Abstract
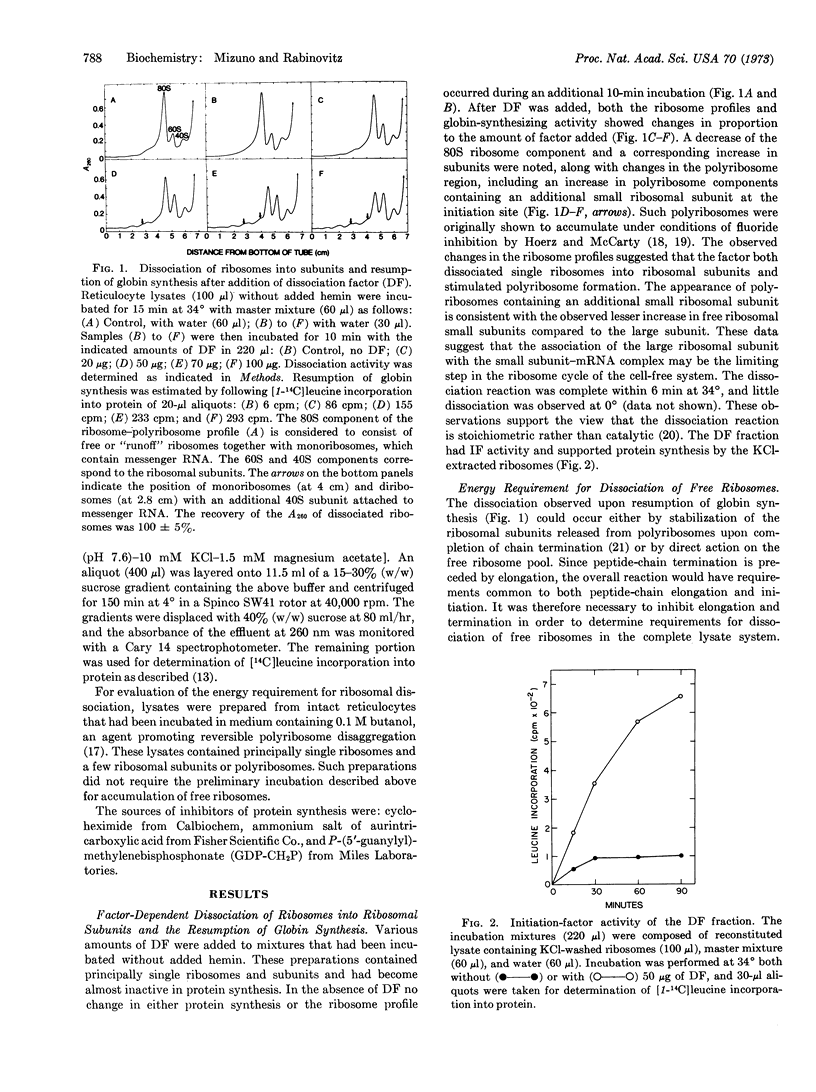
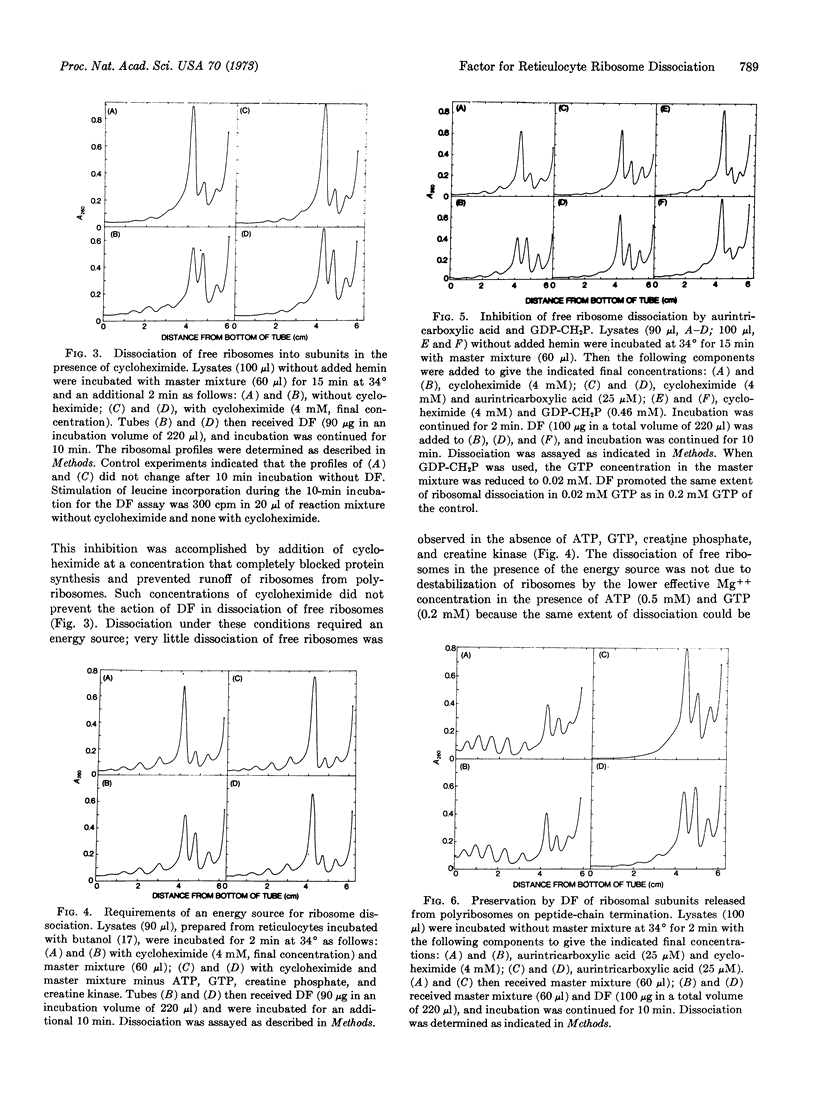
A factor that promoted the dissociation of ribosomes into ribosomal subunits in a complete globin-synthesizing system was isolated from rabbit reticulocyte ribosomes. The factor stimulated both globin synthesis and the association of the small ribosomal subunit with polyribosomes. The dissociation of free ribosomes by the factor could be followed as an independent reaction in the presence of a high concentration of cycloheximide, which inhibited translation and the resulting accumulation of runoff subunits. This reaction required an energy source and was inhibited by P-(5′-guanylyl)-methylenebisphosphonate. These data suggest that energy generated by hydrolysis of GTP may be a requirement for the dissociation reaction. Aurintricarboxylic acid inhibited the factor-mediated dissociation of free ribosomes but not the factor-mediated preservation of subunits formed on peptide-chain termination.
Keywords: ribosome dissociation factor, initiation factor, aurintricarboxylic acid, P-(5′-guanylyl)methylenebisphosphonate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Howard G. A., Herbert E. The ribosome cycle in a reconstituted cell-free system from reticulocytes. Cold Spring Harb Symp Quant Biol. 1969;34:547–554. doi: 10.1101/sqb.1969.034.01.062. [DOI] [PubMed] [Google Scholar]
- Benne R., Voorma H. O. Entry site of formylmethionyl-tRNA. FEBS Lett. 1972 Feb 15;20(3):347–351. doi: 10.1016/0014-5793(72)80104-2. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Woodley C. L., Bose K. K., Gupta K. K. Protein synthesis in rabbit reticulocytes: characteristics of a Met-tRNA Met f binding factor. Biochem Biophys Res Commun. 1972 Jul 11;48(1):1–9. doi: 10.1016/0006-291x(72)90335-x. [DOI] [PubMed] [Google Scholar]
- Colombo B., Vesco C., Baglioni C. Role of ribosomal subunits in protein synthesis in mammalian cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):651–658. doi: 10.1073/pnas.61.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Anderson W. F. Initiation of hemoglobin synthesis: comparison of model reactions that use artificial templates with those using natural messenger RNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):706–711. doi: 10.1073/pnas.69.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnoff J. S., Maitra U. Isolation and properties of polypeptide chain initiation factor FII from Escherichia coli: evidence for a dual function. Proc Natl Acad Sci U S A. 1971 Feb;68(2):318–323. doi: 10.1073/pnas.68.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. II. Exchange of ribosomal subunits at various stages of in vitro polypeptide synthesis. J Mol Biol. 1970 Oct 14;53(1):21–34. doi: 10.1016/0022-2836(70)90043-4. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Hori M., Rabinovitz M. Membranes in polyribosome formation by rabbit reticulocytes. Science. 1967 Jul 21;157(3786):323–325. doi: 10.1126/science.157.3786.323. [DOI] [PubMed] [Google Scholar]
- Gonzalez N. S., Bade E. G., Algranati I. D. Effect of GTP on the dissociation of 70 S ribosomes. FEBS Lett. 1969 Aug;4(4):331–334. doi: 10.1016/0014-5793(69)80268-1. [DOI] [PubMed] [Google Scholar]
- Groner Y., Revel M. A novel form of initiation factors from escherichia coli which binds formyl methionyl tRNA and GTP: "F2-F3 complex". Eur J Biochem. 1971 Sep 13;22(1):144–152. doi: 10.1111/j.1432-1033.1971.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Evidence for a proposed initiation complex for protein synthesis in reticulocyte polyribosome profiles. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1206–1213. doi: 10.1073/pnas.63.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Initiation of protein synthesis in a rabbit reticulocyte lysate system. Biochim Biophys Acta. 1971 Jan 28;228(2):526–535. doi: 10.1016/0005-2787(71)90058-x. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Korner A. Ribosomal subunits of Landschütz ascites cells during changes in polysome distribution. Biochim Biophys Acta. 1968 Nov 20;169(1):129–138. doi: 10.1016/0005-2787(68)90014-2. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Korner A. The role of ribosomal subunits and 80-S monomers in polysome formation in an ascites tumour cell. Biochim Biophys Acta. 1968 Nov 20;169(1):139–149. doi: 10.1016/0005-2787(68)90015-4. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Subunit recycling during translation in a reticulocyte cell-free system. J Biol Chem. 1970 Nov 25;245(22):6237–6239. [PubMed] [Google Scholar]
- Huang M. T., Grollman A. P. Effects of aurintricarboxylic acid on ribosomes and the biosynthesis of globin in rabbit reticulocytes. Mol Pharmacol. 1972 Mar;8(2):111–127. [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- Kabat D., Rich A. The ribosomal subunit--polyribosome cycle in protein synthesis of embryonic skeletal muscle. Biochemistry. 1969 Sep;8(9):3742–3749. doi: 10.1021/bi00837a038. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Control of single ribosome formation by an initiation factor for protein synthesis. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2458–2462. doi: 10.1073/pnas.68.10.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3317–3321. doi: 10.1073/pnas.69.11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford G. R., Kaiser J., Hey W. C. A factor capable of dissociating rat liver ribosomes. Can J Biochem. 1971 Dec;49(12):1301–1306. doi: 10.1139/o71-189. [DOI] [PubMed] [Google Scholar]
- Lockwood A. H., Chakraborty P. R., Maitra U. A complex between initiation factor IF2, guanosine triphosphate, and fMet-tRNA: an intermediate in initiation complex formation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3122–3126. doi: 10.1073/pnas.68.12.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubsen N. H., Davis B. D. A ribosome dissociation factor from rabbit reticulocytes (fluoride-E. coli ribosomes-initiation factors). Proc Natl Acad Sci U S A. 1972 Feb;69(2):353–357. doi: 10.1073/pnas.69.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Schweet R. Isolation of a protein fraction from reticulocyte ribosomes required for de novo synthesis of hemoglobin. Arch Biochem Biophys. 1968 May;125(2):632–646. doi: 10.1016/0003-9861(68)90622-x. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: action of an inhibitor formed in the absence of hemin on the reticulocyte cell-free system and its reversal by a ribosomal factor. Biochim Biophys Acta. 1972 Jul 31;272(4):638–650. [PubMed] [Google Scholar]
- Prichard P. M., Picciano D. J., Laycock D. G., Anderson W. F. Translation of exogenous messenger RNA for hemoglobin on reticulocyte and liver ribosomes. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2752–2756. doi: 10.1073/pnas.68.11.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Whybrow W. A., Clark B. F. Recognition of bacterial initiator tRNA by an initiation factor. Nat New Biol. 1971 May 19;231(20):76–78. doi: 10.1038/newbio231076a0. [DOI] [PubMed] [Google Scholar]
- Sabol S., Sillero M. A., Iwasaki K., Ochoa S. Purification and properties of initiation factor F3. Nature. 1970 Dec 26;228(5278):1269–1273. doi: 10.1038/2281269a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Prichard P. M., Gilbert J. M., Anderson W. F. Separation of two factors, M1 and M2, required for poly U dependent polypeptide synthesis by rabbit reticulocyte ribosomes at low magnesium ion concentration. Biochem Biophys Res Commun. 1970 Feb 20;38(4):721–727. doi: 10.1016/0006-291x(70)90641-8. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D. Activity of initiation factor F3 in dissociating Escherichia coli ribosomes. Nature. 1970 Dec 26;228(5278):1273–1275. doi: 10.1038/2281273a0. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D., Beller R. J. The ribosome dissociation factor and the ribosome-polysome cycle. Cold Spring Harb Symp Quant Biol. 1969;34:223–230. doi: 10.1101/sqb.1969.034.01.028. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]



