Abstract
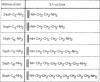
A homologous series of ω-aminoalkylagaroses [Sepharose-NH(CH2)nNH2] that varied in the length of their hydrocarbon side chains was synthesized. This family of agaroses was used for a new type of chromatography, in which retention of proteins is achieved mainly through lipophilic interactions between the hydrocarbon side chains on the agarose and accessible hydrophobic pockets in the protein.
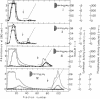
When an extract of rabbit muscle was subjected to chromatography on these modified agaroses, the columns with short “arms” (n = 2 and n = 3) excluded glycogen synthetase (EC 2.4.1.11), but the enzyme was retained on δ-aminobutyl-agarose (n = 4), from which it could be eluted with a linear NaCl gradient. Higher members of this series (e.g., n = 6) bind the synthetase so tightly that it can be eluted only in a denatured form. A column of δ-aminobutyl-agarose, which retained the synthetase, excluded glycogen phosphorylase (EC 2.4.1.1), which in this column series and under the same conditions requires side chains 5-(or 6)-carbon-atoms long for retention. Therefore, it is possible to isolate glycogen synthetase by passage of muscle extract through δ-aminobutyl-agarose, then to extract phosphorylase by subjecting the excluded proteins to chromatography on ω-aminohexyl-agarose (n = 6). On a preparative scale, the synthetase (I form) was purified 25- to 50-fold in one step.
This paper describes some basic features and potential uses of hydrophobic chromatography. The relevance of the results presented here to the design and use of affinity chromatography columns is discussed.
Keywords: protein purification, ω-aminoalkyl-agaroses, glycogen phosphorylase, lipophilic membrane proteins, affinity chromatography
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Young M. Rapid and complete purification of acetylcholinesterases of electric eel and erythrocyte by affinity chromatography. Proc Natl Acad Sci U S A. 1971 Feb;68(2):395–398. doi: 10.1073/pnas.68.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Agarose derivatives for purification of protein by affinity chromatography. Nature. 1970 Dec 26;228(5278):1327–1328. doi: 10.1038/2281327a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Silman I., Kalderon N., Blumberg S. Purification by affinity chromatography of acetylcholinesterase from electric organ tissue of the electric eel subsequent to tryptic treatment. Biochim Biophys Acta. 1972 Apr 7;268(1):138–157. doi: 10.1016/0005-2744(72)90208-2. [DOI] [PubMed] [Google Scholar]
- Er-el Z., Zaidenzaig Y., Shaltiel S. Hydrocarbon-coated sepharoses. Use in the purification of glycogen phosphorylase. Biochem Biophys Res Commun. 1972 Oct 17;49(2):383–390. doi: 10.1016/0006-291x(72)90422-6. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Fischer E. H. On the role of pyridoxal 5'-phosphate in phosphorylase. I. Absence of classical vitamin B6--dependent enzymatic activities in muscle glycogen phosphorylase. Biochemistry. 1965 Jul;4(7):1337–1343. doi: 10.1021/bi00883a018. [DOI] [PubMed] [Google Scholar]
- Miller J. V., Jr, Cuatrecasas P., Thompson E. B. Partial purification by affinity chromatography of tyrosine aminotransferase-synthesizing ribosomes from hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):1014–1018. doi: 10.1073/pnas.68.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold P. C., Harding N. G. Affinity chromatography of dihydrofolate reductase. Biochem J. 1971 Aug;124(1):1–12. doi: 10.1042/bj1240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Soderling T. R., Hickenbottom J. P., Reimann E. M., Hunkeler F. L., Walsh D. A., Krebs E. G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1970 Dec 10;245(23):6317–6328. [PubMed] [Google Scholar]
- Steers E., Jr, Cuatrecasas P., Pollard H. B. The purification of beta-galactosidase from Escherichia coli by affinity chromatography. J Biol Chem. 1971 Jan 10;246(1):196–200. [PubMed] [Google Scholar]
- Yon R. J. Chromatography of lipophilic proteins on adsorbents containing mixed hydrophobic and ionic groups. Biochem J. 1972 Feb;126(3):765–767. doi: 10.1042/bj1260765. [DOI] [PMC free article] [PubMed] [Google Scholar]