group, 16% of eyes in Group 2 and 84% of eyes in Group 3. The CDVA was LogMAR 0.3 (20/40) or better in 100% of eyes in the toric IOL group postoperatively while the figures were 66% and 96% for Group 2 and Group 3, respectively. All three groups had equal distribution of patients with mild ocular co-morbidities including diabetic retinopathy, glaucoma or mild age-related macular degeneration.
The visual acuity data for the Group 2 patients deserve greater attention. The two main findings relating to this group are that i) the post-operative UDVA is no better than the pre-operative CDVA, and ii) the post-operative CDVA appears to be inferior to that achieved by patients in Groups 1 and 3. If these findings were to be repeated in other studies it would be of high significance. The mean post-operative CDVA in Group 2 patients appears to arise from a generalized falling short of LogMAR 0.00 (20/20), and does not seem to be due to a very poor outcome in a small number of patients that may have arisen as a result of a poor post-operative course (for example, occurrence of cystoid macular oedema). It would be unlikely that a large number of patients in a relatively small group would have a poor postoperative outcome due to pathology. We expand this further in the discussion.
Complications
There were no intraoperative complications noted in any of the patients in the three groups. Though patients in the toric group had close monitoring for IOL misalignment, no patient required a return to theatre for re-positioning.
Refraction
The pre-operative mean estimated spherical equivalent (SE) was -0.32D in the Group 1, -0.23D in Group 2 and -0.28D in Group 3 [Table 1]. Postoperatively the mean SE was -0.29D ± 0.72 in the toric IOL group, -0.61D ± 1.13 in Group 2 and -0.19D ± 0.55 in Group 3 and these differences were not statistically significant in any of the three groups.
Astigmatism
Table 1 shows the preoperative and postoperative refractive data in all the three groups. The scatter plot in Figure 1 shows the difference between the keratometric cylinder (measured preoperatively) versus postoperative refractive cylinder. The mean preoperative refractive cylinder was 3.64 D in Group 1, 3.25 D in Group 2 and 0.91 D in Group 3. The mean preoperative keratometric cylinder was 3.77 D in Group 1, 3.41 D in Group 2 and 0.97 D in Group 3 which were quite similar to the mean preoperative refractive values and showed no statistical difference (P = 0.68 for Group 1, P = 0.74 for Group 2 and P = 0.73 for Group 3). Therefore, we are able to confidently refer to the pre-operative refractive cylinder and keratometric cylinder values interchangeably. The postoperative refractive cylinder values were 1.2 D ± 0.68 in Group 1, 3.23 D ± 1.41 in Group 2 and 0.99 D ± 0.51 Group 3. The difference between the preoperative and postoperative refractive cylinder was statistically significant for the toric group only (P= <0.0001). Moreover, the difference was statistically significant for the postoperative refractive cylinder values when comparing Group 1 to Group 2 (P=<0.0001) or when comparing Group 2 to Group 3 (P=<0.0001) but the difference was insignificant between Group 1 and Group 3 (P = 0.23). This suggests that patients with more than 2.5 D of astigmatism can achieve results equivalent to non-astigmats when toric IOL is used but if a non-toric IOL is used then the expected outcome would not be comparable to the non-astigmats.
Figure 1a-c.
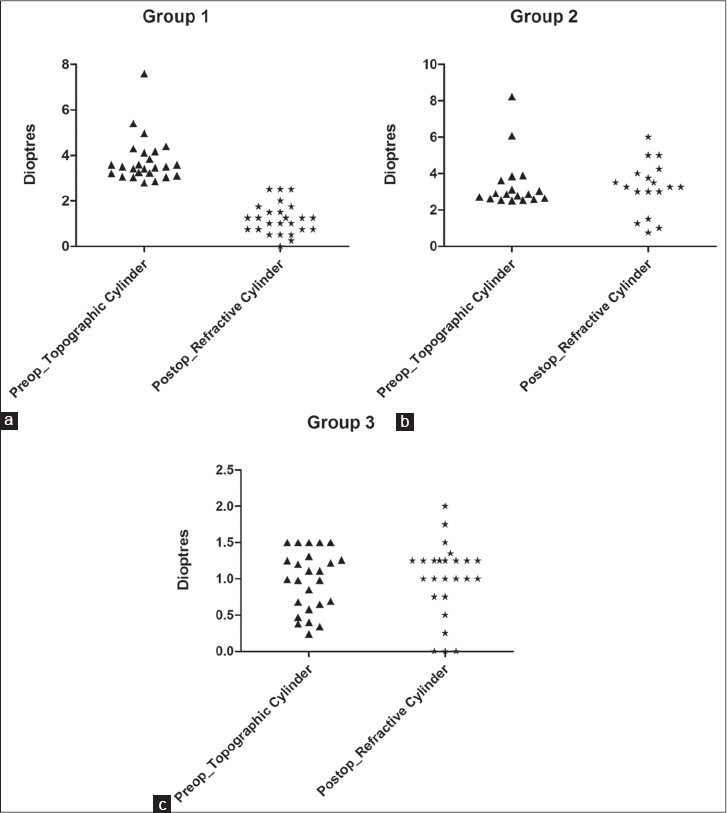
The scatter plot shows the difference between the keratometric cylinder (measured preoperatively) versus postoperative refractive cylinder for all the three groups
Discussion
Corneal astigmatism is frequently seen in a significant proportion of patients attending for cataract surgery[2,3,4] and can greatly influence the refractive outcome of surgery. Residual astigmatism after phacoemulsification can leave the patient symptomatic and significantly decrease vision.[5] Our own data presented in this paper provide further evidence in this regard, showing that uncorrected corneal astigmatism leads to a corresponding manifest refractive cylinder after surgery. In this group of patients our data further demonstrate an outcome of reduced post-operative UDVA and CDVA when compared to astigmatic patients that did receive a toric IOL and when compared to non-astigmatic patients receiving a non-toric IOL.
The data for Group 2 were collected retrospectively for patients that were treated before we adopted out protocol for the use of toric IOLs. Therefore, we do not have objective evidence of absence of cystoid macular oedema or other ocular pathology that may account for the reduced post-operative CDVA in this group. However, the data show that reduced CDVA is not restricted to a small number of patients with very poor outcomes and it would be unlikely that undetected post-operative pathology would be widespread in this group of patients. Nonetheless, a prospective analysis on larger numbers of patients would yield further evidence to shed light on this aspect of our study. The data for Group 2 patients also show a lack of statistically significant difference between pre-operative CDVA and post-operative UCVA, unlike the marked difference enjoyed by patients in Groups 1 and 3.
There are various methods described in the literature to correct pre-existing corneal astigmatism[2,3] at the time of cataract surgery. Toric IOL is one of the possible options with good potential and has been increasingly used by many cataract surgeons to address corneal astigmatism. Corneal astigmatism of more than 1.2 D cannot be corrected by a 2.8 to 3.2 mm incision at the limbus; wider or additional incisions would be needed.[6,7,8] Accurate measurements to calculate IOL power, IOL placement and IOL rotational stability are the key factors for the success and achieving good results following toric IOL implantation. Some studies found a tendency of the studied IOL to rotate in the early postoperative period.[9,10] Rotation by 10 degrees reduces the cylindrical power of a toric IOL by approximately one-third, which increases to two-thirds if the IOL rotates off axis by 20 degrees.[10]
In our study, 76% of eyes achieved UDVA of LogMAR 0.3 (20/40) and all eyes achieved CDVA of LogMAR 0.3 (20/40) or better in the toric IOL group. Entabi et al.[10] recently published their results using the same toric IOL and in their series 69.7% of patients had UDVA of LogMAR 0.3 (20/40) or better. In their study, 36% of eyes had UDVA of LogMAR 0.2 (20/32) or better and 16% of eyes had UDVA of LogMAR 0.0 (20/20) or better. The mean keratometric astigmatism in their study was 2.94 D pre-operatively (Range 2.00 to 5.25 D) and 2.42 D post-operatively (Range 1.75 to 4.75 D). Entabi et al.[10] found CDVA of LogMAR 0.3 (20/40) or better in 96.9%, LogMAR 0.2 (20/32) or better in 81.8%, and LogMAR 0.0 (20/20) or better in 21.1%. Visser et al.[11] published results with the use of AcrySof toric IOL and reported UDVA of LogMAR 0.3 (20/40) or better in 83% of eyes and UDVA of LogMAR 0.0 (20/20) or better in 5% of eyes. In their study the mean keratometric astigmatism was 3.43 D pre-operatively while in our study the mean pre-operative keratometric astigmatism was 3.77 D with a range of 2.8-7.6 D.
There have been significant publications on the use of AcrySof toric IOL with good results though most studies had eyes with low to moderate astigmatism.[12,13] In our study the mean preoperative cylinder was much higher where 92% of eyes in the toric group had more than 3 D of corneal astigmatism. This makes it difficult to compare our results with other published papers. Alioó et al.[14] evaluated the AcriComfort toric IOL implanted in 21 eyes with a mean preoperative astigmatism of 3.73 D; 76% of eyes achieved UDVA of LogMAR 0.3 (20/20) or better postoperatively which is quite similar to our results.
In our study astigmatism reduced from 3.77 D preoperatively to 1.2 D postoperatively in the toric IOL group and 48% of eyes had ≤1 D of refractive astigmatism postoperatively. All 25 eyes had astigmatism reduced in the toric IOL group and none of the eyes had an increase in the astigmatism. In our series, eyes with significant postoperative astigmatism (1 D or more) had high preoperative astigmatism (4 D and above). In general, in our study and in others, astigmatic eyes receiving a toric IOL seem to experience an under correction of the preoperative astigmatism. The possible reasons are not clear but may include imperfections in preoperative measurements and calculation methods for determining the dioptric power of IOL required.
We also compared our results to two other groups in this study who received a non-toric IOL; Group 2 with 18 eyes that had more than 2.5 diopters of corneal astigmatism and Group 3 with 25 eyes that had less than 1.5 D of astigmatism. Group 2 had no significant improvement in the astigmatism, displayed a high manifest refractive cylinder post-operatively and enjoyed a lower UDVA and CDVA than the other groups. However, the toric IOL group and Group 3 had quite similar post-operative refractive cylinder, UDVA and CDVA postoperatively. If we consider that the majority of cataract patients do not present with high corneal astigmatism and will receive a non-toric IOL, then it is the refractive and visual outcomes of these patients against which we should compare the outcomes of astigmatic patients.
We would argue that astigmatic patients should receive the necessary treatment to ensure they achieve the most favorable outcomes possible when compared to non-astigmatic patients.
In conclusion, the use of a toric IOL can significantly improve outcomes in the presence of pre-existing corneal astigmatism, and offer resultant UDVA postoperatively that compares well to the outcomes enjoyed by the majority of patients that do not require a toric IOL. Use of a toric IOL is safe, effective and reasonably predictable though it does require thorough planning and accurate execution. Larger studies with greater patient numbers would be welcomed to add further weight to the body of evidence in respect of toric IOL use in the case of astigmatic patients.
Acknowledgement
Authors are grateful to Dr. D Janicek and Surgical Day Unit of Singleton hospital for helping with the study.
Footnotes
Source of Support: Dr. Khan and Dr Ch’ng have no conflict of interest but Dr. Muhtaseb has since submission become a Consultant for Rayner Ltd UK.
Conflict of Interest: None declared.
References
- 1.Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric Intraocular lens in subjects with cataracts and corneal astigmatism. A randomized, subject-masked parallel group 1 year study. Ophthalmology. 2010;117:2104–11. doi: 10.1016/j.ophtha.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Khan MI, Muhtaseb M. Prevalence of corneal astigmatism in patients attending for routine cataract surgery at a teaching hospital in the United Kingdom. J Cataract Refract Surg. 2011;37:1751–5. doi: 10.1016/j.jcrs.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, González-Méijome JM, Cerviño A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35:70–5. doi: 10.1016/j.jcrs.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Hoffer KJ. Biometry of 7,500 cataractous eyes. Am J Ophthalmol. 1980;90:360–8. doi: 10.1016/s0002-9394(14)74917-7. [DOI] [PubMed] [Google Scholar]
- 5.Nichamin LD. Astigmatism control. Ophthalmol Clin North Am. 2006;19:485–93. doi: 10.1016/j.ohc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Akura J, Kaneda S, Hatta S, Matsuura K. Controlling astigmatism in cataract surgery requiring relatively large self-sealing incisions. J Cataract Refract Surg. 2000;26:1650–9. doi: 10.1016/s0886-3350(00)00484-3. [DOI] [PubMed] [Google Scholar]
- 7.Ben Simon GJ, Desatnik H. Correction of pre-existing astigmatism during cataract surgery: Comparison between the effects of opposite clear corneal incisions and a single clear corneal incision. Graefes Arch Clin Exp Ophthalmol. 2005;243:321–6. doi: 10.1007/s00417-004-1035-3. [DOI] [PubMed] [Google Scholar]
- 8.Mendicute J, Irigoyen C, Ruiz M, Illarramendi I, Ferrer-Blasco T, Monté s-Micó R. Toric intraocular lens versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. J Cataract Refract Surg. 2009;35:451–8. doi: 10.1016/j.jcrs.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Chang DF. Comparative rotational stability of single-piece openloop acrylic and plate-haptic silicone toric intraocular lenses. J Cataract Refract Surg. 2008;34:1842–7. doi: 10.1016/j.jcrs.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Entabi M, Harman F, Lee N, Bloom PA. Injectable 1-piece hydrophilic acrylic toric intraocular lens for cataract surgery: Efficacy and stability. J Cataract Refract Surg. 2011;37:235–40. doi: 10.1016/j.jcrs.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Visser N, Ruíz-Mesa R, Pastor F, Bauer NJ, Nuijts RM, Montés-Micó R. Cataract surgery with toric intraocular lens implantation in patients with high corneal astigmatism. J Cataract Refract Surg. 2011;37:1403–10. doi: 10.1016/j.jcrs.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Bauer NJ, de Vries NE, Webers CA, Hendrikse F, Nuijts RM. Astigmatism management in cataract surgery with the AcrySof toric intraocular lens. J Cataract Refract Surg. 2008;34:1483–8. doi: 10.1016/j.jcrs.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Mendicute J, Irigoyen C, Aramberri J, Ondarra A, Montés-Micó R. Foldable toric intraocular lens for astigmatism correction in cataract patients. J Cataract Refract Surg. 2008;34:601–7. doi: 10.1016/j.jcrs.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Alioì JL, Agdeppa MC, Pongo VC, El Kady B. Microincision cataract surgery with toric intraocular lens implantation for correcting moderate and high astigmatism: Pilot study. J Cataract Refract Surg. 2010;36:44–52. doi: 10.1016/j.jcrs.2009.07.043. [DOI] [PubMed] [Google Scholar]


