Abstract
Introduction
Given the overlapping modes of transmission of HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV), understanding the burden and relationship of these infections is critical for an effective response. Representative data on these infections among males who inject drugs (MWID), the key high-risk population for HIV in Vietnam, are currently lacking.
Methods
Data and stored specimens from Vietnam’s 2009-2010 Integrated Biologic and Behavioral Survey, a cross-sectional study among high-risk populations, were used for this analysis. Plasma samples were tested for HIV, HBV, and HCV using commercial assays. A questionnaire was administered to provide demographic, behavior, and service-uptake information. Provincial-level analyses were conducted to profile MWID enrollees and to provide estimates on the prevalence of HIV, HBV, and HCV infection.
Results
Among 3010 MWID sampled across 10 provinces, the median (range) HIV prevalence was 28.1% (1.0%-55.5%). Median prevalence for current HBV infection (HBsAg+) was 14.1% (11.7%-28.0%), for previous exposure to HBV (total anti-HBc+) was 71.4% (49.9%-83.1%), and for current or past HCV infection (HCV Ag/Ab+) was 53.8% (10.9%-80.8%). In adjusted analysis, HBsAg+ (aOR: 2.09, 1.01-4.34) and HCV Ag/Ab+ (aOR: 19.58, 13.07-29.33) status were significantly associated with HIV infection; the association with total anti-HBc+ approached significance (aOR: 1.29, 0.99-1.68).
Conclusion
The prevalence and association between HIV, HBV, and HCV are high among MWID in Vietnam. These findings indicate the need for integrated policies and practice that for the surveillance, prevention, screening, and treatment of both HIV and viral hepatitis among MWID in Vietnam.
Introduction
Similar modes of transmission of human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) can lead to an increased risk of HIV co-infection with HBV or HCV.[1] Because HIV, HBV, and HCV can be efficiently transmitted via percutaneous exposure to blood, people who inject drugs (PWID) are at especially high risk for infection and co-infection with these viruses and for transmission to others through unsafe needle sharing or sex practices.[2]
Worldwide, of an estimated 240 million chronic HBV infections, 1.2 million (0.5%) occur among people who inject drugs (PWID); of 170 million chronic HCV infections, 10 million (5.9%) occur among PWID.[3] Chronic HBV and HCV infections are associated with increased risk of cirrhosis and liver cancer and are responsible for more than 1 million deaths annually.[3–6] Recent estimates also indicate that a significant amount of hepatitis B, hepatitis C, and HIV burden, as determined by disease specific disability-adjusted life years, can be attributed to illegal injection drug use.[7] The prevalence of HCV co-infection among HIV-infected PWID (HCV/HIV) often exceed 50%.[1,2,8–11] Among people infected with HIV, co-infection with viral hepatitis adversely impacts morbidity and mortality and is becoming a leading cause of death among co-infected PWID, even in the era of anti-retroviral therapy (ART) to treat HIV/AIDS.[12,13]
Up to 335,000 active males who inject drugs (MWID) live in Vietnam, making it one of the six highest burden countries for injecting drug use worldwide.[14,15] Vietnams HIV epidemic is concentrated among high-risk populations, specifically MWID, female sex workers (FSW), and men who have sex with men (MSM).[15] Although these populations are not mutually exclusive, MWID are reported to have the highest HIV burden with national prevalence estimated to be 14.4%MWID [15] The prevalence of current hepatitis B surface antigen (HBsAg), a marker of active HBV infection and infectiousness, varies between 5.7% and 24.7% by population. An estimated 8.4 million (~10% of the population) people in Vietnam are also chronically infected with HBV.[16–18] The prevalence of antibody to HCV (anti-HCV), indicative of current infection or previous exposure to hepatitis C, ranges between 0.38% and 1.7% in the general population in Vietnam but between 31–98.5% among MWID.[2,16]
These hepatitis data come from a variety of relatively small, localized surveys which lack the ability to provide nationally or provincially representative data regarding the epidemiology and frequency of HBV, HCV, or co-infection with HIV for Vietnam. Neither HBV nor HCV is a required reportable disease in Vietnam, and the country currently lacks routine hepatitis surveillance systems.[16,17] Such data are needed about HBV and HCV infection among key populations, such as MWID, for understanding the burden and profile of these key infections to organize appropriate resources for an effective response. The objectives of this analysis are to profile behaviors relevant for HIV, HBV, and HCV acquisition and transmission; to present representative estimates of HIV, HBV, HCV, and co-infection prevalence; and to identify risk factors associated with HIV infection among MWID in Vietnam.
Methods
Study Population and Sampling
Data from the 2009 Integrated Behavioral and Biologic Survey (IBBS) were used for this study. The 2009 IBBS was a cross-sectional survey of populations at increased risk for HIV in Vietnam including male injecting drug users (MWIDs). The main objectives of IBBS were to assess a representative sample of these key populations to determine HIV prevalence, associated behaviors (e.g. condom use and needle-sharing behaviors), and exposure to HIV prevention and care services. During September 2009–February 2010, MWID, defined as individuals self-reporting illicit injecting drug use at least one time in the previous 30 days aged ≥18 years, were sampled across 10 geographically diverse provinces in Vietnam that have received focused support in addressing HIV via the US President’s Emergency Plan for AIDS Relief (PEPFAR) program. MWID were sampled in each province using either respondent driven sampling (RDS) (Hanoi, Da Nang, Ho Chi Minh City (HCMC), Can Tho) or two-staged time-location sampling (TLS) (Hai Phong, Quang Ninh, Nghe An, Yen Bai, Dong Nai, An Giang) based on formative provincial mapping conducted by the study team as described in previous reports.[19]Inclusion of injecting drug users in the Vietnam IBBS is limited to males given the assumption that the vast majority of drug injectors are male and to allow comparability to previous surveys.
Following recruit eligibility screening and informed consent, trained staff conducted individual interviews using a structured questionnaire. Blood samples were collected for HIV testing and remnant samples were stored for potential testing in the future. Participants were provided coupons and encouraged to return to obtain the HIV test results. All participants were compensated between 50,000–100,000 VND ($2.50-$5.00) for their time and travel expenses. A monetary compensation was also provided to RDS recruits who subsequently recruited peers into the study at defined study locations.
Written, informed consent was obtained by reading a standardized study description and consent form that was also provided to the study recruit for their review and signed agreement. This consent process, which included the provision for future testing on stored specimens, was approved by the below-described ethics review boards.
Ethical approval
The study protocol was reviewed and approved by the Vietnam National Institute of Hygiene and Epidemiology (NIHE) Ethics Review Board and the Internal Review Board of the U.S. Centers for Disease Control and Prevention.
Laboratory analysis
HIV status was determined per national guidelines by screening for antibody to HIV using Genscreen HIV Ultra HIV Ag/Ab (Bio-Rad) with confirmatory testing of those screened as HIV-positive by Determine HIV ½ (Alere) and Murex HIV Ag/Ab Combination (DiaSorin). An enrollee was classified as HIV-positive if all three tests were positive for HIV-infection. Ten percent of HIV-negative samples and 5% of HIV-positive samples were randomly selected and re-tested for quality assurance at the National Reference Laboratory at NIHE in Hanoi.[19]
In August 2012, stored plasma from the IBBS study underwent serologic testing for HBV and HCV infection at the Vietnam National Institute of Hygiene and Epidemiology (NIHE) in Hanoi. Testing for HBsAg to determine current HBV infection was conducted by using the Murex HBsAg Version 3; an optical density (OD) value >0.05 over the mean negative control was interpreted as positive for HBsAg.[20] Specimens with indeterminate, weakly positive or negative HBsAg results were also tested for total anti-HBc to determine previous HBV infection by using the ETI-AB-COREK PLUS (DiaSorin). The combination of results was used to classify the HBV infection status of each subject as: ‘current or active HBV infection’ (HBsAg+), ‘prior HBV infection’ (HBsAg-/total anti-HBc+) or ‘susceptible to future HBV’ (HBsAg-/total anti-HBc-). Specimen quantity was insufficient to test for antibody to hepatitis B surface antigen (anti-HBs). As such, a small proportion of MWID classified as susceptible might have been immune from vaccination although we anticipate this to be a small percentage given the low coverage rates of HBV vaccination in this age group and population.[16]
Stored specimens also underwent combined testing for the presence of antibody to HCV or HCV antigen (Murex HCV Ag/Ab Combination, DiaSorin). Specimens that tested indeterminate or weakly positive were re-tested by the same methods. An OD value > 0.20 over the mean negative controls was used as the cut-off definition for a positive result.[21] The subject’s HCV infection status was classified as: ‘past or current HCV infection’ (HCV Ag/Ab+); ‘indeterminate/weakly positive’ (if repeat testing remained indeterminate/weakly positive); or ‘no evidence of HCV infection’ (HCV Ag/Ab-).
Statistical Analysis
Simple frequencies and proportions were calculated for categorical variables by province; and mean and medians were calculated for continuous variables. Prevalence estimates for HIV, HBV, HCV, and co-infections among MWID with 95% confidence intervals (95% CI) were calculated by province. Comparison of categorical data was done using the chi-square test or Fisher’s exact test (if expected frequencies were less than 5). Any indicator missing more than 5% of the total responses by province were reported in the respective output tables. For provinces sampled through RDS, analyses were done by using RDS Analyst (v0.1)[22] with successive sampling estimator [23] applied apriori using mid-range MWID provincial population size estimates approved by the Vietnamese Ministry of Health. [24–25] The remaining six provinces were analyzed using STATA (v.12.0).[26]
Median values and ranges were calculated for the total of provinces sampled. The Mann-Whitney test was used to compare median values for different groups. Pairwise correlations, and their significance levels using t-test probabilities, were calculated and reported to estimate the provincial-level variation between HIV prevalence and HBV and HCV prevalence. For univariate and multivariate risk factor analysis, unweighted odds ratios (OR) and 95% CI were calculated by stratified (conditional) logistic regression with HIV status as the outcome variable and stratified by ‘province’ to protect against any confounding (e.g. Simpson’s paradox) effect that may appear in an unstratified analysis.[27] The rationale for combining data across all provinces was to provide an increased sample size effectively increasing the power to detect key estimates and associations resulting in more robust inferences. All independent variables indicating an association with HIV status (p< = 0.20) in the univariate analysis were entered into a multivariate conditional logistic regression model using backward step-wise selection and the Wald test after estimation to identify the most parsimonious model. Collinearity was assessed by examining the variance inflation factors (VIF) of the model; variables indicating strong collinearity (i.e. VIF >8.0) were removed from the final analysis.
Results
Population characteristics and risk behaviors
Among the 10 provinces, [28] 3010 (3%) of an estimated 98,900 [24] MWID were sampled. The median (range, by province) population was 2.03 (0.76–7.68) million (Table 1.1). The median estimated MWID population across the provinces sampled was 4,520 (1,197–46,213).[24,28] The median age was 30.0 (21.5–35.5) years with the majority (>50%) of enrollees self-reported never being married, but most (>90%) being sexually active.
Table 1.1. Demographic characteristics of MWID in select provinces in Vietnam, 2009.
| Province | Hanoi* | Hai Phong | Quang Ninh | Nghe An | Yen Bai | Da Nang* | Dong Nai | HCMC* | Can Tho* | An Giang | Median (Range) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Province Population | 6,844,100 | 1,904,100 | 1,177,200 | 2,952,000 | 764,400 | 973,800 | 2,720,800 | 7,681,700 | 1,214,100 | 2,153,700 | 2,028,900 (764,400–7,681,700) | |
| Estimated PWID population size | 46,213 | 7,194 | 4,873 | 7,922 | 4,166 | 1,197 | 2,127 | 21,566 | 2,263 | 1,379 | 4,520 (1,197–46,213) | |
| Sampled male MWID (n) | 297 | 298 | 299 | 298 | 348 | 288 | 300 | 310 | 272 | 300 | 298.5 (272–348) | |
| Age (years) | Mean (sd) | 31.5 (8.1) | 35.8 (7.0) | 31.6 (5.7)) | 30.4 (9.3) | 34.6 (7.7) | 24.9 (8.7) | 28.1 (9.1) | 29.2 (7.7) | 32.2 (9.4) | 25.6 (7.3) | 31.0 (24.9–35.8) |
| Median (IQR) | 30.6 (25.7–35.6) | 35.5 (31.2–40.4) | 31.2 (27.5–35.2) | 30.0 (24.6–35.0) | 34.3 (29.2–39.3) | 21.5 (19.4–26.2) | 26.3 (21.0–31.4) | 27.5 (24.0–33.0) | 29.9 (26.0–38.3) | 23.9 (20.0–28.4) | 30.0 (21.5–35.5) | |
| Ethnicity | Kinh (%) | 99.3 | 100 | 98.7 | 99.3 | 82.3 | 100 | 98 | 97.4 | 97.4 | 97.7 | 98.35 (82.3–100) |
| Education (%) | Illiterate | 3 | 0 | 0.3 | 0.3 | 2.9 | 0 | 3.7 | 5 | 12 | 14.1 | 2.95 (0–14.1) |
| Primary | 3.8 | 10.2 | 0.7 | 4.4 | 10.1 | 6.5 | 12.7 | 34.6 | 31.1 | 41.1 | 10.15 (0.7–41.1) | |
| Secondary or High School | 89.6 | 88.2 | 96 | 82.2 | 81.4 | 87.2 | 78.9 | 56.9 | 55.4 | 44.8 | 81.8 (44.8–96) | |
| College/University | 3.6 | 1.7 | 3 | 13.1 | 5.23 | 6.6 | 4.7 | 3.5 | 1.5 | 0 | 3.55 (0–13.1) | |
| Missing (#) | 55 | 1 | 1 | - | 4 | 2 | 1 | 45 | - | 1 | ||
| Marital status (%) | Never Married | 58.6 | 40.1 | 68.2 | 59.4 | 36 | 81.9 | 70 | 69.9 | 51.5 | 66.9 | 63.15 (36–81.9) |
| Currently Married | 30 | 24.2 | 21.4 | 35.2 | 47.6 | 12.5 | 23 | 16.5 | 28.3 | 20.7 | 23.6 (12.5–47.6) | |
| Divorced/Separated or Widowed | 11.8 | 35.7 | 10.3 | 5.4 | 16.5 | 5.5 | 7.1 | 13.6 | 20.2 | 12.4 | 11.45 (5.4–35.7) | |
| Sexually active (i.e. ever had sex) | 92.6 | 96.3 | 80.9 | 95.3 | 97.1 | 90.1 | 77.3 | 93.2 | 95.3 | 82.3 | 92.9 (77.3–97.1) | |
| Household Income (VND * 10,000) | Median (IQR) (VND*10,000) | 200 (190) | 180 (150) | 250 (120) | 150 (150) | 150 (110) | 100 (185) | 150 (150) | 150 (150) | 200 (150) | 150 (110) | 150 (100–250) |
| Unemployed (%) | 0.0 | 0.3 | 0.3 | 9.7 | 23.2 | 0.0 | 1.0 | 0.0 | 1.5 | 0.0 | 0.3 (0–23.2) | |
| Table 1.2: Risk behaviors among MWID in select provinces in Vietnam, 2009 | ||||||||||||
| Average duration (years) of drug use (sd) | 9.5 | 11.30 | 8.7 | 6.1 | 9.4 | 4.7 | 6.6 | 7.6 | 8.8 | 5.6 | 8.2 (4.7–11.3) | |
| Average duration (years) of injection drug use (sd) | 6 | 7.4 | 7.2 | 4.4 | 6.8 | 3.6 | 5.7 | 5.4 | 6.3 | 4.7 | 5.9 (3.6–7.4) | |
| Average age at initiating drug use (sd) | 22.0 (6.6) | 24.5 (6.6) | 22.8 (5.1) | 23.9 (6.2) | 25.2 (6.5) | 20.1 (6.4) | 21.2 (6.0) | 21.6 (7.1) | 24.9 (12.8) | 20.0 (5.9) | 22.4 (20–25.2) | |
| Average age at initiative injection drug use (sd) | 25.6 (7.4) | 28.5 (7.2) | 24.3 (5.4) | 25.7 (6.7) | 27.7 (7.4) | 21.3 (6.5) | 22.1 (6.4) | 23.7 (7.4) | 27.6 (9.2) | 20.9 (6.3) | 25.0 (20.9–28.5) | |
| In past month how often have you injected drugs (%) | > = 4 times/day | 3.7 | 10.1 | 0.3 | 3 | 1.4 | 1 | 0 | 4.8 | 0.7 | 0.67 | 1.2 (0–10.1) |
| 1–3 times/day | 86.4 | 89.6 | 93 | 64.9 | 55.3 | 67.7 | 47.7 | 94.8 | 86.3 | 83 | 84.65 (47.7–94.8) | |
| < 1 time/day | 9.8 | 0.3 | 6.7 | 32.2 | 42.7 | 31.3 | 48 | 0.4 | 12.9 | 16.4 | 14.65 (0.3–48) | |
| Don’t know/no response | 0 | 0 | 0 | 0 | 0.6 | 0 | 4.3 | 0 | 0 | 0 | 0 (0–4.3) | |
| Ever shared needles/syringes with others during injection drug use (95% CI) | 37 (29.5, 45.9) | 31.2 (25.9, 36.5) | 70.2 (65.0, 75.4) | 56.4 50.7, 62.0) | 57.6 (52.4, 62.8) | 43.6 (35.5, 50.9) | 36.7 (31.2, 42.1) | 41.2 (32.8, 50.9) | 36.6 (29.3, 43.9) | 28.1 | 39.1 (28.1–70.2) | |
| Missing (#) | 52 | - | - | - | - | 2 | - | 1 | ||||
| Frequency of needle/syringe sharing in the past 6 months (%) | Always or Most of the time | 2.7 | 0.3 | 0.3 | 1.3 | 0.6 | 4.9 | 3.3 | 3.2 | 5.9 | 4.7 | 3.0 (0.3–5.9) |
| Occasionally | 22.2 | 7.1 | 23.4 | 26.9 | 24.1 | 31.9 | 23.7 | 21.3 | 11 | 10.7 | 22.8 (7.1–31.9) | |
| Never | 22.6 | 23.5 | 46.5 | 28.2 | 32.8 | 6.3 | 9 | 20.3 | 25.7 | 13 | 23.1 (6.3–46.5) | |
| No response | 54.6 | 69.1 | 29.8 | 43.6 | 42.5 | 56.9 | 64 | 55.2 | 57.4 | 71.7 | 56.05 (29.8–71.7) | |
| Received free clean needles and syringes in the past 12 months % (95% CI, n) | 83.3 (71.6, 94.9), n = 80 | 86.6 (81.0, 92.2), n = 142 | 97.7 (95.8, 99.7), n = 220 | 94.3 (90.1, 98.4), n = 122 | 98.3 (96.6, 100), n = 233 | 98.4 (96.9, 99.9), n = 288 | 96.1 (92.7, 99.5), n = 127 | 90.3 (85.4, 95.1), n = 308 | 97.2 (94.4, 99,9), n = 141 | 98.4 (96.9, 100), n = 254 | 97.0 (83.3–98.4) | |
| Median (IQR) IDU network size | 12 (7–20) | 9 (5–17) | 12 (7.5–19.5) | 7 (5–20) | 10 (7–20) | 7 (4–12) | 6 (3–15) | 10 (6–20) | 5 (3–10) | 8 (4–20) | 8.5 (5–12) | |
| Median (IQR) number of different sexual partners in previous 12 months | 2 (1–4) | 0 (0–1) | 1 (0–1) | 1 (1–4) | 1 (1–2) | 3 (1–4) | 1 (1–2) | 1 (0,1) | 1 (0–1) | 1 (1–3) | 1 (0–3) | |
| Missing (#) | - | 13 | 72 | 18 | 13 | - | 83 | 1 | - | 53 | ||
| Proportion (%) reporting sexual relations with a female sex worker in the past 12 months | 45.6 | 33.3 | 23.1 | 47.9 | 35 | 42.8 | 15 | 17 | 20.7 | 31.9 | 32.6 (15–47.9) | |
| Missing (#) | 80 | 169 | 195 | 56 | 71 | - | 120 | - | - | 109 | ||
| Condom used with last sex with female sex worker (%, 95% CI, n) | 70.4 (58.2, 82.6), n = 143 | 91.1 (82.7, 99.5), n = 45 | 84.8 (72.4, 97.3), n = 33 | 81.5 (74.5, 88.5), n = 119 | 83.8 (76.5, 91.1), n = 99 | 77.7 (66.6, 88.8), n = 133 | 70.3 (55.3, 85.2), n = 37 | 49.1 (28.8, 69.5), n = 58 | 67.7 (51.6, 83.7), n = 65 | 81.7 (71.8, 91.6), n = 60 | 79.6 (49.1–91.1) | |
| Ever tested for HIV % (95% CI) | 34.9 (29.2, 40.6) | 58.5 (52.7, 64.4) | 68.4 (63.0, 73.8) | 53.4 (47.7, 59.1) | 38.9 (33.7, 44.1) | 25.3 (20.1, 30.4) | 25.4 (20.2, 30.6) | 26.5 (21.5, 31.4) | 36.8 (30.9, 42.8) | 41.2 (35.2, 47.2) | 38.0 (25.3–68.4) | |
| Ever been in a drug rehabilitation (06) center? | 58.2 (50.5, 65.9) | 62.8 (57.3, 68.3) | 67.2 (61.9, 72.6) | 84.2 (80.1, 88.4) | 61.1 (56.0, 66.2) | 68.8 (63.4, 74.1) | 18.7 (14.3, 23.2) | 64.4 (59.1, 69.8) | 53.7 (47.7, 59.6) | 30.1 (24.9, 35.3) | 62.0 (18.7–84.2) | |
*enrollees sampled using Respondent-driven sampling (RDS)
Key risk behaviors by province for HIV among those sampled are presented in Table 1.2. The median (range, by province) duration of drug use and injecting drug use was 8.2 (4.7–11.3) and 5.9 (3.6–7.4) years respectively. In all provinces except Dong Nai, the majority of enrollees reported injecting drugs one or more times per day with a median of 39.1% (28.1%-70.2%) of MWID reported having ever shared needles or syringes with others during injection drug use. Relatively few (3.0% [0.3%-5.9%]) MWID reported sharing needles ‘always or most of the time’ when they inject drugs, although 56.1% (29.8%-71.7%) of enrollees gave no response for this indicator. The vast majority (97.0% [83.3%-98.4%]) of all MWID sampled did report receiving free clean needles and syringes sometime in the past 12 months. An estimated 32.6% (15.0%-47.9%) of MWID reported having sexual relations with a female sex worker in the previous 12 months, and, of those who had, the majority (79.6% [49.1%-91.1%]) reported having used a condom during the last such encounter. A median of 38% (25.3–68.4%) of MWID reported previously testing for HIV.
HIV, HBV, and HCV prevalence
HIV prevalence among MWID varied widely by province (Fig. 1); the median HIV prevalence was 28.2% (1–55.5%) (Table 2). Across all 10 provinces, 14.1% (11.7%-28.0%) of MWID demonstrated active HBV infection (i.e., HBsAg+) and among those who were HBsAg-, 71.4% (49.9%-83.1%) had evidence of previous HBV infection (61.3% of the overall population sampled). Across provinces, median prevalence of past or current HCV infection among MWID overall was 53.8% (10.9%-87.4%). See the S1 Table for individual provincial-level data related to HIV, HBV, and HCV.
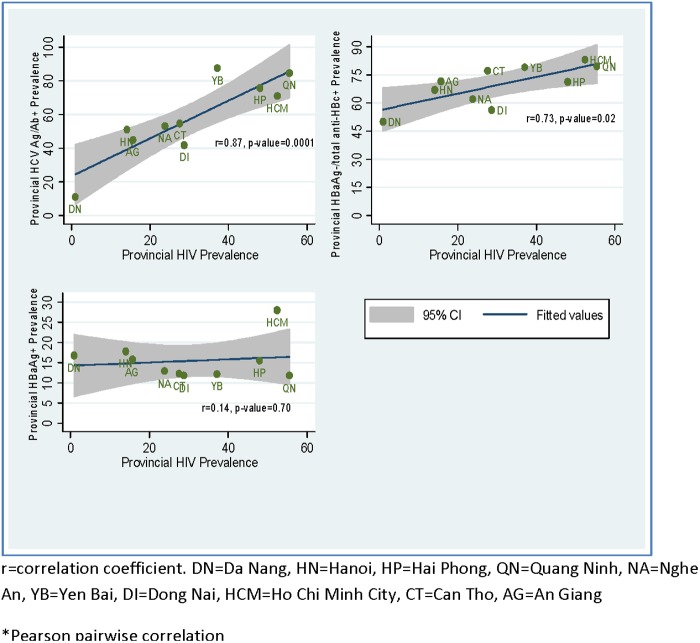
Fig 1. Provincial level correlation* of HIV to HBsAg, total anti-HBc, and HCV in the 2009 IBBS.
Table 2. HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and co-infection prevalence.
| Sero-marker | Median (Range) across sampled provinces% (95% CI) |
|---|---|
| HIV | 28.1 (1–55.5) |
| HBsAg+ | 14.1 (11.7–28) |
| HIV+ | 15.7 (9.6–66.7) |
| HIV- | 16.4 (10.3–22.8) |
| HBsAg-/total anti-HBc+ | 71.4 (49.9–83.1) |
| HIV+ | 81.2 (31.7–100)* |
| HIV- | 64.7 (49–78.1) * |
| HCV Ag/Ab+ | 53.8 (10.9–87.4) |
| HIV+ | 95.8 (35.5–99.4) * |
| HIV- | 52.2 (10.9–80.8) * |
| HBV, HCV and HIV prevalence (among all enrollees with a recorded result) | |
| HBsAg+/HIV+ % (95% CI) | 4.6 (0.9–13.4) |
| anti-HBc+/HIV+ % (95% CI) | 19.7 (0.1–39) |
| HCV Ag/Ab+/HIV+ % (95% CI) | 26.3 (14.1–55.2) |
| HBsAg+/HCV Ag/Ab+/HIV+ % (95% CI) | 1.2 (0.3–2.9) |
* indicate p-value <0.05
HIV-positive participants were more likely than HIV-negative participants to have evidence of previous HBV infection (81.2% vs. 64.7%, p-value<0.005); active HBV infection status did not differ significantly between HIV-positive and HIV-negative MWID (15.7% vs. 16.4%, p-value>0.05). Thus, a median (range) of 4.6% (0.9%-13.4%) of all MWID sampled were HIV-infected and currently co-infected with HBV, and 19.7% (0.1%-39.0%) were HIV-positive and evidenced past HBV infection (Table 2). In all provinces except Hanoi and Da Nang, past or current HCV infection was higher among HIV-positive than HIV-negative respondents (overall: 95.8% vs. 52.2%, p-value<0.0005) (Table 2). Overall, across all provinces, a median of 26.8% (14.1%-55.2%) of sampled MWID tested positive for both past or current HCV infection and HIV, with markedly high prevalence (> = 50%) among MWID in Hai Phong and Quang Ninh. In contrast, no participants in Da Nang province, with its relatively low HIV prevalence (1% [0%-2.4%]), tested positive for both HIV and past or current HCV infection, potentially indicating evidence of low HCV/HIV co-infection prevalence there (See supplemental table).
At the provincial level, HIV prevalence was significantly correlated with past HBV infection prevalence (r = 0.73, p-value = 0.02) and past or current HCV infection prevalence (r = 0.87, p-value = 0.0001) and weakly correlated with active HBV infection prevalence (r = 0.14, p-value = 0.70) (Fig. 1).
Factors associated with HIV status
Bivariate analysis (Table 3a) at the individual level also identified associations between HIV infection and past HBV infection (OR: 2.40, 1.93–2.99) and past or current HCV infection (OR: 29.84, 20.64–43.16). The association between HIV and active HBV infection (OR: 1.19, 0.94–1.49) was statistically not-significant. Several other factors were also associated with HIV status, including: increased duration and frequency of injecting drug use and needle sharing, injecting heroin exclusively compared to also injecting other drugs, increased MWID network size, ever being tested for HIV infection and ever being detained in a drug rehabilitation center.
Table 3. Crude and Adjusted Associations between HIV prevalence (outcome) and select factors among MWID in Vietnam.
| Independent predictor (n, %) | Table 3a: Crude Associations between HIV prevalence and select factors | Table 3b: Adjusted Associations between HIV prevalence and select factors | |||||
|---|---|---|---|---|---|---|---|
| Observations | Odds Ratio (OR) | 95% Confidence Interval | p-value | Adjusted Odds Ratio (aOR) | 95% Confidence Interval | p-value | |
| Age Category | 2986 (% HIV+) | ||||||
| 18–24 | 881 (11.80) | 1 | - | - | 1 | ||
| 24–30 | 706 (41.08) | 3.85 | 2.94, 5.03 | <0.0005 | 1.85 | 1.30, 2.62 | 0.001 |
| > = 30 | 1399 (38.03) | 3.09 | 2.39, 3.98 | <0.0005 | 1.31 | 0.93, 1.83 | 0.12 |
| Ethnicity Category | 3007 | ||||||
| Kinh | 2909 (30.90) | 1 | - | - | |||
| Other | 98 (32.65) | 0.87 | 0.55, 1.37 | 0.55 | |||
| Education | 2982 | ||||||
| Illiterate | 120 (19.17) | 1 | - | - | |||
| Primary | 445 (31.91) | 1.82 | 1.09, 3.04 | 0.02 | |||
| Secondary | 1198 (32.30) | 1.85 | 1.13, 3.02 | 0.01 | |||
| High School | 1087 (31.37) | 1.46 | 0.88, 2.42 | 0.14 | |||
| College, University | 132 (22.73) | 1.34 | 0.72, 2.56 | 0.38 | |||
| Marital Status | 3005 | ||||||
| Never Married | 1800 (28.56) | 1 | - | - | |||
| Currently Married | 789 (32.07) | 1.07 | 0.88, 1.31 | 0.48 | |||
| Divorced | 286 (36.01) | 1.15 | 0.87, 1.53 | 0.32 | |||
| Separated | 110 (43.64) | 1.36 | 0.90, 2.05 | 0.15 | |||
| Widowed | 12 (60.00) | 2.79 | 1.09, 7.15 | 0.03 | |||
| Unemployed | 3000 | ||||||
| Yes | 118 (44.92) | 1 | - | - | |||
| No | 2882 (30.46) | 0.55 | 0.36, 0.83 | 0.004 | |||
| Duration of drug use | 2952 | ||||||
| < 1 year | 202 (4.46) | 1 | - | - | |||
| 1–3 years | 535 (9.35) | 2.3 | 1.10, 4.82 | 0.03 | |||
| 3–5 years | 383 (21.15) | 5.17 | 2.50, 10.67 | <0.0005 | |||
| > = 5 years | 1832 (42.41) | 11.66 | 5.87, 23.18 | <0.0005 | |||
| Duration of injecting drug use (IDU) | 2952 | ||||||
| < 1 year | 391 (7.93) | 1 | - | - | 1 | ||
| 1–3 years | 671 (13.71) | 1.77 | 1.14, 2.74 | 0.01 | 1.13 | 0.66, 1.94 | 0.46 |
| 3–5 years | 458 (28.38) | 3.59 | 2.33, 5.54 | <0.0005 | 1.74 | 1.04, 2.93 | 0.04 |
| > = 5 years | 1415 (46.64) | 7.53 | 5.08, 11.19 | <0.0005 | 3.03 | 1.87, 4.89 | <0.0005 |
| Frequency of IDU (in the past month) | 3007 | ||||||
| > = 4 times per day | 95 (28.42) | 1 | - | - | |||
| 2–3 times per day | 1471 (33.99) | 1.53 | 0.94, 2.47 | 0.09 | |||
| 1 time per day | 832 (26.20) | 1.46 | 0.88, 2.44 | 0.15 | |||
| <1 time per day | 592 (30.07) | 2.17 | 1.28, 3.69 | 0.004 | |||
| Don’t know/no response | 17 (47.06) | 4.07 | 1.36, 12.11 | 0.01 | |||
| Type of drugs injected in the past month | 3009 | ||||||
| Heroin exclusively | 2751 (32.35) | 1 | - | - | 1 | - | - |
| Heroin as well as other drugs | 175 (18.29) | 1 | 0.64, 1.57 | 0.99 | 1.36 | 0.76, 2.43 | 0.31 |
| Drugs other than heroin | 83 (12.05) | 0.41 | 0.20, 0.81 | 0.01 | 0.24 | 0.08, 0.68 | 0.01 |
| Frequency of needle/syringe sharing | 3007 | ||||||
| Never | 1627 (22.56) | 1 | - | - | |||
| Ever shared in the past year | 977 (46.98) | 2.87 | 2.38, 3.47 | <0.0005 | |||
| Ever shared in the past month | 403 (26.05) | 1.47 | 1.13, 1.93 | 0.01 | |||
| MWID network size | 2955 | ||||||
| <5 | 574 (23.87) | 1 | - | - | |||
| 5-<15 | 1318 (29.59) | 1.18 | 0.93, 1.51 | 0.17 | |||
| >15 | 907 (35.75) | 1.47 | 1.15, 1.88 | 0.002 | |||
| Sexually active | 3009 | ||||||
| Yes | 2711 (31.02) | 1 | - | - | |||
| No | 298 (30.54) | 0.98 | 0.74, 1.30 | 0.90 | |||
| Sex with female sex worker in past 12 months | 1947 | ||||||
| No | 1216 (29.19) | 1 | - | - | |||
| Yes | 731 (41.48) | 0.81 | 0.64, 1.02 | 0.07 | |||
| Ever tested for HIV | 2830 | ||||||
| Yes | 1161 | 1 | - | - | |||
| No | 1669 | 0.55 | 0.46, 0.66 | <0.0005 | |||
| Received free needles and syringes in past 12 months | 1361 | ||||||
| Yes | 1297 (35.0) | 1 | - | - | |||
| No | 64 (32.81) | 0.75 | 0.43, 1.32 | 0.32 | |||
| Ever been in a drug rehabilitation center? | 3005 | ||||||
| No | 1999 (25.66) | 1 | - | - | |||
| Yes | 999 (41.54) | 2.21 | 1.86, 2.64 | <0.0005 | |||
| Don’t know/no response | 7 (42.86) | 2.39 | 0.53, 10.79 | 0.26 | |||
| HBsAg+ | 3009 | ||||||
| No | 2554 (30.50) | 1 | - | - | 1 | ||
| Yes | 455 (33.63) | 1.19 | 0.94, 1.49 | 0.14 | 2.09 | 1.01, 4.34 | 0.05 |
| total anti-HBc+ | 2585 | ||||||
| No | 785 (17.45) | 1 | - | - | 1 | ||
| Yes | 1800 (36.56) | 2.40 | 1.93, 2.99 | <0.0005 | 1.29 | 0.99, 1.68 | 0.06 |
| HCV Ag/Ab+ | 3004 | ||||||
| No | 1,224 (40.8) | 1 | - | - | 1 | - | - |
| Yes | 1780 (59.3) | 29.84 | 20.64, 43.16 | <0.0005 | 19.58 | 19.46, 132.84 | <0.0005 |
In adjusted analysis, which controlled for controlled for age category, duration of injecting drug use, type of drug use, current HBV infection, previous HBV infection status, and past or current HCV infection status, HIV infection was positively associated with MWID aged 24–30 years (relative to those aged 18–24 years; aOR: 1.85, 1.30–2.62), those injecting drugs longer than 3 years (relative to those injecting less than 3 years; aOR (3–5 years): 1.74, 1.04–2.93, aOR (> = 5years): 3.01, 1.87–4.89), and negatively associated with MWID who do not use heroin (aOR: 0.24, 0.08–0.68) (Table 3b). Both HBsAg+ (aOR: 2.09, 1.01–4.34) and HCV Ag/Ab+ (aOR: 19.58, 13.07–29.33) were also significantly associated with HIV infection in the adjusted analysis. While not statistically significant at the defined level, total anti-HBc+ among those HBsAg-(aOR: 1.29, 0.99–1.68) appears to indicate an association with HIV infection. No collinearity was identified among the variables included in the final adjusted analysis.
Conclusion
This study indicates that the prevalence of HIV, HBV, HCV and their co-infection among MWID in Vietnam are high in the majority of provinces sampled. HIV infection is associated with previous HBV infection and with current or past HCV infection (HCV Ag/Ab+) in both provincial and individual-level analyses. Injection of heroin, as opposed to other drugs, is associated with HIV infection as is an intermediate age range (24–30 years of age) and longer duration of injection drug use, in-line with expectations.
Our findings support previous, but localized studies, which indicate that the burden of HIV, HBV, and HCV infection is high among MWID in Vietnam.[2,16,17,29,30] While the point prevalence of HIV at the provincial level appears to be higher than what is reported through the Vietnam annual sentinel surveillance, the wide variation by province is similar for this study and the sentinel surveillance data. This supports the geographically heterogeneous characterization [15] of the HIV epidemic among MWID that may be due to a variety of factors in each province including: key population sizes (e.g. MWID), availability of HIV/AIDS prevention and care services (e.g. coverage of needles and syringe exchange programs, HIV counseling and testing, and HIV/AIDS treatment), duration of risk (e.g. duration of injecting use), and risk behaviors (e.g. types of drugs used, frequency of needle sharing among MWID), and drug trafficking routes that often enter Vietnam from the north and north-west potentially leading to increasing injecting drug use and associated HIV. This study provides a more geographically and population-representative profile of these associations and correlations than had previously been available for MWID in Vietnam and provides both provincial-specific estimations of HIV, HCV, and HBV burden and relationship that may also be considered for the national context. Specifically, it provides previously unavailable estimation about the significant association between HIV infection and current, active HBV infection (HBsAg+) and indication of an association between HIV infection with previous infection with HBV (total anti-HBc+) when controlling for other key factors. The absolute burden of HCV and its substantial association with HIV infection further indicates a clear clinical and public health issue for MWID in Vietnam, particularly given the relative efficiency of HCV transmission via blood from needle sharing among MWID.[31]MWID
The association between HIV infection and infection with HBV and HCV when adjusted for demographics, behavior, and service uptake provide evidence that MWID in Vietnam should be counseled on risks and risk reduction for HIV, HBV, and HCV (e.g. cessation, reduction of injection drug use, non-sharing of needles and syringes, consistent condom use). Services such as opioid substitution therapy should be provided as early as possible in their injecting lifetime to prevent acquiring and transmitting HIV, HBV, and HCV. Intervening early (e.g. within 1 year of injecting drug use initiation and at an earlier age) with MWIDs may have a substantially positive impact not only on HIV infection but also on associated HBV and HCV infection or HCV re-infection. This may be relevant especially among heroin injectors who make up an estimated 85% of drug users in Vietnam.[32]
Given that Vietnam’s estimated 335,000 MWID represent the most-at-risk-population for acquiring and transmitting HIV and HBV and HCV, accurate and timely data are critical for understanding the epidemiologic profile as well as designing and expanding appropriate intervention and care programs for those at risk for such HBV/HIV and HCV/HIV. We believe that this study provides an example to the Government of Vietnam (GVN) and other stakeholders of more integrated, and potentially more efficient, disease surveillance, particularly among populations such as MWID that are at increased risk for HIV and viral hepatitis.
HIV/AIDS prevention, care, and treatment services in Vietnam have expanded considerably over the past decade, primarily through initiatives supported by PEFPAR) the Global Fund Against HIV/AIDS, TB, and Malaria, as well as projects supported by the World Bank and the UK Department for International Development. These initiatives have supported the GVN to expand HIV treatment, medical methadone therapy (MMT), and various harm-reduction efforts for MWID. The Vietnam government has set an ambitious goal to initiate 80,000 injecting drug users on MMT by 2015 although significant barriers exist towards achieving that including conflicting policies for the treatment or incarceration of PWID as well as decreasing financial support from domestic and international sources.[32] There were 22,000 PWIDs that had initiated MMT as of late 2014.[33] Despite progress in HIV/AIDS prevention and control in Vietnam, a limited number of programs and strategies focus on other key co-infections, such as HBV or HCV.[34,35] The potential impact of integrated prevention interventions are further supported by data from this study indicating that, in addition to its association with HIV infection, increased duration of injecting drug use (i.e. > = 1 year) among enrolled MWID injecting heroin is significantly associated with prior HBV infection (data not shown).
Treatment programs for HIV and HBV and HCV should be fully integrated and evaluated. In February 2011, revision to the Vietnam ART guidelines recommended the use of tenofovir (TDF) and lamivudine (3TC) containing regimens that would provide an additional antiviral effect against HBV and also potentially improve HCV-related outcomes.[36,37] Such control efforts are especially critical given a recent study from Vietnam that reports a high (15.4%) of hepatitis D virus among those with current HBV infection which may have a negative impact on HBV-related outcomes.[38] International guidelines also indicate the need for hepatitis B vaccination, screening and the appropriate care of HBV and HCV as a key component of comprehensive HIV prevention, care, and treatment among MWID [5,39–41] This will be particularly important as increased access to HIV treatment in Vietnam will likely contribute to longer life-spans of HIV-infected people, resulting in a positive impact on the chronic morbidity and mortality of HBV and HCV-associated liver disease.[10,42,43]
There are several limitations and potential biases to consider with these findings. We are unable to make inferences about the temporal relationships among injecting drug use, infection with HIV, and infection with HBV or HCV due to the cross-sectional design of the study and because we did not have sufficient serum to test for markers of acute HBV or HCV infection. However if one assumes that injecting drug use is a significant mode of infection for HIV, HBV, and HCV among MWID, then HCV and HBV infection likely precede HIV infection due to their ease of transmission relative to HIV. [39,44,45] Also because of insufficient serum, we did not test for anti-HBs, so we were unable to identify occult HBV or to define the frequency of immunity to HBV obtained through vaccination. However, given the low coverage rates of HBV vaccination in this age group and population [16], few participants in this study are likely to have been previously vaccinated. Factors associated with viral infection were analyzed with all provinces combined under the assumption that associations were similar across provinces and to increase power of the study to provide valid inferences that could more readily be translated into policy and practice. In addition, sexual orientation was not assessed in this survey which limits any analysis of male-male sex behavior as a risk factor. Such data should be considered for future research. Finally, our sampling frame, while larger than any previous survey, was not designed to be nationally representative which might limit the generalizability of these findings to all provinces in Vietnam.
In conclusion this study provides important information on the profile and burden of HIV, HBV, HCV, and their associated co-infection among MWID in Vietnam. Such estimates are important for advocating for funding and political commitment and for developing, and monitoring comprehensive, effective harm-reduction and universal vaccination programs that simultaneously address viral hepatitis and HIV/AIDS. Our results support the conclusion that adequately responding to HIV and viral hepatitis among MWID requires integrated policies, surveillance, and prevention, screening, and treatment programs.
Supporting Information
(DOCX)
Acknowledgments
The authors would like to thank the Vietnam Provincial HIV/AIDS Committees (PACs) who contributed to the completion of this study. We would like to thank staff at The National Institute of Hygiene and Epidemiology (NIHE) in Hanoi and The Pasteur Institute in Ho Chi Minh City for their valuable technical contribution and management of this study. We would also like to acknowledge the CDC Division of Viral Hepatitis, specifically, Jan Drobeniuc, Saleem Kamili, and Chong-Gee Teo for their technical input to this effort as well as Dr. Sheryl Lyss, the Vietnam CDC Associate Director for Science, for her technical review and suggested edits to the manuscript. As a disclaimer, the findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. All authors declare no conflict of interest.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC) under the terms of 5U2GGH000116. CDC staff were directly engaged in the study design, analysis, decision to publish, and preparation of the manuscript.
References
- 1. Sulkowski MS. Viral hepatitis and HIV coinfection. J. Hepatol. 2008;48(2):353–67. 10.1016/j.jhep.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 2. Quan VM, Go VF, Nam le V, et al. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: a case-control study. AIDS Care 2009;21(1):7–16. 10.1080/09540120802017610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378(9791):571–83. 10.1016/S0140-6736(11)61097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perz JF, Armstrong GL, Farrington L a, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45(4):529–38. 10.1016/j.jhep.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global policy report on the prevention and control of viral hepatitis in WHO member states. 2013. 10.1016/j.jhep.2013.06.029 [DOI] [PubMed]
- 6.Prevention C for DC and. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Mmwr Morb. Mortal. Wkly. Rep. 1998;47(No. RR-19). [PubMed]
- 7. Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: fi ndings from the Global Burden of Disease Study 2010. Lancet 2013;6736(13):1–11. 10.1016/S0140-6736(13)61530-5 [DOI] [PubMed] [Google Scholar]
- 8. Garten RJ, Lai S, Zhang J, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int. J. Epidemiol. 2004;33(1):182–8. 10.1093/ije/dyh019 [DOI] [PubMed] [Google Scholar]
- 9. Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J. Infect. Dis. 2013;207 Suppl (Suppl 1):S1–6. 10.1093/infdis/jis927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clin. Infect. Dis. 2012;55 Suppl 1(Suppl 1):S33–42. 10.1093/cid/cis367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidance on Prevention of Viral Hepatits B and C Among People Who Inject Drugs.; 2012. [PubMed]
- 12. Hernando V, Perez-Cachafeiro S, Lewden C, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J. Hepatol. 2012;57(4):743–51. 10.1016/j.jhep.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 13. Weber, Sabin R, Friis-Moller N CA et al. Liver-Related Deaths in Persons Infected With the Human Immunodeficiency Virus. Arch Intern Med 2006;166:1632–1641. [DOI] [PubMed] [Google Scholar]
- 14. Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008;372(9651):1733–45. 10.1016/S0140-6736(08)61311-2 [DOI] [PubMed] [Google Scholar]
- 15.Vietnam Ministry of Health. Viet Nam HIV/AIDS Estimates and Projections 2011–2015.; 2012.
- 16. Nguyen VTT. Hepatitis B infection in Vietnam: current issues and future challenges. Asia. Pac. J. Public Health 2012;24(2):361–73. 10.1177/1010539510385220 [DOI] [PubMed] [Google Scholar]
- 17. Sereno L, Mesquita F, Kato M, Jacka D, Nguyen TT Van, Nguyen TN. Epidemiology, Responses, and Way Forward: The Silent Epidemic of Viral Hepatitis and HIV Coinfection in Vietnam. J. Int. Assoc. Physicians AIDS Care (Chic). 2012;11(5):311–20. 10.1177/1545109712453939 [DOI] [PubMed] [Google Scholar]
- 18. Nguyen CH, Ishizaki A, Chung PTT, et al. Prevalence of HBV infection among different HIV-risk groups in Hai Phong, Vietnam. J. Med. Virol. 2011;83(3):399–404. 10.1002/jmv.21978 [DOI] [PubMed] [Google Scholar]
- 19.Vietnam Ministry of Health NI of H and E. Integrated Biological and Behavioral Surveillance (IBBS) in Vietnam-Round II 2009.; 2011.
- 20.DiaSorin S.p.A. Technical Assay Details and Performance Summary: Murex HBsAg v.3; 2013. 10.1016/j.jfo.2013.06.003 [DOI] [PMC free article] [PubMed]
- 21.DiaSorin S.p.A. Technical Assay Details and Performance Summary: Murex HCV Ag/Ab Combination; 2013. 10.1016/j.jfo.2013.06.003 [DOI] [PMC free article] [PubMed]
- 22.Mark S. Handcock Ian E. Fellows KJG. Deducer RDSAnalyst: Graphical User Interface to the RDS package for Respondent-Driven Sampling., Version 0.1, Available: http://hpmrg.org. 2012.
- 23. Giles K, Handcock MS. Respondent-Driven Sampling: An Assessment of Current Methodology. Sociol. Methodol. 2010;40(1):1–34. 10.1111/j.1467-9531.2010.01223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vietnam Ministry of Health (MOH). Vietnam HIV/AIDS Estimates and Projections.; 2012.
- 25.Volz E, Wejnert C, Cameron C, et al. Respondent-Driven Sampling Analysis Tool (RDSAT). Respondent-Driven Sampl. Anal. Tool Version 7.1. Ithaca, NY Cornell Univ 2012. Available: http://www.respondentdrivensampling.org/. Accessed 2013 August 14.
- 26.StataCorp. Stata Statistical Software: Release 12. 2011.
- 27. Ameringer S, Serlin RC, Ward S. Simpson’s Paradox and Experimental Research Suzanne. Nurs Res 2009;58(2):123–127. 10.1097/NNR.0b013e318199b517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vietnam General Statistics Office. Population and Employment Statistics. Available: http://www.gso.gov.vn/default_en.aspx?tabid=467&idmid=3&ItemID=12941. Accessed 2013 August 14.
- 29. Nguyen VT, Mclaws M, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. Hepatology 2010;22(2007):2093–2100. 10.1111/j.1440-1746.2007.05010.x [DOI] [PubMed] [Google Scholar]
- 30. Nguyen VTT, McLaws M-L, Dore GJ. Prevalence and risk factors for hepatitis C infection in rural north Vietnam. Hepatol. Int. 2007;1(3):387–93. 10.1007/s12072-007-9008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sy T, Jamal MM. Epidemiology of Hepatitis C Virus (HCV) Infection. 2006;3(2):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen TTM, Nguyen LT, Pham MD, Vu HH, Mulvey KP. Methadone maintenance therapy in Vietnam: an overview and scaling-up plan. Adv. Prev. Med. 2012;2012:732484 10.1155/2012/732484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Few addicts get methadone relief in Vietnam. Asia News Network http://www.asianewsnet.net/news-68034.html. Published 2014 November 24.
- 34.Vietnam Ministry of Health. Evaluation of the Epidemiological Impact of Harm Reduction Programs on HIV in Vietnam.; 2011.
- 35.Vietnam Ministry of Health. National Strategy on HIV/AIDS Prevention and Control: 2010–2020.; 2010.
- 36. Anderson JP, Tchetgen Tchetgen EJ, Lo Re V, et al. Antiretroviral Therapy Reduces the Rate of Hepatic Decompensation Among HIV- and Hepatitis C Virus-Coinfected Veterans. Clin. Infect. Dis. 2013:1–9. 10.1093/cid/cit779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents; 2013. Available: http://www.ncbi.nlm.nih.gov/pubmed/12617573. [PubMed]
- 38. Sy BT, Ratsch B a, Toan NL, et al. High prevalence and significance of hepatitis D virus infection among treatment-naïve HBsAg-positive patients in Northern Vietnam. PLoS One 2013;8(10):e78094 10.1371/journal.pone.0078094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR. Recomm. Rep 2012;61(RR-5):1–40. Available: http://www.ncbi.nlm.nih.gov/pubmed/23135062. [PubMed]
- 40.World Health Organization. WHO, UNODC, UNAIDS Technical Guide for Countries to Set Targets for Universal Access to HIV Prevention, Treatment and Care for Injecting Drug Users; 2009. 10.1016/j.drugpo.2007.12.002 [DOI] [PubMed]
- 41. Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010;24(10):1537–48. 10.1097/QAD.0b013e32833a0918 [DOI] [PubMed] [Google Scholar]
- 42. Taylor LE, Swan T, Matthews G V. Management of hepatitis C virus/HIV coinfection among people who use drugs in the era of direct-acting antiviral-based therapy. Clin. Infect. Dis. 2013;57 Suppl 2(Suppl 2):S118–24. 10.1093/cid/cit326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun H-Y, Sheng W-H, Tsai M-S, Lee K-Y, Chang S-Y, Hung C-C. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: A review. World J. Gastroenterol. 2014;20(40):14598–14614. 10.3748/wjg.v20.i40.14598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Centers for Disease Control and Prevention. Guidelines for Prevention of Transmission of Human Immunodeficiency Virus and Hepatitis B Virus to Health-Care and Public-Safety Workers A Response to P.L. 100–607 The Health Omnibus Programs Extension Act of 1988. MMWR. Morb. Mortal. Wkly. Rep. Suppl. 38(S-6):3–37. 22695457 [Google Scholar]
- 45. Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected patient. Clin. Liver Dis. 2003;7(1):179–94. Available: http://www.ncbi.nlm.nih.gov/pubmed/12691466. Accessed 2014 January 19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.