Abstract
We investigated the effects of hypoxia on spontaneous (SP)- and activin A (AA)-induced definitive endoderm (DE) differentiation of mouse embryonic stem cells (mESCs) and their subsequent differentiation into distal pulmonary epithelial cells. SP differentiation for 6 days of mESCs toward endoderm at hypoxia of 1% O2, but not at 3% or 21% (normoxia), increased the expression of Sox17 and Foxa2 by 31- and 63-fold above maintenance culture, respectively. Treatment of mESCs with 20 ng/mL AA for 6 days under hypoxia further increased the expression of DE marker genes Sox17, Foxa2, and Cxcr4 by 501-, 1,483-, and 126-fold above maintenance cultures, respectively. Transient exposure to hypoxia, as short as 24 h, was sufficient to enhance AA-induced endoderm formation. The involvement of hypoxia-inducible factor (HIF)-1α and reactive oxygen species (ROS) in the AA-induced endoderm enrichment was assessed using HIF-1α−/− mESCs and the ROS scavenger N-acetylcysteine (NAC). Under SP conditions, HIF-1α−/− mESCs failed to increase the expression of endodermal marker genes but rather shifted toward ectoderm. Hypoxia induced only a marginal potentiation of AA-induced endoderm differentiation in HIF-1α−/− mESCs. Treatment of mESCs with AA and NAC led to a dose-dependent decrease in Sox17 and Foxa2 expression. In addition, the duration of exposure to hypoxia in the course of a recently reported lung differentiation protocol resulted in differentially enhanced expression of distal lung epithelial cell marker genes aquaporin 5 (Aqp5), surfactant protein C (Sftpc), and secretoglobin 1a1 (Scgb1a1) for alveolar epithelium type I, type II, and club cells, respectively. Our study is the first to show the effects of in vitro hypoxia on efficient formation of DE and lung lineages. We suggest that the extent of hypoxia and careful timing may be important components of in vitro differentiation bioprocesses for the differential generation of distal lung epithelial cells from pluripotent progenitors.
Introduction
Chronic respiratory diseases are responsible for about 4 million deaths every year [1], causing ∼7% of all mortality worldwide [2]. Common pharmacological approaches to treating chronic lung diseases, such as pulmonary hypoplasia and emphysema, aim at controlling the symptoms and are largely ineffective in eliminating the causes [3]. Organ transplantation is the last resource for end-stage diseases and is hampered by limited donor availability [4].
Alternative approaches to treating lung diseases include, but are not limited to, lung cell transplantation and engineering of lung tissue [5]. Epithelial cells of the distal lung are the prime target cell population required for the regeneration and reconstruction of functional lung tissue. Therefore, distal lung-specific cells derived from both embryonic stem (ES) and induced pluripotent stem (iPS) cells may serve as a promising candidate cells [6]. One of the available approaches for directed differentiation of ES or iPS cells into distal lung epithelial cells is to recapitulate in vitro some of the critical steps of embryonic lung development [7].
Previous studies have shown that the first critical step toward the directed pulmonary differentiation of pluripotent progenitor cells is their differentiation into definitive endoderm (DE), followed by induction of lung lineage specialization and maturation [8–13]. Activin A (AA) is a potent inducer of endoderm differentiation from pluripotent stem cells, whereas fibroblast growth factor 2 (FGF-2) promotes subsequent lung epithelial cell derivatization [12,14,15]. However, in the context of lung-specific differentiation in vitro, little attention has been paid to the possible effects of physical parameters in the microenvironment, such as oxygen tension.
Reduced oxygen tension is an important physiological cue during development, regulating embryonic development of several organs, including heart, lung, limb buds, bone, and pancreas [16–20]. During normal development in utero, embryonic stem cells (ESCs) (eg, both mouse and human) exist in a microenvironment with an oxygen tension of <5% O2 [21]. Moreover, before the circulation develops, the ESCs undergo differentiation at progressively decreasing oxygen tension as the embryo mass increases. The oxygen tension can be as low as 1.5% or even lower depending on the location of the cells inside the embryo [22]. However, ESCs are most often cultured in vitro under an atmospheric oxygen tension (∼21%). A growing body of evidence suggests that reduced oxygen tension promotes both self-renewal/maintenance of pluripotent stem cells [23] and their differentiation into many cell types, for example, neurons [24,25], cardiomyocytes [26], endothelial cells [27], chondrocytes [28], and rod cells of the retina [29].
As mentioned above, oxygen tension plays a key role in lung development [20,30]. In vivo studies suggest that DE as well as embryonic and fetal lung development occurs at a relatively low oxygen (O2) tension (<3% O2) [31–33]. Differentiation into DE, in rodents and humans, occurs very early during the developmental process in the absence of vascularization, that is, in a low oxygen tension environment. By contrast, subsequent lung specification and branching occur in the presence of an advanced circulation system [31–33].
We hypothesized that the initial endoderm development occurs in and may be driven by low oxygen tension and that higher oxygen tension may be required for the subsequent regulation of lung differentiation and maturation. We posit that manipulation of the ambient oxygen level could be a possible strategy for improving the efficiency of ESC differentiation in vitro toward DE and lung epithelial cells. Indeed, several recent studies indicated a possible role of low oxygen tension in enrichment of visceral endoderm [34] and DE formation [35].
In this study, we first investigated the effects of hypoxia on the directed endoderm differentiation of mouse embryonic stem cells (mESCs) as induced by exposure to AA [14,15]. We found that hypoxia of 1% O2 tension, but not 3% (mild hypoxia) or 21% (normoxia), significantly increased both the spontaneous (SP)- and the AA-induced differentiation of mESCs toward endoderm lineages, as assessed by both the enhanced expression of DE-specific marker genes and gene products as well as the percentage of cells differentiated into DE. Transient exposure to hypoxia, as short as 24 h, was sufficient to significantly augment the AA-induced increase in DE formation. Mechanistically, we demonstrated the involvement of hypoxia-inducible factor (HIF)-1α and reactive oxygen species (ROS) in the hypoxia-induced DE enrichment.
Using a recently reported stepwise differentiation protocol that mimics embryonic development [10], we demonstrated that transient exposure of mESCs to hypoxia of various durations leads to a differential increase in the expression of distal lung cells marker genes aquaporin 5 (Aqp5), surfactant protein C (Sftpc), and secretoglobin 1a1 (Scgb1a1 formerly known as CC10) for alveolar epithelium type I, type II, and club cells, respectively.
Our study describes, for the first time, the effects of low levels of oxygen on enhancing the formation of DE and diverse lung lineages in vitro from pluripotent stem cells. Our results suggest that inclusion of hypoxia may positively impact in vitro bioprocessing protocols aimed at optimizing the generation of DE and lung-specific cell types for regenerative lung engineering and cell-based therapies.
Materials and Methods
mESC maintenance culture
The mESC line, E14-12ΔS (further referred to as mESCs), triple transfected with hCD4 under Foxa2, hCD25 under Foxa3, and GFP under brachyury T promoters [36], was kindly provided by Dr. Paul Gadue, Children's Hospital of Philadelphia (Philadelphia, PA) and maintained as previously described [13]. Briefly, undifferentiated mESCs were grown in a serum-free/feeder-free culture system on 0.1% gelatin-coated (Millipore) tissue culture-treated plastic. The serum-free media composed of knockout Dulbecco's modified Eagle medium (KO-DMEM)/F12 medium supplemented with 0.5× N2, 0.5× B27, and 0.05% bovine serum albumin (BSA; all from Invitrogen), 50 IU/mL penicillin and 50 μg/mL streptomycin (Cellgro), 2 mM l-glutamine (Invitrogen), 10 ng/mL human recombinant bone morphogenetic protein (BMP-4; R&D Systems), 1,000 U/mL ESGRO® mouse leukemia inhibitory factor (mLIF) (Millipore), and 0.15 mM 1-Thioglycerol (Sigma). Some preliminary experiments were carried out with mouse ES-D3 cells, which were cultured feeder-free, as previously described [37]. Details are provided in Supplementary Materials and Methods (Supplementary Data are available online at www.liebertpub.com/scd).
To study the role of HIF-1α in the hypoxia-mediated enhanced directed differentiation of mESC, we used HIF-1α knockout mESCs (HIF-1α−/− mESCs) and the corresponding wild type (WT, HIF-1α+/+ mESCs), which were originally developed by Dr. Peter Carmeliet, VIB KU, Leuven, Belgium. These cells were kindly provided by Dr. Celeste Simon (University of Pennsylvania, Philadelphia, PA) and maintained in a feeder-free culture on 0.1% gelatin-coated (Millipore) tissue culture-treated plastic, as previously described [38]. The maintenance media composed of the DMEM supplemented with 4.5 g/L glucose without sodium pyruvate (Invitrogen), 15% fetal bovine serum (Biowest), 1% nonessential amino acid (Invitrogen), 1,000 U/mL ESGRO mouse LIF (Millipore), 100 IU/mL penicillin and 100 μg/mL streptomycin (Cellgro), 2 mM l-glutamine (Invitrogen), and 0.1 mM β-mercaptoethanol (Invitrogen).
For all cell lines, the maintenance media were changed daily. The cells were split every 2–3 days (upon reaching 80% confluence) using TrypLE Express (Invitrogen) and plated for subculture at ∼28,000 cells/cm2. The cells were maintained in a humidified incubator at 37°C in 95% air/5% CO2 atmosphere. The cell cultures were evaluated visually and photomicrographs were taken using the Nikon Eclipse TE 2000-U (Nikon) inverted microscope connected to the Hitachi KP-D50 digital camera.
mESC differentiation
Differentiation toward DE
The DE differentiation protocol of both WT and knock-out mESCs required 6 days in total. To initiate DE differentiation, mESCs were trypsinized, as described previously, resuspended in the appropriate maintenance media, seeded at a density of 1,000 cells/cm2 in the wells of 0.1% gelatin-coated six-well tissue culture-treated plates and cultured overnight (considered as day 1). The next day, the cells were switched to the SP differentiation media (consisting of LIF- and BMP-4-free maintenance media), which was used either as is or supplemented with 20 ng/mL AA to induce SP differentiation and directed DE differentiation, respectively. The cells were kept at 37°C under differentiative conditions for additional 5 days, unless indicated otherwise, at either atmospheric oxygen tension (21% O2) in a standard humidified air-regulated incubator with 5% CO2 or transferred 24 h after seeding into a reduced oxygen tension humidified hypoxia chamber with 5% CO2 and a balance of N2 at 37°C (InVIVO2 300; Ruskin Technologies). Before replenishing, the media was preequilibrated for 24 h in the hypoxia chamber. Cells were processed (fixed or harvested) inside the hypoxia chamber for further analysis.
Differentiation toward lung lineages
DE progenitors derived under normoxic or hypoxic conditions were subsequently specified to lung lineage according to the developmental biology-based protocol of Longmire et al. [10]. Briefly, following differentiation of the mESC to DE (as described previously), differentiation toward anterior foregut endoderm (anteriorization) was initiated by switching the culture to the SP media supplemented with 100 ng/mL mNoggin (R&D Systems) and 10 μM SB431542 (R&D Systems) for 24 h. Next, to induce lung-specification, the early lung/thyroid progenitors were cultured in the SP media supplemented with 100 ng/mL mWnt3a (R&D Systems), 10 ng/mL mouse keratinocyte growth factor (mKGF) (R&D Systems), 10 ng/mL human fibroblast growth factor 10 (hFGF10) (R&D Systems), 10 ng/mL mBMP4 (R&D Systems), 20 ng/mL human epidermal growth factor (hEGF) (R&D Systems), 500 ng/mL mouse fibroblast growth factor 2 (mFGF2) (R&D systems), and 100 ng/mL heparin sodium salt (Sigma) for 7 days. Thereafter, the cells were cultured for 6 days in the induction media (induction) supplemented with 100 ng/mL hFGF10, 500 ng/mL mFGF2, and 100 ng/mL heparin, followed by culture for 3 days in the distal lung differentiation media supplemented with 50 nM dexamethasone (Sigma), 0.1 mM cAMP (Sigma), 0.1 mM 3-isobutyl-1-methylxanthine (IBMX) (Sigma), and 10 ng/mL mKGF. All non-water-soluble compounds were dissolved in dimethylsulfoxide and the final v/v fraction of the solvent was kept below 0.1%. The control cultures were maintained in the SP media comprising KO-DMEM/F12 media supplemented with 0.5× N2, 0.5× B27, and 0.05% BSA and the solvent only.
Cell viability assay
Cells cultured under different treatments and various oxygen levels were dislodged by brief (<1 min) trypsinization using TrypLE Express. Cell viability was evaluated by staining the cells with the Guava ViaCount cell viability assay kit (Millipore) according to the manufacturer's instructions, and data were acquired with the Guava PCA flow cytometer (Millipore). Cell numbers and cell viability in triplicate samples were determined using the Guava ViaCount software (1,000 events/sample).
Immunofluorescence
The mESCs were immunostained as previously described in detail [39]. Briefly, the cells were washed, fixed for 15 min in 4% paraformaldehyde, washed with 1× phosphate-buffered saline (PBS), and then permeabilized for 15 min using 0.1% Triton-X (Sigma). All procedures were performed at room temperature. Nonspecific binding was reduced by blocking for 1 h at room temperature with the blocking solution (PBS supplemented with 10% chicken serum, 1% BSA, 0.1% Triton-X), followed by incubation overnight at 4°C with primary antibody against Foxa2 (1:100; Abcam) or species-specific immunoglobulin G (IgG, 1:100; Abcam) diluted in the blocking solution. Thereafter, the cells were washed three times and incubated for 1 h in the dark at room temperature with an Alexa 594-labeled secondary antibody (1:1,000; Invitrogen) diluted in the blocking solution. The samples were then incubated for 15 min with 1 μg/mL 4′,6-diamidino-2-phenylindole (Invitrogen), washed three times, and mounted with ProLong Gold antifade reagent (Invitrogen). Unless stated otherwise, all phase contrast and fluorescent photomicrographs were acquired using the inverted Nikon TE 2000U microscope (Nikon Instrument, Inc.) equipped with Northern Eclipse imaging software (P3I).
Flow cytometry
During the course of differentiation, the cells were periodically dislodged by gentle trypsinization (<5 min) and stained for flow cytometric analysis as previously described in detail [39]. Briefly, the expression of CD4-Foxa2 and Cxcr4 was probed using anti-human CD4-Allophycocyanin (APC, 1:5; BD Pharmingen) and anti-mouse Cxcr4-phycoerythrin (PE, 1:20; eBioscience), respectively. Some cells were stained with mouse IgG1 κ-APC (BD Pharmingen) and rat IgG2b κ-PE (eBioscience) as isotype controls for APC and PE, respectively, and processed in a similar way. Data were acquired using the FACSCalibur flow cytometer (BD) and analyzed using Cyflogic 1.2.1 software (CyFlo Ltd.).
Gene expression analyses
Expression of select genes was quantitated using reverse transcription–quantitative PCR (qPCR) as previously described in detail [39]. Briefly, total RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. RNA gels were run using 2% agarose (RNAse free) to ensure that RNA was intact before complementary DNA (cDNA) synthesis. RNA concentration was determined using the NanoDrop 3300 Fluorospectrometer with ND-3300 software (Thermo Scientific). Five micrograms of total RNA was then reverse-transcribed into cDNA with random primers using the TaqMan high-capacity cDNA reverse transcription kit (Applied Biosystems). qPCR analysis was performed using the Eppendorf Mastercycler Ep Realplex II system (Eppendorf) with fast thermal cycling using TaqMan Fast Universal Master Mix. The Taqman inventoried primers and probes (Applied Biosystems) are listed in Supplementary Table S1. Reactions were performed in triplicate, the gene expression levels were normalized to an endogenous housekeeping gene Pp1a, and relative expression levels were calculated using the 2−ΔΔCT method and are expressed as a fold of undifferentiated mESCs, unless mentioned otherwise.
Statistical analyses
All experiments were performed in triplicates with at least three independent repeats, unless specified otherwise. Where appropriate, the results are presented as mean±standard error of the mean. Statistically significant differences between two treatment groups were determined using the unpaired two-tailed Student t-test and between several groups using analysis of variance test followed by Bonferroni post-hoc test, unless mentioned otherwise. All statistical analyses were performed using Prism software (version 5.0a; GraphPad Software, Inc.). Significance was considered for P<0.05.
Results
Effects of hypoxia on endoderm enrichment under SP and AA differentiation conditions
To study the effects of hypoxia on mESC differentiation, we first characterized the viability of the cells cultured under various oxygen tensions. The viability of mESC cultures maintained for up to 3 weeks under hypoxic conditions at 3% or 1% O2 was similarly high (∼90%) and statistically indistinguishable from that of the cultures at 21% O2 tension, further referred to as “normoxia” (Fig. 1A). Moreover, exposure of mESCs for up to 6 days to 1% O2 tension (further referred to as “hypoxia”) when differentiating under either SP conditions following LIF/BMP-4 withdrawal, or upon directed endoderm induction with 20 ng/mL AA, did not affect their viability compared to maintenance cultures (Fig. 1B). However, the rate of proliferation of mESCs cultured in hypoxia under maintenance, SP or AA conditions, was significantly reduced compared to the same conditions in normoxia (Supplementary Fig. S1). These findings cumulatively indicate that mESCs cultured under reduced oxygen tensions as low as 1% O2 remain viable and continue to proliferate, albeit at a reduced pace.
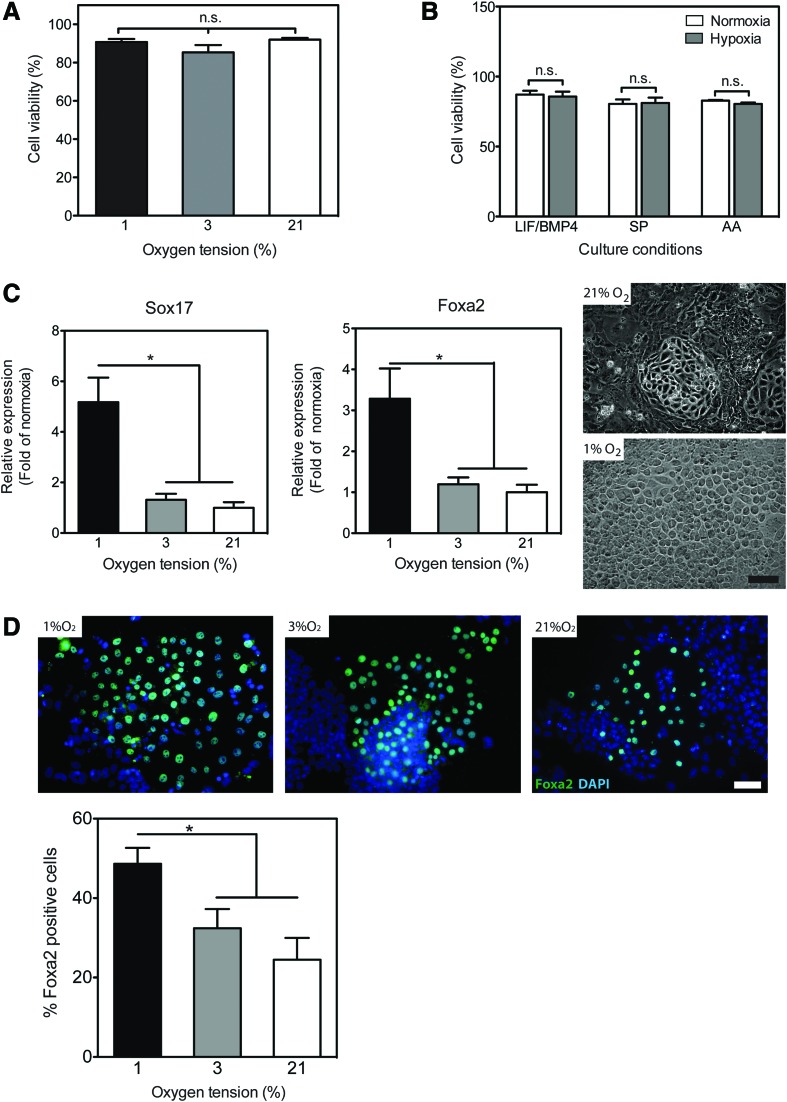
FIG. 1.
Hypoxia promotes the enrichment of endoderm progenitors under spontaneous (SP) differentiation conditions. (A) Mouse embryonic stem cells (mESCs) were cultured for 6 days in the maintenance media under various oxygen tensions. The viability was measured by flow cytometry using the Guava ViaCount cell viability assay kit. The data are presented as the percentage of viable cells from the total cell count [mean±standard error of the mean (SEM), n=9]. n.s., not significant. (B) mESCs cultured under normoxia (21% O2, white bars) or hypoxia (1% O2, gray bars) conditions in the maintenance media (leukemia inhibitory factor/bone morphogenetic protein 4), SP differentiation media, or SP media supplemented with 20 ng/mL activin A (AA). The viability was measured by flow cytometry, as above. The data are presented as the percentage of viable cells (mean±SEM, n=18–23). (C) mESCs were cultured for 6 days in the SP media under various oxygen tensions. The relative expression of Sox17 and Foxa2, measured using quantitative polymerase chain reaction (qPCR), is presented as fold of normoxia (mean±SEM, n=10, *P<0.05). Right panel: representative phase-contrast photomicrographs at day 6 of SP-differentiated cultures (original magnification 200×, scale bar 50 μm). (D) mESCs were cultured for 6 days in the SP media under various oxygen tensions and processed for immunofluorescent staining with the anti-Foxa2 antibody (green) and nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Upper panel: representative fluorescent photomicrographs at day 6 of SP-differentiated cultures acquired at 200× magnification, size bar 50 μm. Lower panel: the number of Foxa2+ cells was counted manually and is presented as percentage of total cell number per field of view (mean±SEM, n=21–31, *P<0.05). Color images available online at www.liebertpub.com/scd
Initiation of differentiation of mESCs under normoxic conditions either by LIF/BMP-4 removal or in the presence of AA resulted in the downregulation of the mRNA levels of marker genes for stemness, whereas select markers for all three germ layers were upregulated (Supplementary Fig. S2). In terms of the magnitude of the latter effect, both SP and AA conditions seemed to favor endodermal differentiation. For example, when cultured under SP conditions (LIF/BMP-4 withdrawal) in normoxia for 6 days, mESCs exhibited a 9.2±0.6- and 32.9±5.4-fold increase in mRNA expression of Sox17 and Foxa2, which are marker genes of endoderm [13], compared to the maintenance culture.
In the presence of AA, the expression of Sox17 and Foxa2 mRNA was further augmented by 2.9±0.6- and 5.5±1.0-fold over SP differentiation (Supplementary Fig. S2), which amounts to an overall increase of ∼30- and ∼180-fold over the maintenance culture, respectively (Supplementary Fig. S2). Similarly, under normoxic conditions, AA also enhanced the expression levels of Cxcr4, a marker for the DE [13] as well as that of Brachury T, a marker for the mesoendoderm [13], over those induced by SP. By contrast, AA did not significantly change the expression of mesodermal markers [mesenchyme homeobox1 (Meox1) and Vimentin (Vim)], or of Sox7, a marker for the extraembryonic endoderm [13].
Next, we studied the effects of hypoxia on SP- and AA-induced mESC differentiation. In SP cultures, exposure to moderate hypoxia (5–12% O2) did not significantly affect the levels of Sox17 and Foxa2 (data not shown). Even at 3% O2, the expression of mRNA levels of both these transcription factors, while showing a trend, was statistically indistinguishable from that in SP cultures in normoxia. However, lowering the oxygen tension to 1% significantly enhanced Sox17 and Foxa2 expression in SP cultures by 5.2±1.0- and 3.3±0.7-fold above the SP cultures in normoxia, respectively (Fig. 1C).
Morphological evaluation of mESCs following 6 days of SP differentiation upon LIF/BMP-4 withdrawal indicated enrichment in epithelial-like cellular phenotypes in hypoxia-treated cultures compared with normoxia (Fig. 1C). We then stained the cultures with antibodies against Foxa2 protein and found that 48.6±4% of cells undergoing SP differentiation under hypoxic conditions (1% O2) were positive for Foxa2 expression, which is tantamount to essentially a doubling of the number of Foxa2-positive cells under normoxia (Fig. 1D). The number of Foxa2-positive cells in 3% O2, while showing a trend for an increase, was statistically indistinguishable from that in normoxia (Fig. 1D), further supporting the gene expression results. Based on these results, we concluded that exposure to 1% O2 had a substantial effect on spontaneously differentiating mESCs resulting in significant enrichment in endoderm progenitors; therefore, all subsequent experiments were performed at this level of hypoxia.
Our previous study indicated that under normoxia, AA-induced endodermal differentiation of mESCs yielded ∼50% Foxa2+ cells compared to ∼25% positive cells in SP differentiating cultures [12]. Therefore, we hypothesized that concomitant exposure to hypoxia and AA will further enhance the enrichment in endodermal progenitors. Indeed, treatment of mESCs with 20 ng/mL AA in hypoxia increased Sox17 and Foxa2 gene expression by 501±108- and 1,483±169-fold compared to the maintenance cultures, respectively (Fig. 2A). This increase in the expression of Sox17 and Foxa2 genes was significantly larger in hypoxia than in normoxia in both AA-treated cultures [by 19.0±4.1- and 8.3±0.9-fold, respectively (Fig. 2A and Supplementary Fig. S2)] and the SP cultures [by 3.3±0.2- and 1.9±0.3-fold, respectively (Fig. 2A and Supplementary Fig. S2)].
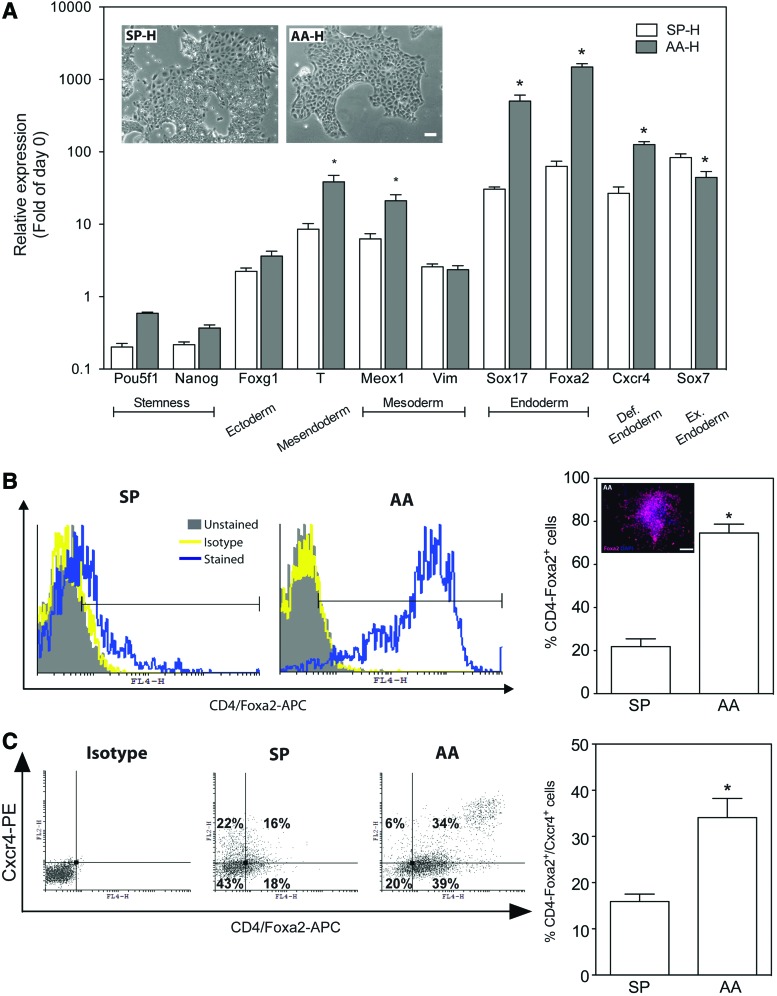
FIG. 2.
Hypoxia enhances AA-induced differentiation toward definitive endoderm. (A) mESCs were cultured for 6 days in the SP media or 20 ng/mL AA-containing media under hypoxia (H; 1% O2). The relative expression of marker genes was measured using qPCR and is presented as fold of day 0 (mean±SEM, n=7–9, *P<0.05 AA-H vs. SP-H). Inset: representative photomicrographs at day 6 of SP- and AA-differentiated cultures under hypoxia acquired at 100× magnification, scale bar 50 μm. (B) Flow cytometric analysis of Foxa2 protein expression in mESCs cultured for 6 days in the SP media or 20 ng/mL AA media under hypoxia (1% O2). Immunostained cultures with isotype control antibody were used to assign the appropriate gates. The data in the right panel are presented as the percentage of positive events out of the total number of collected events in the cell gate (mean±SEM, n=8–10, *P<0.05 AA vs. SP). Inset: immunofluorescent staining against Foxa2 (red) and nuclear stain with DAPI (blue) of mESCs treated for 6 days with AA under hypoxia. (C) Flow cytometric analysis of Foxa2 and Cxcr4 protein expression in mESCs cultured for 6 days in the SP media or 20 ng/mL AA media under hypoxia (1% O2). Immunostained cultures with isotype control antibody were used to assign the appropriate gates. The data in the right panel are presented as the percentage of double-positive events out of the total number of collected events in the cell gate (mean±SEM, n=4, *P<0.05 AA vs. SP). Color images available online at www.liebertpub.com/scd
Similarly, the AA-induced mRNA expression of Cxcr4 was increased in hypoxia by 125.9±12.5-fold compared to the maintenance culture, which is a 4.7±0.5-fold increase compared to SP cultures under hypoxia (Fig. 2A), and by 7.3±0.7-fold over AA-treated cultures under normoxia (Supplementary Fig. S2). In hypoxia, the expression of the visceral (extraembryonic) endoderm marker gene Sox7 in AA-treated cultures was slightly but significantly downregulated by ∼47% compared to SP cultures.
Considering that concomitant expression of Sox17, Foxa2, and Cxcr4 indicates differentiation toward DE [36], our findings of higher expression of these three gene markers under hypoxia, lower expression of Sox7, in concert with the morphological evaluation of the cultures (Fig. 2A, inset), suggest that AA treatment leads to further enrichment in DE progenitors, with concomitant reduction of visceral endoderm.
In parallel, we also analyzed expression of marker genes for other germ layers to account for the heterogeneic nature of the mESC differentiation [13]. As depicted in Fig. 2A, AA treatment during hypoxia had no effect on the expression of a mesenchymal maker Vim as opposed to the upregulation of another marker gene Meox1 and an early mesoendodermal marker brachyury T (T). The slight upregulation of an ectodermal marker gene Foxg1 was statistically insignificant. The marker genes for stemness, Pou5f1 and Nanog, were significantly downregulated in differentiating mESCs compared with maintenance culture but remained higher in AA-treated cultures under hypoxia than in the SP cultures.
To confirm the gene expression results at the protein level, we analyzed select gene products by flow cytometry in cells cultured in hypoxia under either SP or AA conditions. In hypoxia, 75±4% and 22±4% of the cells were Foxa2+ in AA- and SP-cultures, respectively (Fig. 2B). The robust presence of Foxa2+ cells in AA-treated cultures was also validated using immunostaining (Fig. 2B, inset); overall, these findings support our hypothesis that hypoxia increases the proportion of Foxa2+ cells in AA-treated cultures. Moreover, 34±4% and 15.9±1.6% of the AA and SP cultures were double stained for Foxa2+ and Cxcr4+, respectively (Fig. 2C), supporting our previous observations on the genes expression level that hypoxia significantly increases the fraction of DE cells.
Involvement of HIF and ROS in hypoxia-enhanced endoderm differentiation
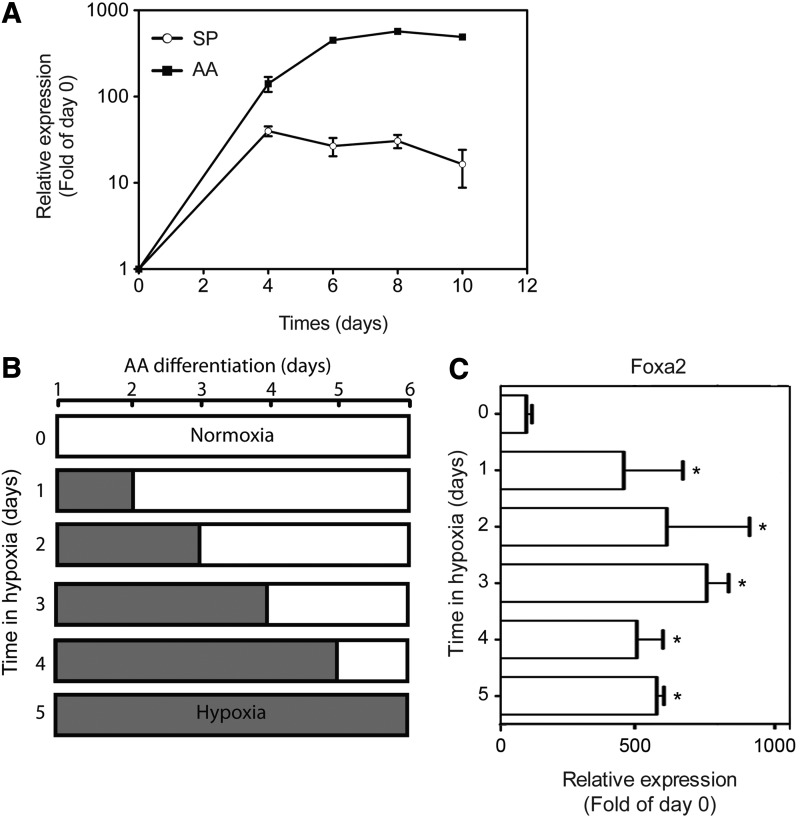
AA-induced endoderm differentiation in normoxia reached a plateau by day 6 of treatment, as inferred from the kinetics of Foxa2 mRNA expression (Fig. 3A). Thus, while keeping the duration of AA treatment constant (6 days), we sought to identify in the next set of experiments the minimal duration of exposure to hypoxia that would result in increased expression of endoderm marker genes, such as Foxa2. As depicted in Fig. 3B and C, exposure to hypoxia for 24 h sufficed to enhance AA-induced Foxa2 upregulation by 461.1±210.7-fold compared to the maintenance culture, which is 4.1±1.9-fold increase over AA-treated cultures under normoxia (0 day in hypoxia). Longer exposure to hypoxia did not lead to a statistically significant additional increase in the gene expression levels. These findings imply that exposure to hypoxia for a relatively short time period (≤24 h) is sufficient to trigger a strong and persistent induction of endodermal differentiation.
FIG. 3.
Time course of hypoxia-mediated enhancement of AA-induced expression of Foxa2. (A) mESCs were cultured under hypoxia in the SP media or 20 ng/mL AA media for up to 10 days. At the indicated time points, relative expression of Foxa2 was measured using qPCR. The data are presented as fold of the maintenance culture (mean±SEM, n=5–11). (B) Schematic presentation of the experimental protocol to examine the role of the duration of hypoxia on endoderm differentiation. White bars: duration of normoxia; gray bars: duration of hypoxia. (C) mESCs were cultured in normoxia/hypoxia according to the scheme depicted in (B) in 20 ng/mL AA-containing media for a total of 6 days. Relative expression of Foxa2 was measured by qPCR for all experimental setups at day 6 in culture. The data are presented as fold of maintenance culture (mean±SEM, n=5–14, *P<0.05 vs. 0 day in hypoxia).
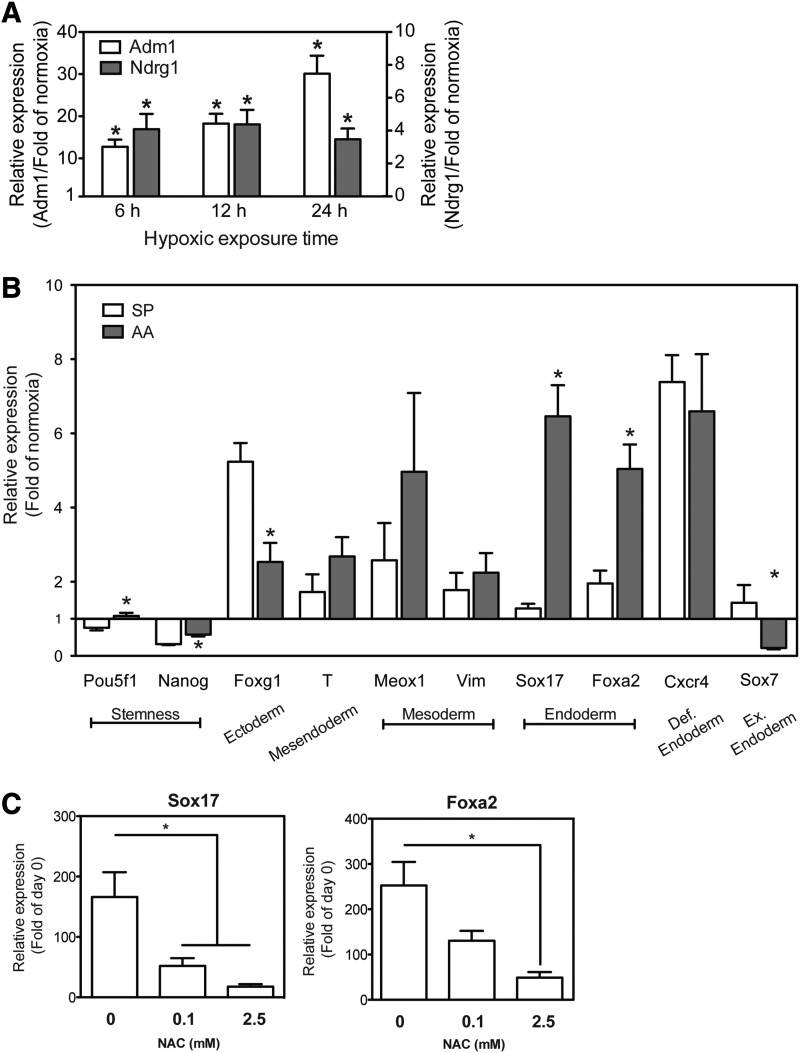
Based on these results, we hypothesized that the observed effects might involve activation of hypoxia-sensitive transcription factors, such as HIF-1α [40]. For technical reasons, the activation of HIF-1α is generally assessed by evaluating the induction of downstream targets, such as Adm1 and Ndrg1, which are considered as surrogate markers for HIF-1α activation due to the presence of HIF-1α binding sites in their promoter regions [41]. As depicted in Fig. 4A, exposure of AA-treated mESCs to as little a 6 h of hypoxia resulted in a statistically significant increase in the gene expression of Adm1 and Ndrg1.
FIG. 4.
Contribution of hypoxia-inducible factor (HIF)-1α and reactive oxygen species (ROS) to enhance endoderm differentiation. (A) Wild-type mESCs were cultured under hypoxia for the indicated periods of time in the SP media. The relative expression of Adm1 (white bars, left y-axis) and Ndrg1 (gray bars, right y-axis) was measured using qPCR. The data are presented as fold of the respective normoxia culture (mean±SEM, n=10–11, *P<0.05 hypoxia vs. normoxia). (B) HIF-1α−/− mESCs were cultured for 6 days in the SP media or 20 ng/mL AA-containing media under normoxia or hypoxia (1% O2). The relative expression of marker genes, as measured using qPCR, is presented as fold of the respective normoxia culture (mean±SEM, n=8–14, *P<0.05 AA vs. SP). (C) Wild-type mESCs were cultured for 6 days in 20 ng/mL AA-containing media under hypoxia (1% O2) in the presence of increasing concentrations of the ROS scavenger N-acetylcysteine (NAC). The relative gene expression of Sox17 and Foxa2, as measured using qPCR, is presented as fold of day 0 (mean±SEM, n=6–8, *P<0.05).
To further examine the involvement of HIF-1α in hypoxia-induced endoderm differentiation, we utilized an established HIF-1α-knock out mESC line (HIF-1α−/− mESCs) and their corresponding WT cells [41]. In preliminary studies, we established that under normoxic conditions, the AA-induced levels of marker genes for endodermal differentiation were similar in the WT mESCs as in E14-12ΔS cells (data not shown). As seen in Fig. 4A, the expression of Adm1 mRNA in WT mESCs increased by ∼12- to 30-fold following exposure of the cells to hypoxia for 6–24 h. Similarly, at 6 h exposure to hypoxia, the expression of Ndrg1 mRNA was about 15-fold higher than under normoxia but then remained relatively constant over time. There was no change in the expression levels of Adm1 and Ndrg1 over time under normoxia. These data suggest that the 1% oxygen tension used in our study induced a typical hypoxic response mediated by increased HIF-1α activity.
SP differentiation of HIF-1α−/− mESCs in hypoxia for 6 days resulted in only minimal, statistically insignificant, upregulation in the expression of Foxa2 and Sox17, compared with normoxia cultures (Fig. 4B) and in contrast to the E14-12ΔS mESCs (Fig. 2A). We conclude that in HIF-1α−/− mESCs, hypoxia did not augment SP endodermal differentiation beyond what was already obtained in normoxia. On the other hand, the expression of Foxg1 (neuronal ectoderm marker gene) and Cxcr4 (Fig. 4B) in HIF-1α−/− mESCs was significantly upregulated in hypoxia by comparison to maintenance cultures. Since concomitant expression of Foxg1 and Cxcr4 indicates differentiation toward neuronal progenitor cells [42], this finding implies that in the absence of HIF-1α SP differentiation of the cells in hypoxia was shifted toward ectoderm.
Treatment of HIF-1α−/− mESCs with AA in hypoxia resulted in a significant attenuation of the upregulation in the expression of DE markers Sox17, Foxa2, and Cxcr4 compared with WT-mESCs, which in the HIF-1α−/− mESCs increased only by 6.5±0.8-, 5.0±0.7-, and 6.6±1.5-fold, respectively (Fig. 4B and Supplementary Fig. S3). In other words, exposure of the HIF-1α−/− mESCs to AA in hypoxia resulted in more than 100-fold lower gene expression levels of Sox17 and Foxa2 and more than 10-fold lower gene expression of Cxcr4 than in the corresponding WT cells (Supplementary Fig. S3). Cumulatively, these data suggest a pivotal role of HIF-1α in the hypoxia-facilitated increase in the AA-induced expression of endodermal marker genes.
The fact that the effects of hypoxia on DE marker expression in AA-treated HIF-1α−/−-mESCs were significantly attenuated, but not entirely abrogated, suggests that other factors may be involved in the hypoxia-mediated upregulation of mESC differentiation. An important component of the hypoxic microenvironment is the formation and presence of ROS [43,44]. Therefore, we explored the contribution of ROS to hypoxia-induced endoderm differentiation.
Differentiation of (wild-type) mESCs by AA under hypoxia and in the presence of increasing concentrations of the ROS scavenger, N-acetylcysteine (NAC), resulted in a dose-dependent decrease in the levels of hypoxia-induced expression of Sox17 and Foxa2 expression (Fig. 4C). At 2.5 mM NAC, the hypoxia-induced increase in the expression of Sox17 and Foxa2 genes was attenuated by ∼80% (Fig. 4C), suggesting that the mechanism underlying the hypoxia-induced endoderm enrichment may be a combination of HIF-1α-dependent and ROS-mediated transcriptional changes.
Effects of hypoxia on distal lung differentiation
Recently, Longmire et al. described a developmental biology-inspired differentiation protocol for the differentiation of mESCs into distal lung epithelial cells [10]. This protocol, originally carried out for 23 days under atmospheric oxygen tension, entails a multistep sequel: induction of DE, anteriorization, lung specification, induction of lung progenitors, and differentiation into distal lung epithelial cells. We hypothesized that exposure to hypoxia during this protocol will further enhance the differentiation of mESCs into distal lung cells. In the course of mouse embryonic development, oxygen availability gradually increases as the development of cardiovascular system progresses and the blood begins to circulate around E9.5 [45].
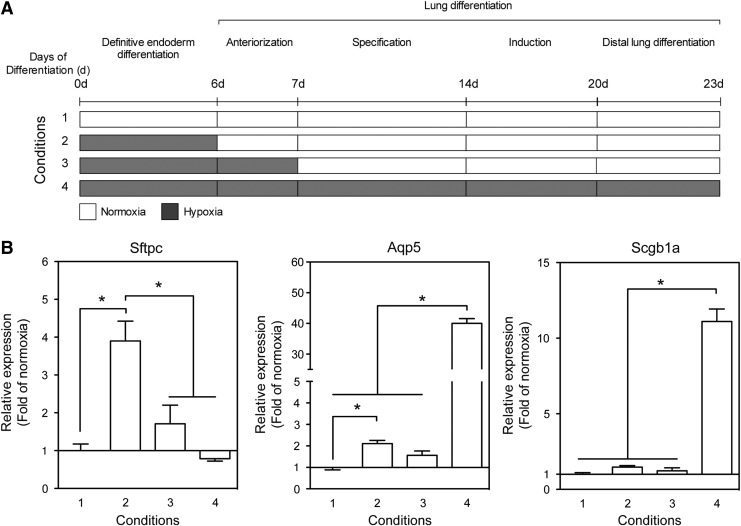
To more closely recapitulate the in vivo microenvironment, we limited the exposure to hypoxia in our experimental system to the duration of DE differentiation and anteriorization (up to day 7), followed by normoxia, while one control group was exposed to hypoxia for the duration of the DE differentiation, that is, only up to day 6 and other control groups were cultured during the entire 23 days in either normoxia or hypoxia (Fig. 5A). As seen in Fig. 5B, the duration of exposure of mESCs to hypoxia differentially affected the expression of marker genes for alveolar epithelial (AE) cell type II (Sftpc), AE type I (Aqp5), and club cells (club cell secretory protein, Scgb1a1). Exposure of the cultures to hypoxia during the DE formation step (6 days in culture) followed by normoxia for additional 17 days led to increased expression of Sftpc by 3.9±0.5-fold compared to cultures differentiated in normoxia only (Fig. 5B).
FIG. 5.
Duration of exposure to hypoxia modulates the differentiation into diverse distal lung cells. (A) Schematic presentation of the experimental protocols to evaluate the contribution of the duration of hypoxia to the differentiation of mESCs into diverse distal lung cells. Normoxia (white area), hypoxia (gray area). (B) mESCs (E14-12ΔS) were cultured according to the indicated condition number. The relative expression of Sftpc, Aqp5, and Scgb1a1 was measured using qPCR. The data are presented as fold of cultures differentiated under normoxia (condition 1) (mean±SEM, n=7–9, *P<0.05).
Exposure to hypoxia for one more day, until the end of the anteriorization step (7 days in culture) followed by normoxia for additional 16 days, essentially abolished the hypoxia effect resulting in statistically insignificant upregulation in Sftpc expression by 1.7±0.5-fold. Moreover, culturing the cells for the entire period of 23 days in hypoxia prevented upregulation of Sftpc beyond the levels found when the cells were cultured in normoxia only. By contrast, induction of marker genes for AE type I (Aqp5) and club cells (Scgb1a1) was maximal when the cells were cultured in hypoxia for the duration of the entire differentiation protocol and increased by 40.0±1.5- and 11.1±0.8-fold, respectively, beyond the levels found in normoxia. Variations in the duration of exposure to hypoxia at the beginning of the differentiation protocol only marginally affected the degree of upregulation of Aqp5 and Scgb1a1 (Fig. 5B). These data suggest that timing and duration of the in vitro exposure to hypoxia can lead to a directed enrichment in specific distal lung cell types.
Discussion
Mammalian embryonic development occurs at reduced intrauterine O2 levels that can be as low as 1% [46,47]. This observation, initially made in a variety of animal models, was also confirmed for early stages of human development, where the oxygen tension in the embryo undergoes dynamic changes from ∼2.5% O2 in the first trimester toward 8% O2 starting from the second trimester and until birth [48]. This suggests that early embryonic development and subsequent organogenesis in mammalian embryos takes place in a low O2 environment (less than ∼2.5%). To the best of our knowledge, this is the first study to demonstrate enrichment in DE cells derived from AA-treated mESCs by hypoxia through concomitant activation of the HIF-1α pathway and increased presence of ROS. Moreover, using a recently described developmental biology-based distal lung differentiation protocol [10], we demonstrate that timing and duration of exposure to hypoxia differentially modulated the enrichment of endodermal distal lung derivatives, such as alveolar epithelium type I, type II, and club cells.
During early embryonic development, cell proliferation, differentiation, and function occur under low O2 tension [48]. Some of the stem cells in adult organs also reside in poorly oxygenated niches, for example, heart epicardium [49], kidney [50], and bone marrow [51]. In this study, we found that mESCs remain viable and continue to proliferate over the entire range of 1–21% O2 tension (Fig. 1 and Supplementary Fig. S1). This finding is in line with reports that under low O2 tension murine and human ESCs (hESCs) continue to proliferate, exhibiting lower rates of SP differentiation [52,53] and a greater capacity for self-renewal by expressing higher levels of pluripotency marker genes (POU5F1, SOX2, and NANOG) [54]. However, some other studies suggest that in vitro hypoxic conditions (1–4% O2) efficiently promote differentiation of diverse pluripotent stem cells toward ectodermal cell types (eg, neurons [24], retinal progenitors [55]) or cells of mesodermal origin (eg, cardiomyocytes [26], endothelial cells [27], and chondrocytes [28]).
Regarding the effects of low O2 levels on differentiation toward the endoderm germ layer and its derivatives, the literature is surprisingly limited. To the best of our knowledge, the few existing reports describe the formation of visceral endoderm from hESCs under 3% O2 tension [34], retinoic acid-induced DE differentiation from mESCs under 5% O2 tension [35], AA-induced DE differentiation in normoxia followed by hepatic specification under 4% O2 tension [56], lack of enhanced pancreatic specification under 5% O2 tension [57], and hypoxia-mediated priming toward DE differentiation [52]. Here, we report that Foxa2 and Sox17 expressions, both at the gene and the protein levels, are significantly enriched by lowering the O2 tension from 3% to 1% (Fig. 1). In line with the recent report by Lim et al. [34], a visceral endoderm marker gene (Sox7) was upregulated by hypoxia in our experimental system in SP cultures. Low oxygen levels (1% O2) enhanced the efficiency of DE cell generation in AA-treated cultures, as evident from the increased coexpression of Foxa2/Cxcr4 and invariant expression of Sox7 when compared to normoxia and SP cultures, respectively (Fig. 2).
The molecular mechanisms involved in transducing low O2 tension into differential gene regulation rely mainly on the combination between the stabilization of HIF family of transcription factors and formation of ROS [58]. To date, three major isoforms of HIFα proteins were identified, which include 1α, 2α, and 3α [59]. The following activities of HIFα proteins are of particular relevance for ESCs: (1) HIF-3α upregulates the expression of HIF-2α and in parallel prevents the expression of HIF-1α [60]; (2) under low oxygen tension, HIF-2α is a positive regulator of hESC pluripotency genes and proliferation; and (3) HIF-1α is not expressed under normoxia and temporally translocates to the nucleus during the first 48 h of hypoxia [60]. Therefore, we hypothesized that the rapid upregulation of Foxa2 upon transient exposure of AA-treated cultures to hypoxia (Fig. 3) is mediated by HIF-1α. In support of our hypothesis, upregulation of the expression of a series of marker genes for DE, such as Foxa2, Sox17, and Cxcr4, following AA treatment under hypoxic conditions was significantly impaired in HIF-1α−/− mESCs (Fig. 4 and Supplementary Fig. S3) compared with the control WT mESCs (Supplementary Fig. S3).
The observed enrichment in endoderm progenitors in AA-treated mESCs in hypoxia is consistent with the reported HIF-1α-mediated increase of an early endoderm marker gene (Krt8) expression in embryoid bodies cultures primed by exposure (48 h) to hypoxia of 1% O2 tension [40], that is, the same O2 level as also used in this study. A recent report described the effect of hypoxic preconditioning (at 2% O2) on enhanced formation of DE, where the actual differentiation was performed in normoxia [52], and supports our findings that short-term hypoxic exposure leads to upregulation of Foxa2 gene expression (Fig. 3). Our study provides additional insight into the effects of longer time periods of hypoxic exposure by demonstrating that continuous exposure for up to 6 days to hypoxia results in enhanced differentiation toward DE, as assessed from the upregulation of coexpressed Foxa2 and Cxcr4 markers and downregulation of Sox7 (Fig. 2).
To investigate the involvement of HIF-1α and ROS in the hypoxia-mediated endodermal differentiation, we employed both HIF-1α−/− mESCs and a ROS scavenger (NAC). While the overall levels of DE marker genes in AA-treated HIF-1α−/− mESCs were significantly downregulated compared to the corresponding WT cells (Supplementary Fig. S3), the expression levels of Foxa2 and Sox17 were still significantly higher than in the SP cultures (Fig. 4B). AA is a member of transforming growth factor β (TGFβ) superfamily and exerts its biological activities in part through TGFβ receptors [61].
ROS are an integral part of the TGFβ-induced signalization and gene expression [62]. A chemically induced increase in intracellular ROS levels under normoxic conditions reportedly enhances the formation of early mesoendoderm [63] and visceral endoderm [64,65]. The reduction in AA-induced DE differentiation of mESCs treated with ROS scavengers under hypoxia (Fig. 4C) suggests that this effect is at least in part mediated by the formation and subsequent signaling of ROS and does not solely rely on HIF stabilization.
One of our long-term goals is to optimize ESC bioprocessing by leveraging in vivo-like microenvironmental conditions for the efficient generation of diverse lung-relevant cell populations. We note that there is a solid basis of knowledge regarding the role of hypoxia during in vivo embryonic lung development [66], which is in contrast to the limited information available for hypoxia-induced endoderm differentiation in vitro and the current lack of published reports on the effects of hypoxia on differentiation of murine or hESCs toward lung phenotypes (PubMed search performed on September 11, 2014). Therefore, we applied a set of hypoxic conditions to a recently published protocol that differentiates mESCs into lung progenitors by following a sequel of defined embryonic development stages [10]. Our data indicate that depending on the timing and duration of exposure of the cells to hypoxia, this protocol will differentially induce the expression of lung cell-specific markers for AE type I, AE type II, and club cells (Fig. 5).
We posit that the differential responses to the duration and timing of exposure of mESCs to hypoxia may arise from differential sensitivity of gene expression patterns to hypoxic preconditioning, a hypothesis that deserves further research. Interestingly, Van Haute et al. reported that culturing of human ESCs at the air–liquid interface (ALI) under atmospheric oxygen tension promotes differentiation into diverse lung-specific and other (mesenchymal) cells and facilitates the in vitro assembly of this mixed population into lung-like tissue [67]. Therefore, the enhanced ability to manipulate the outcome of specific differentiation protocols using specific culture conditions (eg, ALI) or by merely changing the level of oxygen tension may lead to more efficient protocols for the targeted enrichment in specific lung cell types for future regenerative applications.
Taken together, these results suggest that to efficiently generate lung-relevant cells optimal in vitro bioprocessing will involve differential exposure to oxygen tension as a critical component of a multifactorial differentiation protocol.
There is a growing body of in vivo evidence supporting pulmonary integration and therapeutic efficacy of ESC-derived lung-specific cell types [68]. In terms of translating the outcomes of our in vitro differentiation protocol into preclinical and clinical studies, we envisage a future application of lung-specific epithelial cells for the ex vivo reseeding of decellularized lungs [69] or for transplantation in vivo, both of which are beyond the scope of this article. Indeed, following our initial in vivo studies of intratracheal delivery of fetal pulmonary cells in a mouse model [70], Roszell et al. demonstrated the integration of intratracheally delivered mESC-derived AE type II cells into the lungs of mice [12].
A critical issue will be to demonstrate the efficacy and uniformity of cell engraftment along the lines recently described by us for in vitro reseeding of decellularized lungs [69]. Another projected application of our findings will be the use of in vitro-derived lung epithelial cells for mechanistic studies in an in vitro model of engineered pulmonary organoids [71,72]. An important milestone toward clinical translation, currently under investigation in our laboratory, will be to demonstrate the applicability of our protocols and findings to the directed pulmonary differentiation of human pluripotent cells.
Conclusions
In this study, we provide evidence for the beneficial effects of hypoxia on the enrichment in DE differentiation of AA-treated mESCs. We further demonstrate important roles of both HIF-1α and ROS in mediating the effects of hypoxia using HIF-1α−/− mESCs and a ROS scavenger, respectively. The increase in the expression of endodermal markers is evident upon a short transient exposure to hypoxia, suggesting a major role of hypoxia-sensitive transcriptional processes in the regulation of endoderm gene expression and subsequent pulmonary differentiation. We also demonstrate that the timing and duration of exposure to hypoxia differentially enhances the expression of Aqp5, Sftpc, and Scgb1a1, that is, marker genes for AE type I, type II, and club cells, respectively.
Our findings position hypoxia as a critical component of bioprocessing protocols aimed at optimizing the directed differentiation and enrichment of endoderm and DE cells in general and distinct lung-relevant phenotypes in particular. We suggest that careful timing and duration of exposure to hypoxia will be an important element of an optimized in vitro bioreactor-based differentiation protocol for the efficient generation of lung-specific cells for drug discovery and cell therapy.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Paul Gadue (Children's Hospital of Philadelphia), Dr. Peter Carmeliet (VIB Vesalius Research Center Leuven, Belgium), and Dr. Celeste Simon (University of Pennsylvania School of Medicine, Philadelphia, PA) for providing the mESCs used in this study. This study was funded, in part, by a grant from NIH (5R01 HL-104258-02). P.I.L. is the Laura H. Carnell Professor for Bioengineering; E.S.S. is supported by Margaret Wolf Memorial Fund; C.T.S. is a NASA graduate student research program (GSRP) fellow.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rattue P. (2012). Lung diseases leading cause of death, most people don't know. In: Medical News Today. Hutchings J, ed. MediLexicon, Intl.www.medicalnewstoday.com/articles/247293.php [Google Scholar]
- 2.Gibson GJ, Loddenkemper R, Sibille Y. and Lundbäck B. (eds.). (2014). The European Lung White Book: Ch. 1—The burden of lung disease. ERS, Sheffield [Google Scholar]
- 3.Jungebluth P. and Macchiarini P. (2011). Stem cell-based therapy and regenerative approaches to diseases of the respiratory system. Br Med Bull 99:169–187 [DOI] [PubMed] [Google Scholar]
- 4.Lau AN, Goodwin M, Kim CF. and Weiss DJ. (2012). Stem cells and regenerative medicine in lung biology and diseases. Mol Ther 20:1116–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, Hoffman AM. and Weiss DJ. (2013). Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18:895–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MD, Huang SX. and Snoeck HW. (2013). Stem cells of the respiratory system: from identification to differentiation into functional epithelium. Bioessays 35:261–270 [DOI] [PubMed] [Google Scholar]
- 7.Wong AP. and Rossant J. (2013). Generation of lung epithelium from pluripotent stem cells. Curr Pathobiol Rep 1:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin YM, Zhang A, Rippon HJ, Bismarck A. and Bishop AE. (2010). Tissue engineering of lung: the effect of extracellular matrix on the differentiation of embryonic stem cells to pneumocytes. Tissue Eng Part A 16:1515–1526 [DOI] [PubMed] [Google Scholar]
- 9.Kadzik RS. and Morrisey EE. (2012). Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell 10:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, et al. (2012). Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10:398–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H, Quan Y, Yan Q, Peng X, Mao Z, Wetsel RA. and Wang D. (2013). Isolation and characterization of alveolar epithelial type II cells derived from mouse embryonic stem cells. Tissue Eng Part C Methods 20:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI. and Finck CM. (2009). Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A 15:3351–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecht S, Gerstenhaber JA, Stabler CT, Pimton P, Karamil S, Marcinkiewicz C, Schulman ES. and Lelkes PI. (2014). Heterogeneous mixed-lineage differentiation of mouse embryonic stem cells induced by conditioned media from A549 cells. Stem Cells Dev 23:1923–1936 [DOI] [PubMed] [Google Scholar]
- 14.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ. and Keller G. (2004). Development of definitive endoderm from embryonic stem cells in culture. Development 131:1651–1662 [DOI] [PubMed] [Google Scholar]
- 15.Kim PT. and Ong CJ. (2012). Differentiation of definitive endoderm from mouse embryonic stem cells. Results Probl Cell Differ 55:303–319 [DOI] [PubMed] [Google Scholar]
- 16.Dunwoodie SL. (2009). The role of hypoxia in development of the Mammalian embryo. Dev Cell 17:755–773 [DOI] [PubMed] [Google Scholar]
- 17.Patterson AJ. and Zhang L. (2010). Hypoxia and fetal heart development. Curr Mol Med 10:653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinis M, Simon MT, Ilc K, Mazure NM, Pouyssegur J, Scharfmann R. and Duvillie B. (2010). Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 59:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ. and Schipani E. (2007). Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol 177:451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D. and Post M. (2005). Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 288:L167–L178 [DOI] [PubMed] [Google Scholar]
- 21.Greer SN, Metcalf JL, Wang Y. and Ohh M. (2012). The updated biology of hypoxia-inducible factor. EMBO J 31:2448–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ufer C. and Wang CC. (2011). The roles of glutathione peroxidases during embryo development. Front Mol Neurosci 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins KE, Sharp TV. and McKay TR. (2013). The role of hypoxia in stem cell potency and differentiation. Regen Med 8:771–782 [DOI] [PubMed] [Google Scholar]
- 24.Kim TS, Misumi S, Jung CG, Masuda T, Isobe Y, Furuyama F, Nishino H. and Hida H. (2008). Increase in dopaminergic neurons from mouse embryonic stem cell-derived neural progenitor/stem cells is mediated by hypoxia inducible factor-1alpha. J Neurosci Res 86:2353–2362 [DOI] [PubMed] [Google Scholar]
- 25.Mondragon-Teran P, Lye GJ. and Veraitch FS. (2009). Lowering oxygen tension enhances the differentiation of mouse embryonic stem cells into neuronal cells. Biotechnol Prog 25:1480–1488 [DOI] [PubMed] [Google Scholar]
- 26.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E. and Zandstra PW. (2009). Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng 102:493–507 [DOI] [PubMed] [Google Scholar]
- 27.Purpura KA, George SH, Dang SM, Choi K, Nagy A. and Zandstra PW. (2008). Soluble Flt-1 regulates Flk-1 activation to control hematopoietic and endothelial development in an oxygen-responsive manner. Stem Cells 26:2832–2842 [DOI] [PubMed] [Google Scholar]
- 28.Koay EJ. and Athanasiou KA. (2008). Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis Cartilage 16:1450–1456 [DOI] [PubMed] [Google Scholar]
- 29.Garita-Hernandez M, Diaz-Corrales F, Lukovic D, Gonzalez-Guede I, Diez-Lloret A, Valdes-Sanchez ML, Massalini S, Erceg S. and Bhattacharya SS. (2013). Hypoxia increases the yield of photoreceptors differentiating from mouse embryonic stem cells and improves the modeling of retinogenesis in vitro. Stem Cells 31:966–978 [DOI] [PubMed] [Google Scholar]
- 30.Jarecki J, Johnson E. and Krasnow MA. (1999). Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99:211–220 [DOI] [PubMed] [Google Scholar]
- 31.Morrisey EE. and Hogan BL. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18:8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam PP. and Behringer RR. (1997). Mouse gastrulation: the formation of a mammalian body plan. Mech Dev 68:3–25 [DOI] [PubMed] [Google Scholar]
- 33.Gebb SA. and Jones PL. (2003). Hypoxia and lung branching morphogenesis. Adv Exp Med Biol 543:117–125 [DOI] [PubMed] [Google Scholar]
- 34.Lim HJ, Han J, Woo DH, Kim SE, Kim SK, Kang HG. and Kim JH. (2011). Biochemical and morphological effects of hypoxic environment on human embryonic stem cells in long-term culture and differentiating embryoid bodies. Mol Cells 31:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuda T, Teratani T, Chowdhury MM, Ochiya T. and Sakai Y. (2013). Hypoxia efficiently induces differentiation of mouse embryonic stem cells into endodermal and hepatic progenitor cells. Biochem Eng J 74:95–101 [Google Scholar]
- 36.Gadue P, Gouon-Evans V, Cheng X, Wandzioch E, Zaret KS, Grompe M, Streeter PR. and Keller GM. (2009). Generation of monoclonal antibodies specific for cell surface molecules expressed on early mouse endoderm. Stem Cells 27:2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bidez PRI, Kresh YJ, Wei Y. and Lelkes. PI. (2011). Enhanced cardiac differentiation of mouse embryonic stem cells by electrical stimulation. In: Stem Cell Engineering. Artmann G, Hecheler J, Minger S, eds. Springer, Heidelberg, pp 119–142 [Google Scholar]
- 38.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, et al. (1998). Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485–490 [DOI] [PubMed] [Google Scholar]
- 39.Pimton P, Sarkar S, Sheth N, Perets A, Marcinkiewicz C, Lazarovici P. and Lelkes PI. (2011). Fibronectin-mediated upregulation of alpha5beta1 integrin and cell adhesion during differentiation of mouse embryonic stem cells. Cell Adh Migr 5:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SW, Jeong HK, Lee JY, Yang J, Lee EJ, Kim SY, Youn SW, Lee J, Kim WJ, et al. (2012). Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med 4:924–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA. and Simon MC. (2006). Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol 26:3514–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang DJ, Oh SH, Lee N, Choi C, Jeon I, Kim HS, Shin DA, Lee SE, Kim D. and Song J. (2013). Contralaterally transplanted human embryonic stem cell-derived neural precursor cells (ENStem-A) migrate and improve brain functions in stroke-damaged rats. Exp Mol Med 45:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giaccia AJ, Simon MC. and Johnson R. (2004). The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev 18:2183–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K, Zhang T, Dong Q, Nice EC, Huang C. and Wei Y. (2013). Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis 4:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones R. and Reid. LM. (2004). Development of the pulmonary vasculature. In: The Lung: Development, Aging and the Environment. Harding R, Pinkerton KE, and Plopper CG, eds. Elsevier Ltd, Oxford, United Kingdom, pp 81–104 [Google Scholar]
- 46.Fischer B. and Bavister BD. (1993). Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil 99:673–679 [DOI] [PubMed] [Google Scholar]
- 47.Gassmann M, Fandrey J, Bichet S, Wartenberg M, Marti HH, Bauer C, Wenger RH. and Acker H. (1996). Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci U S A 93:2867–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN. and Burton GJ. (2000). Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157:2111–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kocabas F, Mahmoud AI, Sosic D, Porrello ER, Chen R, Garcia JA, DeBerardinis RJ. and Sadek HA. (2012). The hypoxic epicardial and subepicardial microenvironment. J Cardiovasc Transl Res 5:654–665 [DOI] [PubMed] [Google Scholar]
- 50.Oliver JA, Maarouf O, Cheema FH, Martens TP. and Al-Awqati Q. (2004). The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parmar K, Mauch P, Vergilio JA, Sackstein R. and Down JD. (2007). Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A 104:5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fynes K, Tostoes R, Ruban L, Weil B, Mason C. and Veraitch FS. (2014). The Differential effects of 2% oxygen preconditioning on the subsequent differentiation of mouse and human pluripotent stem cells. Stem Cells Dev 23:1910–1922 [DOI] [PubMed] [Google Scholar]
- 53.Ezashi T, Das P. and Roberts RM. (2005). Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 102:4783–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forristal CE, Christensen DR, Chinnery FE, Petruzzelli R, Parry KL, Sanchez-Elsner T. and Houghton FD. (2013). Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS One 8:e62507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae D, Mondragon-Teran P, Hernandez D, Ruban L, Mason C, Bhattacharya SS. and Veraitch FS. (2012). Hypoxia enhances the generation of retinal progenitor cells from human induced pluripotent and embryonic stem cells. Stem Cells Dev 21:1344–1355 [DOI] [PubMed] [Google Scholar]
- 56.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S. and Duncan SA. (2010). Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hakim F, Kaitsuka T, Raeed JM, Wei FY, Shiraki N, Akagi T, Yokota T, Kume S. and Tomizawa K. (2014). High oxygen condition facilitates the differentiation of mouse and human pluripotent stem cells into pancreatic progenitors and insulin-producing cells. J Biol Chem 289:9623–9638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giordano FJ. (2005). Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu YZ, Moran SM, Hogenesch JB, Wartman L. and Bradfield CA. (1998). Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr 7:205–213 [PMC free article] [PubMed] [Google Scholar]
- 60.Forristal CE, Wright KL, Hanley NA, Oreffo RO. and Houghton FD. (2010). Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinck AP. (2012). Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily. FEBS Lett 586:1860–1870 [DOI] [PubMed] [Google Scholar]
- 62.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR. and Chandel NS. (2013). Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem 288:770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji AR, Ku SY, Cho MS, Kim YY, Kim YJ, Oh SK, Kim SH, Moon SY. and Choi YM. (2010). Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp Mol Med 42:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen JW, Hwang JT. and Kelly GM. (2012). Reactive oxygen species and Wnt signalling crosstalk patterns mouse extraembryonic endoderm. Cell Signal 24:2337–2348 [DOI] [PubMed] [Google Scholar]
- 65.Sandieson L, Hwang JT. and Kelly GM. (2014). Redox regulation of canonical Wnt signaling affects extraembryonic endoderm formation. Stem Cells Dev 23:1037–1049 [DOI] [PubMed] [Google Scholar]
- 66.Shimoda LA. and Semenza GL. (2011). HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 183:152–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Haute L, De Block G, Liebaers I, Sermon K. and De Rycke M. (2009). Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res 10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matthay MA, Anversa P, Bhattacharya J, Burnett BK, Chapman HA, Hare JM, Hei DJ, Hoffman AM, Kourembanas S, et al. (2013). Cell therapy for lung diseases. Report from an NIH-NHLBI workshop, November13–14, 2012. Am J Respir Crit Care Med 188:370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lecht S, Stabler CT, Rylander AL, Chiaverelli R, Schulman ES, Marcinkiewicz C. and Lelkes PI. (2014). Enhanced reseeding of decellularized rodent lungs with mouse embryonic stem cells. Biomaterials 35:3252–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crisanti MC, Koutzaki SH, Mondrinos MJ, Lelkes PI. and Finck CM. (2008). Novel methods for delivery of cell-based therapies. J Surg Res 146:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI. and Finck CM. (2006). Engineering three-dimensional pulmonary tissue constructs. Tissue Eng 12:717–728 [DOI] [PubMed] [Google Scholar]
- 72.Mondrinos MJ, Jones PL, Finck CM. and Lelkes PI. (2014). Engineering de novo assembly of fetal pulmonary organoids. Tissue Eng Part A. [Epub ahead of print]; DOI: 10.1089/ten.tea.2014.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.