Abstract
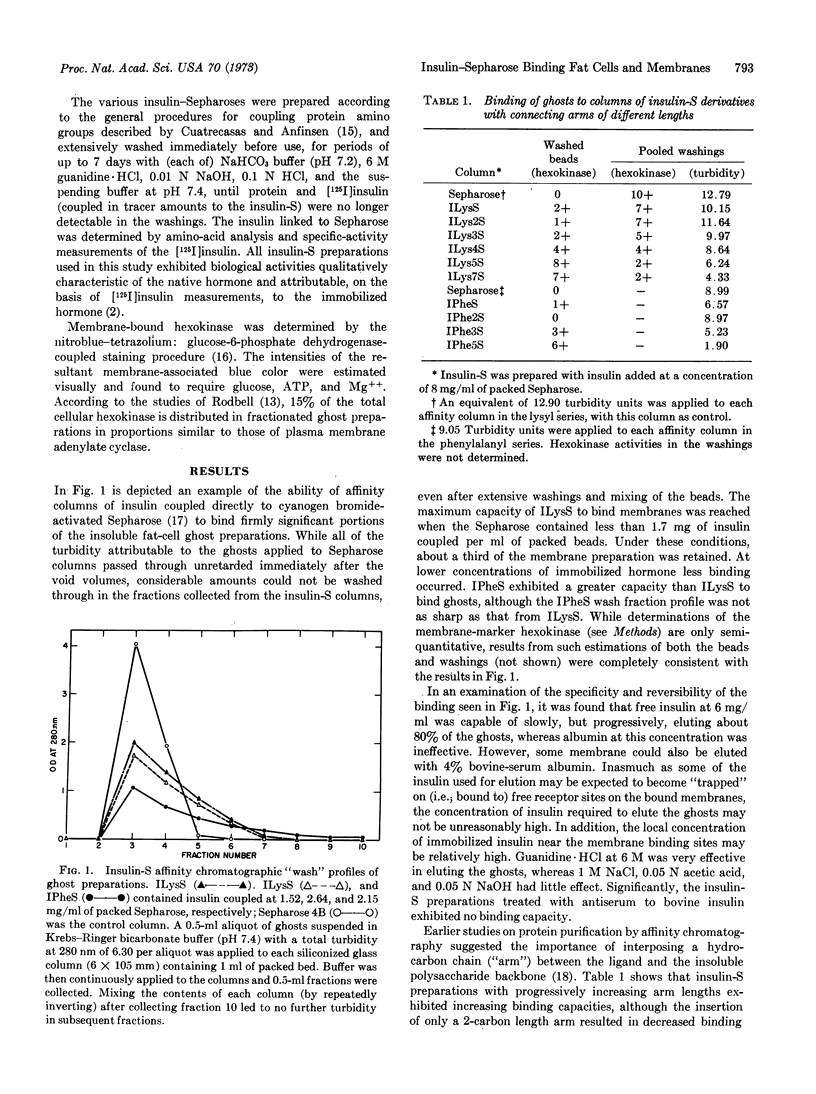
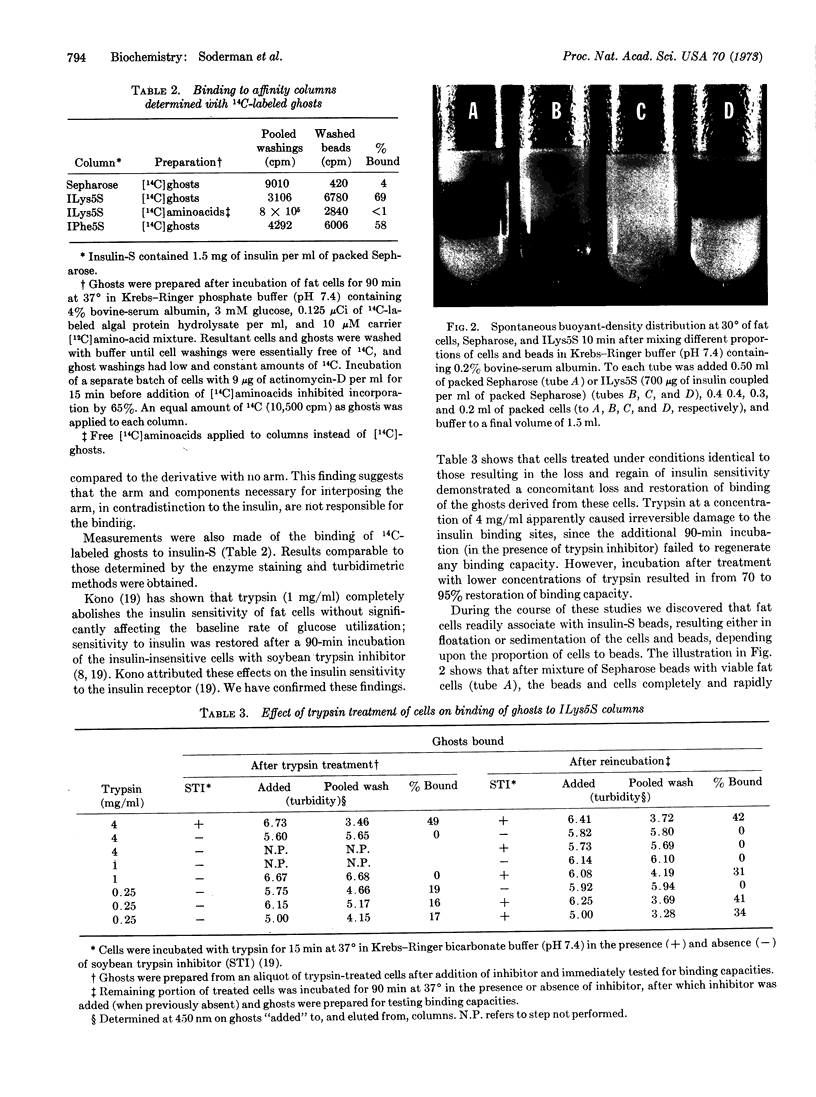
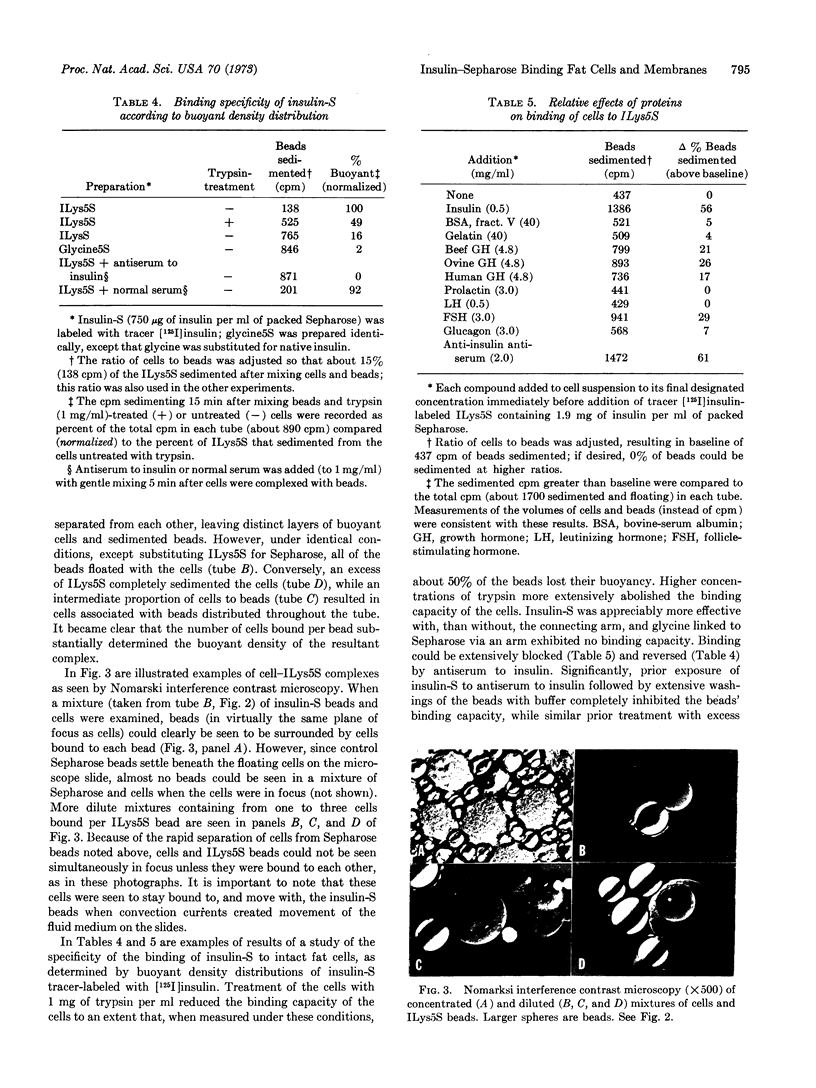
Immobilized insulin, prepared by coupling insulin directly to agarose or through hydrocarbon “connecting arms,” was demonstrated to be capable of firmly binding intact adipocytes and their ghosts. Various lines of evidence indicate that the insulin receptor on the plasma membrane, in addition to the insulin coupled to the agarose, was responsible for the observed binding. This evidence includes: (a) the finding that increasing the “arm” length increased the binding capacities of insulin-Sepharose affinity chromatographic columns, (b) specific inhibition and reversal by insulin and antiserum to insulin of the binding, as compared to lesser effects by other peptide hormones, (c) the indication that only the plasma membrane sacs, not the other cellular contaminants in the crude ghosts, are capable of binding, and (d) the impairment and restoration of trypsin-sensitive membrane binding sites that are also required for insulin biosensitivity. These findings support the idea that the insulin receptor is the trypsin-sensitive site. By use of the differential buoyant densities of the various cell-bead complexes that resulted from the interaction of adipocytes with insulin-Sepharose, a new procedure was developed to demonstrate and study the binding. These complexes could also be demonstrated by interference contrast microscopy. Binding readily occurred under conditions favorable for insulin stimulation of the cells. By coupling tracer amounts of [125I]insulin to Sepharose or insulin-Sepharose, the effects of anti-insulin antisera, free insulin, and other peptide hormones and supplemental factors on the buoyant-density distribution of the complexes could be measured, as well as the effects of other ligands coupled to Sepharose.
Keywords: insulin receptor, insulin-Sepharose, affinity chromatography, buoyant density, plasma membranes
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Blatt L. M., Kim K. H. Regulation of hepatic glycogen synthetase. Stimulation of glycogen synthetase in an in vitro liver system by insulin bound to sepharose. J Biol Chem. 1971 Aug 25;246(16):4895–4898. [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Katzen H. M., Soderman D. D., Wiley C. E. Multiple forms of hexokinase. Activities associated with subcellular particulate and soluble fractions of normal and streptozotocin diabetic rat tissues. J Biol Chem. 1970 Aug 25;245(16):4081–4096. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- Lambert B., Sutter B. C., Jacquemin C. Effect of iodination on the biological activity of insulin. Horm Metab Res. 1972 May;4(3):149–151. doi: 10.1055/s-0028-1094089. [DOI] [PubMed] [Google Scholar]
- Oka T., Topper Y. J. Insulin-sepharose and the dynamics of insulin action. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2066–2068. doi: 10.1073/pnas.68.9.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]





