Abstract
Purpose:
The purpose was to compare transepithelial versus epithelium off technique of corneal collagen crosslinking (CXL) in patients of keratoconus.
Materials and Method:
Totally, 40 eyes (40 patients) with progressive keratoconus were subjected to transepithelial CXL (20 eyes; Group I), and conventional CXL (20 eyes; Group II). Patients were evaluated for uncorrected distance visual acuity, best corrected visual acuity (BCVA), slit lamp-biomicroscopy, keratometry, 9-point pachymentry, orbscan II, and endothelial cell count at baseline and post CXL at 1, 3, and 6 months.
Results:
The two groups were similar with respect to the evaluated parameters both at baseline and at the end of 6 months. There was an improvement in mean BCVA from Log Mar 0.327 ± 0.1 (Group 1), 0.36 ± 0.08 (Group 2) to 0.23 ± 0.08 (Group 1; P < 0.001), 0.22 ± 0.06 (Group 2; P < 0.001), respectively, at 6 months. Mean Sim K astigmatism decreased from 6.6 ± 1.93 D (Group 1), 6.64 ± 1.93 D (Group 2) to 5.14 ± 1.86 D (Group 1; P = 0.001), and 4.77 ± 0.06 (Group 2; P = 0.001), respectively, at 6 months. The mean pachymetry increased from 432.05 ± 19.36 μm (Group 1), 429.91 ± 16.66 μm (Group 2) to 447.8 ± 16.09 μm (Group 1; P < 0.001), 440.25 ± 11.18 um (Group 2; P = 0.002), respectively, at 6 months. All cases showed stabilization of keratoconus two eyes in epithelium off group developed persistent stromal haze. Most of the patients in Group II experienced pain and photophobia during first 2 days, but not of Group I.
Conclusion:
Trans-epithelial technique offers visual and topographic outcomes similar to the conventional method with superior patient comfort postintervention.
Keywords: Keratoconus, riboflavin, trans-epithelial technique of collagen cross-linking, ultraviolet a radiation
Introduction
Corneal collagen cross-linking (CXL) aided by photosensitized riboflavin helps stabilize progressive keratoconus and may delay the need for keratoplasty.[1] Conventionally, epithelial debridement is done before exposing the corneal stroma to ultraviolet-A (UVA) in order to enhance the stromal penetration of photo-activated riboflavin. However debriding the epithelium involves the inherent risk of corneal infection, sub-epithelial haze, sterile corneal infiltrates, corneal scarring, endothelial damage, and herpetic activation.[1,2,3,4,5] Thus, trans-epithelial technique came into vogue, which was thought to combine the advantages of the conventional technique in addition to maintaining a higher safety profile.[6,7]
With this background, we carried out CXL in 20 keratoconic eyes by transepithelial technique (Group I) and conventional technique in other 20 eyes (Group II) and compared the visual outcome, change in topographic pattern, corneal thickness, and endothelial cell density (ECD) between the two groups at baseline and at the end of follow-up (6 months).
Materials and Methods
Ethical clearance was obtained from the Institutional Review Board prior to conducting this study. 40 consecutive patients presenting to cornea services at a tertiary care center with documented keratoconus progression on topography over at least 1-year follow-up were selected, and informed consent for the procedure was obtained. These patients were then randomly allocated to either of the two groups according to odd even number method (randomized control trial). Inclusion criteria included patients with age more than 18 years, documented keratoconus progression (>1 D increase in steep K/12 months or >0.5 D in 6 months), no evidence of corneal scarring, keratometry between 47 D and 55 D and corneal thickness at the thinnest point ≥400 μm.
Pre-crosslinking evaluation included uncorrected distance visual acuity (UCDVA), Contact lens corrected distance visual acuity (CDVA), corneal topography (on Orbscan II [Bausch and Lomb, Salt Lake City, Utah]), central corneal thickness (CCT) using ultrasonic pachymenty and ECD by center to center method (Konan Specular Microscope, Konan Medical USA., Torrance, CA). Values for steepest keratometry (K max), flattest keratometry (K min) and keratometric astigmatism were obtained from Orbscan II.
The procedure was carried out under strict aseptic conditions in the operation theatre. In the epithelium on method, Proparacaine (0.5%) anesthetic drops were instilled thrice every 5 min before the introduction of isotonic solution of 0.1% riboflavin in 20% dextran (Nano XL, New Taipei City, Taiwan). The eye was then cleaned and draped, riboflavin drops were instilled every 3-5 min for 30 min, along with frequent proparacaine eye drops. End point was confirmed by observing anterior chamber fluorescence on slit lamp biomicroscopy at the end of ½ h. It was followed by UVA radiation using two UV diodes, with a desired irradiance of 3 mW/cm2 controlled with a UVA meter at 1 cm distance (wavelength 765 nm; CL-UVR machine, Appasamy Associates, Chennai, Tamil Nadu, India) for next 30 min with associated use of riboflavin and proparacaine eye drops every 3-5 min.
In Group II, after loosening the epithelium with proparacaine drops instilled thrice every 5 min, central 7 mm corneal epithelium marked using a disposable corneal trephine (Storz Ophthalmics, St Louis, Missouri, USA) was scraped off with a merosel sponge. Similar protocol for CXL as mentioned above was then applied. A soft bandage contact lens was prescribed to Group 2 patients which was removed approximately at day 3 after ensuring complete epithelial healing. Patients were prescribed topical drops moxifloxacin 0.3% four times a day (Cipla, India) 4 times a day, predacetate 1% QID to Group 1 (tapered gradually and stopped at 1 month) since day 1 and after epithelial healing in Group II and oral nonsteroidal anti-inflammatory drug three times daily for 1 week. The patients were followed-up daily until complete re-epithelialization in Group II and at day 1 and day 7 in Group I, and subsequently at 1, 3 and 6 months post cross-linking. UCDVA, CDVA, topography (Videokeratography, orbscan II), pachymetry, and ECD were documented at each followup visit. Subjective pain analysis as experienced by the patients in each group was done.
Data analysis
Visual acuity was measured using Snellen's chart and was converted to logarithm of the minimum angle of resolution (logMAR) for calculation of means. All variables were fitted into a normal distribution and parametric analysis using meanand standard deviation was employed for quantitative data and percentages for qualitative data. The significance between parameters was assessed by Student's t-test for paired values and Chi-square test for nonparametric variables. For calculating the difference between the values of the two groups at baseline and after therapy, two sample t-test was applied. Significance was set at P < 0.05.
Results
The mean age of patients in Group 1 was 22.35 ± 3.95 years, with a range of 18-26 years. In Group 2, the mean age was 23.95 ± 4.08 years (range: 18-30 years). There was no significant difference in the age of patients between the two groups (P = 0.21). Eighty percent of the study population was male, and 20% were female. There were 17 males (85%) and three females (15%) in Group 1 while there were 15 males (75%) and five females (25%) in Group 2. The gender distribution was thus comparable (P = 0.7).
The CDVA, expressed in logMAR units showed statistically significant improvement (P < 0.001) at all points in time when the baseline acuity was compared with post-CXL acuity in both groups. Vision was 0.321 ± 0.1 logMAR at baseline in Group 1, improved to 0.32 ± 0.09 1 month later and changed to 0.23 ± 0.08 at the end of 6 months (P < 0.001). Similarly, in Group 2, baseline CDVA was 0.36 ± 0.08 logMAR at baseline which improved to 0.22 ± 0.06 at 6 months (Group 2; P < 0.001). Notably, there was no difference between the two groups in relation to visual acuity at baseline (P = 0.27) and at any time post CXL till 6 months (P = 0.7; two sample t-test) [Table 1].
Table 1.
CDVA, keratometric indices between groups I and II at each follow-up
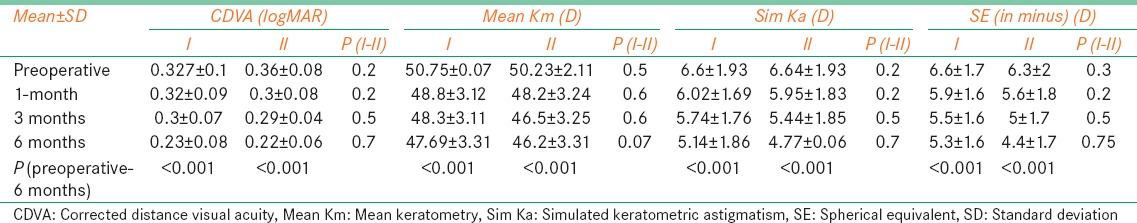
Mean Km in Group 1 preoperatively was 50.75 ± 0.07 D (range 46.05 D to 50.94 D) and that in Group 2 was 50.23 ± 2.11 D (range 46.9 D to 50.9 D). The difference between two groups at baseline was not significant (P = 0.48). Mean sim K astigmatism in Group 1 preoperatively was 6.6 ± 1.9 D (range 4.7 D to 10.7 D) and in Group 2 was 6.64 ± 1.93 D (range 4.4 D to 10.7 D). The difference between two groups was not significant (P = 0.2). Mean spherical equivalent (SE) refraction in Group 1 preoperatively was −6.6 ± 1.76 DS (range − 2.25 DS to − 8.5 DS) and in Group 2 was −6.28 ± 1.97 DS (range − 2.55 DS to − 8.75 DS) (P = 0.2; two-sample t-test). There was no difference in any of the calculated topographic indices at baseline between the two groups.
The mean baseline steepest keratometry, flattest keratometry, and keratometric astigmatism in Group 1 were 53.64 ± 4.1 D, 47.74 ± 4.72 D, and 5.9 ± 2.25 D, respectively [Table 1]. On the contrary, the mean baseline steepest keratometry, flattest keratometry, and keratometric astigmatism in Group 2 were 53.91 ± 3.77 D, 47.17 ± 4.6 D, and 6.73 ± 2.81 D, respectively. There was no difference in any of the calculated topographic indices at baseline between the two groups. Post cross-linking in Group 1, there was a significant decline in the steepest as well as the flattest K values at 1, 3 and 6 months (P < 0.05). However, no such statistically significant difference was observed in keratometric astigmatism at 1 and 3 months, but was seen at 6 months when compared with baseline in Group 1. Steepest K declined from 53.64 ± 4.1 D at baseline to 50.39 ± 4.57 D at the end of 6 months (P = 0.002), flattest K decreased from 47.74 ± 4.72 D to 46.1 ± 4.58 D (P = 0.015) and keratometric astigmatism changed from 5.9 ± 2.25 D to 4.29 ± 2.28 D at the end of 6 months in Group 1 subjects (P = 0.014) [Table 1]. Similarly, patients in Group 2 experienced decline in steepest K from 53.91 ± 3.77 D at baseline to 50.51 ± 4.49 D at the end of 6 months (P = 0.008), lowest K decreased from 47.17 ± 4.6 D to 46.05 ± 4.58 D (P = 0.014) and keratometric astigmatism changed from 6.73 ± 2.81 D to 4.46 ± 2.84 D (P = 0.014) at the end of 6 months [Table 1].
The mean baseline CCT in Group I was 432.05 ± 19.36 μm. At 3 months and 6 months, the values were 435.9 ± 16.58 μm and 447.8 ± 16.09 μm respectively. There was a statistically significant difference between the baseline and 3 months (P = 0.001); and between the baseline and 6 months (P < 0.001) after the procedure. Similarly in the conventional group, the mean CCT was 429.91 ± 16.66 μm at baseline, 435.1 + 19.02 um at 3 months and the end of 6 months it was 440.25 ± 11.18 um. In this group, though the corneal thickness increased significantly between baseline and at last follow-up of 6 month (P = 0.002), there was no change at 3 months in corneal thickness when compared to the baseline values (P = 0.2). There was no significant difference in the CCT values between the two groups at baseline (P = 0.7; two sample t-test), 3 months (P = 0.8) or at 6 month follow-up (P = 0.09).
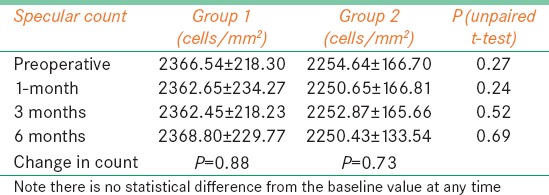
The mean baseline endothelial cell count in Group I was 2366.5 ± 218.3 cells/mm2. One month after the procedure, it was 2362.65 ± 234.3 cells/mm2; at 3 months 2362.45 ± 218.2 cells/mm2; at 6 months 2368.8 ± 229.8 cells/mm2 and after 12 months 2364.67 ± 208.3 cells/mm2 [Table 2]. The difference between the baseline and 12 months after the procedure was not statistically significant (P = 0.88), indicating that CXL did not induce endothelial damage during follow-up. Baseline mean endothelial cell count in the conventional group was 2254.64 ± 166.7 cells/mm2. At 1 month, it was 2250.65 ± 166.8, at 3 months post treatment, it was 2252.9 ± 165.7 cells/mm2 and at 6 months, 2250.4 ± 133.54 cells/mm2. The difference between endothelial cell count at any time during follow-up and between baseline and at 6 months was not significant (P = 0.73).
Table 2.
Endothelial cell density in the two groups at different points in time
In trans-epithelial group none of the eyes showed any complication, however, in the epithelium off group, there was stromal haze in two eyes in the posterior stroma which appeared early in the post-operative course and persisted till 3-4 months. Most of the patients in Group II experienced pain and photophobia during first 2 days, but none in Group I. None of the eyes presented with post cross-linking infection, sterile infiltrates, edema or significant rise in intraocular tension. No other adverse systemic events were noted in the treatment population.
Discussion
Corneal collagen cross-linking is one of the most promising procedures to halt the progression of keratoconus. It strengthens the cornea by covalently cross-linking the collagen fibres, increases rigidity by 300%, reduces the morbidity of progressive disease and ultimately may decline the need for corneal transplantation.[1] Trans-epithelial method of CXL, a recent technique, was introduced with the intention to reduce the risk of inherent complications associated with the conventional debridement method.[1,2,3] CXL with epithelium-on technique theoretically reduces the risk of corneal infection (it obviates the need for soft contact lens use), subepithelial haze, sterile infiltrates, herpetic reactivation, and endothelial damage. Moreover in the absence of epithelium debridement, the procedure becomes more comfortable for the patient, saves time and thinner corneas with CCT lesser than 400 μm can also be subjected to CXL.[9] Confocal microscopic studies have shown the preservation of sub basal nerve plexus in patients with epithelium on technique unlike in those with a conventional method.[10] However because of reduced penetration by riboflavin through the intact epithelium, it involves greater risk of treatment failure.[10]
Baiocchi et al. had concluded that a theoretically safe and effective riboflavin concentration could be achieved for CXL only after the epithelium was removed and not in intact epithelium.[11] In order to increase the permeability of riboflavin through intact epithelium, different innovations in the standard protocol have been suggested by separate authors. Stojanovic et al. used multiple methods to enhance permeability of riboflavin in transepithelial technique of CXL namely, chemical disruption of epithelial tight junctions (use of benzalkonium chloride-containing local medication and hypotonic riboflavin solution), mechanical disruption of the superficial epithelium, and use of prolonged riboflavin-induction.[6] They found that the epithelium on method is effective in reducing SE, cylindrical equivalent, K-max, and improving CDVA without any significant adverse effects. Boxer Wachler et al. used tetracaine and critical micelle concentration in their modification of the transepithelial technique and reported that their method is effective with a very low 4-year retreatment rate.[12] Proparacaine drops were preserved with 0.01% benzalkonium chloride which enhances the penetration of riboflavin by chemical disruption of tight junctions in the epithelium.[8] Preservatives act by loosening and disrupting the corneal epithelium and enhance corneal penetration of topically applied substances through intact epithelium.[8,13] Raiskup et al. found that a higher (0.02%) and a lower concentration of blood alcohol content (0.01%) both achieve statistically similar extent of riboflavin absorption through the corneal epithelium.[13]
In our study, we used 0.1% riboflavin in 20% dextran along with frequent instillation of anesthetic drops enabling riboflavin to penetrate the corneal stroma. Though using this technique clinically, beneficial effects were not observed by some groups,[14] we found that the change in mean Km and Sim Km was equivalent to that seen after conventional CXL in our series of patients and also similar to that quoted in literature.[15,16,17] Further, our results revealed that all eyes showed halt in the progression of the disease, an increase in the corneal thickness along with the improvement in CDVA.
We found that both the methods of CXL caused an increase in the central pachymetry values at 3 and 6-month follow-up when compared to the pre-intervention values. There is conflicting evidence in the literature regarding the corneal thickness change after CXL. Few studies report that CXL causes an initial decline in CCT at 1 month following the procedure after which it recovers to normal thickness at 3 and 6 months.[18,19,20,21] Some others report that there is no change in corneal thickness over long-term follow-up.[22,23] However, the eventual increase in corneal thickness has also been documented by some studies.[23,24,25]Choi et al. also showed in ex vivo models of cornea that there is an increase in corneal thickness to 107% post CXL administration.[26] We observed increased corneal thickness over a follow-up period of 6 months. This increased pachymetry could be due to lamellar remodeling of the treated cornea or due to stromal edema following therapy.[26]
However, it is certain from our study that epithelial debridement does not influence the final pachymetry reading. Similarly, none of the participating eyes in either group experienced a significant decrease in endothelial cell count post cross-linking. These results are in agreement with past studies.[9,19,27,28]
The posterior stromal haze seen in two of our eyes in the epithelium off group is known to be secondary to a myofibroblast generation.[29,30] It has maximum intensity at 1 month, and it significantly decreases between 3 and 12 months. The presence/absence of haze does not correlate with clinical results.[30] The risk of haze development is the greatest in cases with advanced keratoconus.[31] There is no study so far which proves whether one method of CXL is superior to another in relation to preventing corneal haze formation. Though we saw haze formation in 10% of our cases with epithelium off, we need a larger population size in order to prove this relationship in statistical terms. Furthermore, there was superior ocular comfort in patients of Group I with respect to pain experienced.
The limitations of our study include a small number of patients in each group and short follow-up. Nevertheless, in this study, we observed that the trans-epithelial technique of CXL using isotonic riboflavin in 20% dextran in conjunction with liberal use of anesthetic drops containing preservatives (0.05% BAC in our case) is as effective as the conventional technique in halting the progression of keratoconus. With the exception of persistent stromal haze in 10% eyes with epithelium off CXL, the two treatment modalities were equivalent in terms of biometric results and post-operative complications. However, since superior comfort was experienced by patients in the trans-epithelial group, this method may replace the conventional method of CXL as the standard technique in the near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hovakimyan M, Guthoff RF, Stachs O. Collagen cross-linking: Current status and future directions. J Ophthalmol 2012. 2012:406850. doi: 10.1155/2012/406850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spoerl E, Hoyer A, Pillunat LE, Raiskup F. Corneal cross-linking and safety issues. Open Ophthalmol J. 2011;5:14–6. doi: 10.2174/1874364101105010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhawan S, Rao K, Natrajan S. Complications of corneal collagen cross-linking. J Ophthalmol 2011. 2011:869015. doi: 10.1155/2011/869015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kymionis GD, Portaliou DM, Bouzoukis DI, Suh LH, Pallikaris AI, Markomanolakis M, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg. 2007;33:1982–4. doi: 10.1016/j.jcrs.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009;35:540–6. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Stojanovic A, Chen X, Jin N, Zhang T, Stojanovic F, Raeder S, et al. Safety and efficacy of epithelium-on corneal collagen cross-linking using a multifactorial approach to achieve proper stromal riboflavin saturation. J Ophthalmol 2012. 2012:498435. doi: 10.1155/2012/498435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri K, Hammersmith KM, Nagra PK. Corneal collagen cross-linking: Ectasia and beyond. Curr Opin Ophthalmol. 2012;23:280–7. doi: 10.1097/ICU.0b013e328354865e. [DOI] [PubMed] [Google Scholar]
- 8.Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38:1000–5. doi: 10.1016/j.jcrs.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Filippello M, Stagni E, Buccoliero D, Bonfiglio V, Avitabile T. Transepithelial cross-linking in keratoconus patients: Confocal analysis. Optom Vis Sci. 2012;89:e1–7. doi: 10.1097/OPX.0b013e318269c8e5. [DOI] [PubMed] [Google Scholar]
- 10.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Paradiso AL. Transepithelial corneal collagen crosslinking for keratoconus: Qualitative investigation by in vivo HRT II confocal analysis. Eur J Ophthalmol. 2012;22(Suppl 7):S81–8. doi: 10.5301/ejo.5000125. [DOI] [PubMed] [Google Scholar]
- 11.Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: Riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009;35:893–9. doi: 10.1016/j.jcrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Boxer Wachler BS, Pinelli R, Ertan A, Chan CC. Safety and efficacy of transepithelial crosslinking (C3-R/CXL) J Cataract Refract Surg. 2010;36:186–8. doi: 10.1016/j.jcrs.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Raiskup F, Pinelli R, Spoerl E. Riboflavin osmolar modification for transepithelial corneal cross-linking. Curr Eye Res. 2012;37:234–8. doi: 10.3109/02713683.2011.637656. [DOI] [PubMed] [Google Scholar]
- 14.Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010;26:942–8. doi: 10.3928/1081597X-20100212-09. [DOI] [PubMed] [Google Scholar]
- 15.Goldich Y, Marcovich AL, Barkana Y, Mandel Y, Hirsh A, Morad Y, et al. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: Results after 2 years of follow-up. Cornea. 2012;31:609–14. doi: 10.1097/ICO.0b013e318226bf4a. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal VB. Corneal collagen cross-linking with riboflavin and ultraviolet-a light for keratoconus: Results in Indian eyes. Indian J Ophthalmol. 2009;57:111–4. doi: 10.4103/0301-4738.44515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saffarian L, Khakshoor H, Zarei-Ghanavati M, Esmaily H. Corneal Crosslinking for Keratoconus in Iranian Patients: Outcomes at 1 year following treatment. Middle East Afr J Ophthalmol. 2010;17:365–8. doi: 10.4103/0974-9233.71600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mencucci R, Paladini I, Virgili G, Giacomelli G, Menchini U. Corneal thickness measurements using time-domain anterior segment OCT, ultrasound, and Scheimpflug tomographer pachymetry before and after corneal cross-linking for keratoconus. J Refract Surg. 2012;28:562–6. doi: 10.3928/1081597X-20120703-02. [DOI] [PubMed] [Google Scholar]
- 19.Vinciguerra P, Camesasca FI, Albè E, Trazza S. Corneal collagen cross-linking for ectasia after excimer laser refractive surgery: 1-year results. J Refract Surg. 2010;26:486–97. doi: 10.3928/1081597X-20090910-02. [DOI] [PubMed] [Google Scholar]
- 20.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Alió JL, Toffaha BT, Piñero DP, Klonowski P, Javaloy J. Cross-linking in progressive keratoconus using an epithelial debridement or intrastromal pocket technique after previous corneal ring segment implantation. J Refract Surg. 2011;27:737–43. doi: 10.3928/1081597X-20110705-01. [DOI] [PubMed] [Google Scholar]
- 22.Goldich Y, Barkana Y, Wussuku Lior O, Marcovich AL, Hirsh A, Avni I, et al. Corneal collagen cross-linking for the treatment of progressive keratoconus: 3-year prospective outcome. Can J Ophthalmol. 2014;49:54–9. doi: 10.1016/j.jcjo.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Cinar Y, Cingü AK, Turkcu FM, Yüksel H, Sahin A, Yildirim A, et al. Accelerated corneal collagen cross-linking for progressive keratoconus. Cutan Ocul Toxicol. 2014;33:168–71. doi: 10.3109/15569527.2013.816724. [DOI] [PubMed] [Google Scholar]
- 24.Dong Z, Zhou X. Collagen cross-linking with riboflavin in a femtosecond laser-created pocket in rabbit corneas: 6-month results. Am J Ophthalmol. 2011;152:22–271. doi: 10.1016/j.ajo.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 25.He X, Spoerl E, Tang J, Liu J. Measurement of corneal changes after collagen crosslinking using a noninvasive ultrasound system. J Cataract Refract Surg. 2010;36:1207–12. doi: 10.1016/j.jcrs.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Lee SC, Lee HJ, Cheong Y, Jung GB, Jin KH, et al. Structural response of human corneal and scleral tissues to collagen cross-linking treatment with riboflavin and ultraviolet A light. Lasers Med Sci. 2013;28:1289–96. doi: 10.1007/s10103-012-1237-6. [DOI] [PubMed] [Google Scholar]
- 27.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: Preliminary results. J Refract Surg. 2008;24:S720–5. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 28.Vinciguerra P, Albè E, Trazza S, Rosetta P, Vinciguerra R, Seiler T, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–78. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 29.Kymionis GD, Portaliou DM, Diakonis VF, Kontadakis GA, Krasia MS, Papadiamantis AG, et al. Posterior linear stromal haze formation after simultaneous photorefractive keratectomy followed by corneal collagen cross-linking. Invest Ophthalmol Vis Sci. 2010;51:5030–3. doi: 10.1167/iovs.09-5105. [DOI] [PubMed] [Google Scholar]
- 30.Salomão MQ, Chaurasia SS, Sinha-Roy A, Ambrósio R, Jr, Esposito A, Sepulveda R, et al. Corneal wound healing after ultraviolet-A/riboflavin collagen cross-linking: A rabbit study. J Refract Surg. 2011;27:401–7. doi: 10.3928/1081597X-20101201-02. [DOI] [PubMed] [Google Scholar]
- 31.Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009;25:S824–8. doi: 10.3928/1081597X-20090813-12. [DOI] [PubMed] [Google Scholar]


