Abstract
Articular cartilage damage is a persistent and increasing problem with the aging population. Strategies to achieve complete repair or functional restoration remain a challenge. Photopolymerizing-based hydrogels have long received an attention in the cartilage tissue engineering, due to their unique bioactivities, flexible method of synthesis, range of constituents, and desirable physical characteristics. In the present study, we have introduced unique bioactivity within the photopolymerizing-based hydrogels by copolymerizing polyethylene glycol (PEG) macromers with methacrylated extracellular matrix (ECM) molecules (hyaluronic acid and chondroitin sulfate [CS]) and integrin binding peptides (RGD peptide). Results indicate that cellular morphology, as observed by the actin cytoskeleton structures, was strongly dependent on the type of ECM component as well as the presence of integrin binding moieties. Further, CS-based hydrogel with integrin binding RGD moieties increased the lubricin (or known as superficial zone protein [SZP]) gene expression of the encapsulated chondrocytes. Additionally, CS-based hydrogel displayed cell-responsive degradation and resulted in increased DNA, GAG, and collagen accumulation compared with other hydrogels. This study demonstrates that integrin-mediated interactions within CS microenvironment provide an optimal hydrogel scaffold for cartilage tissue engineering application.
Introduction
Articular cartilage repair utilizing bioactive scaffolds have recently attracted enormous attention in the field of regenerative medicine.1 Cartilage tissue engineering strategies seek to overcome the cartilage self-repair limitation through the development of cellular scaffolds that closely mimic the complex structure of articular cartilage. Cartilage is consisted of dense fibrillar network of collagen type II and negative-charged glycosaminoglycan.2 Further, articular cartilage is organized in a functional manner, in which superficial (or boundary) layer of articular cartilage secrets lubricating factors, such as lubricin (superficial-zone proteins) and hyaluronic acids (HAs).3
Hydrogels exhibit a physical structure that is similar to native tissue.4 They serve as a foundation template for increased cell–cell interactions and the formation of extracellular matrix (ECM) to provide structural support to the newly formed tissue. In particular, polyethylene glycol diacrylate (PEGDA) photopolymerizing hydrogels have been extensively utilized as scaffolds for cartilage tissue engineering applications primarily because of creating the particular functional group to the hydrogel,5 ease of cell encapsulation, and simplicity of handling.6 For example, PEG-based hydrogel system has been used to encapsulate mouse mesenchymal stem cells for inducing chondrogenic commitment.7 However, major limitations of PEG-based hydrogel system as scaffold for tissue engineering are the lack of cell-specific adhesion and the lack of in vivo degradation.8,9 Overcome these limitations, ECM-derived cell adhesive peptides have been important targets for cell-adhesive modification of PEG-based hydrogel. Further, Nuttelman et al. confirmed that the RGD-modified PEG-based hydrogel provided niches that promote human mesenchymal stem cell viability.10 RGD-modified PEG-based hydrogels were also reported to induce chondrogenic commitment of embryonic-stem-cell-derived cells.5,11 In addition to peptide modification on PEG-based hydrogels, methacrylated biological macromolecules have been photocrosslinked along with PEG-based system to provide appropriate biological properties for stem cell differentiation.12
Recently we have reported that chondrocytes isolated from different zones (superficial/middle/deep) seeded within ECM-based hydrogels showed varying cellular response.13 Therefore, to mimic innate cellular microenvironment, improved ECM compositions are necessary to ensure the long-term maintenance of the chondrocyte phenotype. The present study tests the hypothesis that the incorporation of RGD into ECM-based hydrogels may serve as a biologically active microenvironment that supports chondrocyte phenotype.
In addition to support of cellular phenotype, the expression of lubricin and hyaluronic acid synthase (HAS) is a substantial challenge for articular cartilage tissue engineering. In this regard, we show that the expression of lubricin and HAS genes was modulated by ECM-specific microenvironments and integrin interactions. This study provides a framework for understanding the interactions between chondrocytes and the ECM components and integrin-mediated cellular functions.
Materials and Methods
Isolation and culture of bovine chondrocytes
Bovine chondrocytes were isolated, cultured, and characterized as previously described.14 Briefly, full-thickness bovine cartilage was obtained from the slaughterhouse. To isolate chondrocytes, the cartilage chunks were cut into small pieces and they were digested in Dulbecco's modified Eagle's medium (DMEM; Gibco), 5% fetal bovine serum (FBS; Cat. No. 10438-026; Gibco), and 0.2% collagenase (Worthington Biochemical Corporation) for 14–18 h. The resulting chondrocytes were then filtered with using 70-μm cell strainers and washed with phosphate-buffered saline (PBS). Isolated chondrocytes were maintained in chondrocyte culture medium containing DMEM (Gibco), 10% FBS (Cat. No. 10438-026; Gibco), 10,000 U/mL penicillin-streptomycin, 1 mM of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Gibco), 100 μM of nonessential amino acid (NEAA; Gibco), 0.4 μM of proline, and 50 μg/mL of vitamin C. We have utilized the same batch of FBS throughout the experiments to minimize the FBS-dependent variations. Culture medium was changed every other day.
Methacrylation of chondroitin sulfate and HA
The hydroxyl function groups of chondroitin sulfate (CS; Sigma-Aldrich) and hyaluronic acid (HA; MW of 1.6×107; Genzyme Corp.) were reacted with the glycidyl methacrylate (GMA; Sigma-Aldrich) for methacrylate group incorporations.15 Briefly, for methacrylation of CS (MeCS), 1.0 g of CS dissolved in PBS (10%) was reacted with 1 mL of GMA (73 mM) for 11 days. For methacrylation of HA (MeHA), 1.0 g of HA dissolved in PBS (1%) was reacted with 2 mL of GMA (73 mM) for 8 days. After the reaction, MeCS and MeHA were purified for 48 h against 1000-MW dialysis membrane. The purified molecules were lyophilized and stored at −20°C until use.
Synthesis of RGD/RDG-modified poly (ethylene glycol) acrylate
Tyrosine-arginine-glycine-aspartate-serine (YRGDS) and tyrosine-arginine-aspartate-glycine-serine (YRDGS) were synthesized using solid-phase peptide synthesis (SPPS). High-performance liquid chromatography (HPLC) was performed to confirm RGD and RDG peptide purity (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Sample purities were analyzed by RP-HPLC (Thermo Scientific Spectra System AS300; Thermo-Fisher) using C18 reverse-phase column (120 Å, 5 μm, 4.6×250 mm2; AAPPTec) using the following conditions: gradient elution with A: 0.1% TFA/water, B: 0.1% TFA/acetonitrile; from 10% to 90% over 30 min, a flow rate: 1.0 mL/min; detection: UV, 230 nm. For peptide-modified polymers, YRGDS and YRDGS were reacted with acrylate-PEG-N-hydroxysuccinimide-ester (acrylate-PEG-NHS, 3500 MW; Jenkem Technology) in 50 mM TRIS buffer (pH 8.2) for 2 h at room temperature. The product was lyophilized and stored at −20°C until use.
Photoencapsulation of bovine chondrocytes
Isolated primary bovine chondrocytes were encapsulated into (YRGDS)-modified poly (ethylene glycol)-diacrylate (PEGDA, MW 3400; SunBio, Inc.) or YRGDS-modified PEGDA, as previously described.5 PEGDA solution (20% w/v) was mixed in 1:1 ratio with Acrylate-PEG-YRGDS or Acrylate-PEG-YRDGS in sterile PBS for 2.5 mM final peptide concentration. For RGD/RDG-modified ECM hydrogels, YRGDS-PEGDA or YRDGS-PEGDA solution was mixed with MeCS (10% w/v in PBS) and MeHA (1% w/v in PBS), respectively, in 1:1 ratio to formulate precursor solution. Cells were gently mixed with the polymer solution at a concentration of 2×106 cells/construct and photopolymerized with photoinitiator (Igacure 2959, 0.05% w/v) using UV light (3.5 mW/cm2) for 5 min. The constructs (diameter=5 mm, height=2.3 mm, and total volume=75 μL) were maintained at 37°C with 5% CO2 with chondrocyte culture medium for 3 weeks.
Swelling and mechanical properties of hydrogels
For each hydrogel, gel samples (n=3) were swollen and weighted in equilibrium swollen state. The gels were subsequently lyophilized to remove the water weight from the samples and the dry weight was measured. Swelling ratio (Q) was then calculated from the mass swelling ratio.16
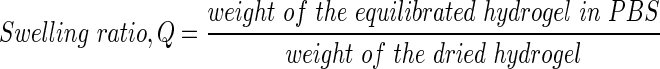 |
Hydrogel degradation was examined by monitoring weight loss upon incubation in enzyme-containing solutions. Wet weight of hydrogels (Wt) was monitored as a function of incubation time in hyaluronidase (100 U/mL, H3506; Sigma-Aldrich) or chondrotinase ABC (1 U/mL, C3667; Sigma-Aldrich) solution at 37°C up to 4 days. The weight-loss-ratio percent was defined as the following equation, where Wt indicates initial weight of hydrogel.
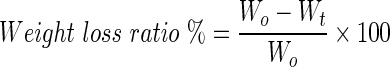 |
For mechanical characterization, hydrogels were equilibrated in PBS for 24 h and compression tests were performed using Instron Model 5966 (Instron Corporation) with a 10-kN-load cell. The load, stress, and strain values recorded were used to calculate the stress and strain from the measured dimensions of each sample. Compressive modulus was calculated from the linear region of the stress–strain curve.
Cellular viability and morphological analysis
Cell viability in hydrogels was determined by live/dead cell viability/cytotoxicity kit (Molecular Probes; L-3224) that contains calcein-AM (“Live” dye) and ethidium homodimer-1 (EthD-1, “Dead” dye). Images were collected using CKS41 fluorescence microscope (Olympus). For cellular morphological analysis, cells were fixed at 4% paraformaldehyde and cut into 500-μm section. After permeabilized with 0.1% Triton X-100 for 30 min, sections were stained with 4′6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 10 min and Alexa Fluor® 488 Phalloidin (A12379, 1:150 dilutions; Life Technologies) for 2 h. Images were obtained using LSM 510-Meta confocal microscope (Zeiss).
Biochemical analysis of hydrogels
Biochemical assays were performed on RGD/RDG-modified ECM-based hydrogel constructs. Constructs (n=3) were collected at 3 weeks, lyophilized, mechanically crushed, and digested in papainase solution (1 mL/construct; 125 μg/mL; Worthington Biomedical) for 16 h at 60°C.14 DNA content was quantified using Quant-iT™ PicoGreen®dsDNA assay kit (Invitrogen™) according to the manufacturer's instruction. The GAG content was quantified using dimethylmethylene blue (DMMB) spectrophotometric assay at A525, as previously described.17 For measurement of GAGs produced by cells in cell-laden PEG-CS hydrogels, we have subtracted off the GAG value of acellular PEG-CS hydrogels from the total value (i.e., GAG from CS-PEG+ cells). Total collagen content was determined by measuring the amount of hydroxyproline within the constructs after acid hydrolysis and reaction with p-dimethylaminobenzaldehyde and chloramine-T, as previously described.18
Histological and immunostaining analyses
Cell-laden hydrogel constructs were fixed with 4% paraformaldehyde solution at 4°C for overnight. Prior to the embedding in optimal temperature cutting compound (OCT), samples were incubated in 20% sucrose solution for 2 h at room temperature, and OCT-embedded samples were placed in isopentane and frozen in liquid nitrogen. Using a cryostat (Leika; CM3050), sample was cryosectioned into 15-μm-thick slices. For H&E staining, frozen sections were rehydrated in PBS at room temperature for 10 min and stained with hematoxylin (Ricca Chemical Company; Gill 2, Cat. No. 3536-16) for 5 min. The stained sections were thoroughly rinsed with DI water. Sections were then stained with Eosin-Y (Richard-Allan Scientific; Cat. No. 7111) for 1 min, followed by multiple washes in DI water. For Safranin-O staining, the rehydrated sections were stained with 0.1% Safranin-O (ScholAR Chemistry) for 5 min at room temperature. The stained samples were gradually dehydrated in series of ethanol, followed by CitriSolv (Fisher Scientific; Cat. No. 22-143975). For immunofluorescence staining of collagen types I and II and F-actin, rehydrated sections were blocked and permeabilized in PBS containing 0.3% Triton X-100 and 3% bovine serum albumin (BSA; Sigma; Cat. No. A7906) for 1 h at room temperature. Next, the sections were incubated with primary antibodies against type I and type II collagens (1:200, rabbit polyclonal; Fitzgerald; Cat. No. 70R-CR007X and Cat. No. 70R-CR008X, respectively) at 4°C for overnight. After washing in PBS, sections were incubated with secondary antibodies (1:250; goat anti-rabbit Alexa-Fluor 488 and goat anti-rabbit Alexa-Fluor 546; Life Technologies) and Alexa-Fluor 488 Phalloidin (1:100; Life Technologies) for F-actin. The nuclei were stained with Hoechst 33342 (2 μg/mL; Life Technologies) for 5 min at room temperature, followed by mounting sections with Vectashield (Vector Laboratories). Imaging was performed using a fluorescence microscope (Carl Zeiss; Axio Observer A1).
Real-time PCR
Total RNAs were extracted from cell-laden hydrogel (n=6) with Trizol, and reverse transcribed into cDNA using the SuperScript Synthesis System (Invitrogen). Real-time PCRs were performed using the SYBR Green PCR Mastermix and the ABI StepOnePlus™ Real-Time PCR system (Applied Biosystems). cDNA samples (1 μL for total volume of 20 μL per reaction) were analyzed for the genes of interest, and GAPDH was used as a reference gene. The level of expression of each target gene was then calculated as −2ΔΔCt.19 Each sample was repeated at least three times for the gene of interest. The PCR primers are listed in Table 1.
Table 1.
Primer List
| Gene | Primer 5′-3′ |
|---|---|
| GAPDH | F: TGT TGT GGA TCT GAC CTG CC |
| R: TTC TCA GTG TGG CGG AGA TG | |
| Type-II collagen | F: CTG TCC TCT GCG ACG ACA TAA |
| R: CCA TCT GGG CAG CAA AGT TTC | |
| Aggrecan | F: CGG TGA GAC GTC AGC CTA TC |
| R: ATT CTG GGA TGG TGG TGC TG | |
| Lubricin | F: CGC AGA AAC CAA CCA AAG CA |
| R: TTG ATG CCG AAG CCT TGA CT | |
| HAS2 | F: GGA TTA TGT ACA GGT TTG TGA TTC AGA |
| R: ACC TCC AAC CAT GGG ATC TTC | |
| Link protein | F: GCT CTG TGC AAT ATC CCA TC |
| R: CCC ACT TTT GCA ATC TGA GC |
Statistical analysis
All data are presented as mean±standard deviation (SD). Statistical significance was determined by analysis of variance (ANOVA single factor) with *p<0.05.
Results
Synthesis and characterization of RGD/RDG-modified ECM-based hydrogels
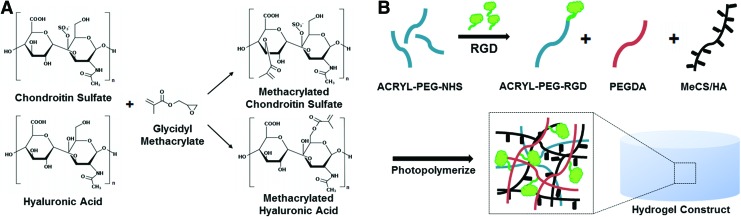
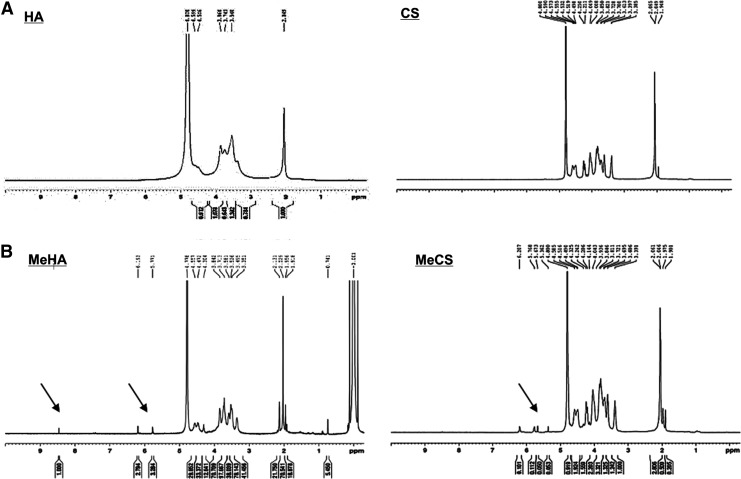
ECM-based photopolymerizing hydrogels were fabricated by introducing methacrylated functional groups to CS and HA followed by copolymerizing modified CS or HA with PEGDA/Acrylate-PEG-RGD solution. CS and HA have hydroxyl functional group that can react with glycidyl methacrylate, resulting in incorporation of photoreactive groups to CS and HA (Fig. 1A). To confirm the incorporation of methacrylate functional group on CS and HA, we performed 1H-NMR analysis (Fig. 2). Two 1H-NMR peaks of MeCS representing vinyl protons at 6.207 ppm and 5.768 ppm and MeHA of 6.193 ppm and 5.771 ppm were observed in the 1H-NMR spectra. For further RGD/RDG incorporation into the ECM-based hydrogels, PEGDA with Acrylate-PEG-RGD or Acrylate-PEG-RDG was mixed 1:1 with MeCS or MeHA prior to photopolymerization (Fig. 1B).
FIG. 1.
Schematic illustration of RGD/RDG-modified extracellular matrix (ECM) hydrogels. (A) Chondroitin sulfate (CS) and hyaluronic acid (HA) were reacted with glycidyl methacrylate (GMA) to form methacrylated CS and methacrylated HA. (B) ACRL-PEG-RGD/RDG was mixed with MeCS and MeHA, respectively, to make different groups of hydrogel. Color images available online at www.liebertpub.com/tea
FIG. 2.
1H-NMR spectra image of CS, HA, MeCS, and MeHA. (A) 1H-NMR spectra image of CS and HA before reacting with pGMA. (B) 1H-NMR spectra image of acrylate peaks at ∼5.3 ppm to ∼6.2 ppm indicate the presence of methacrylation in MeCS. Acrylate peaks at ∼5.7 ppm to ∼6.2 ppm indicate the presence of methacrylation in MeHA.
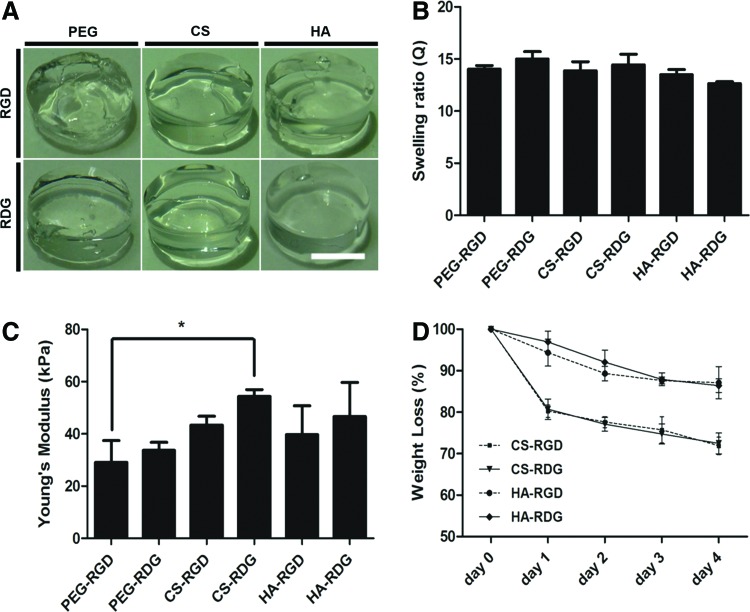
Each type of acellular hydrogel formed into homogenous cylindrical shape (Fig. 3A). Swelling studies indicated that equilibrium swelling of the hydrogels had been reached after being immersed in PBS for 24 h. Swelling ratios of the hydrogels remained the same afterward for the 3 weeks of culture period (data not shown). The equilibrium swellings of acellular hydrogels are shown in Figure 3B. PEG-RGD had a swelling ratio of 15.01, which was higher than that of all the other hydrogel compositions. However, there was no statistical difference between PEG-RGD and PEG-RDG. The results indicated that exchange of RGD to RDG groups into the PEG-based hydrogel did not cause any change in swelling ratio. Further, among the ECM-based hydrogels, HA-RDG had a swelling ratio of 12.64, which was the lowest. However, swelling ratio differences among each ECM-based hydrogel were not statistically significant.
FIG. 3.
RGD/RDG-modified ECM-based hydrogel characterization. (A) Gross image of the acellular hydrogels. Scale bar=250 μm. (B) Swelling ratio of six hydrogel groups after swollen in PBS for 1 day. Similar swelling ratio was observed between hydrogel groups. Error bars represent the standard deviation on the mean for n=3. (C) Young's modulus values for six acellular hydrogel groups. Error bars represent the standard deviation on the mean for n=3: *p-value<0.05. (D) Degradation rate of each type of hydrogel material. Error bars represent the standard deviation of the mean for n=3. Viability test within hydrogel after cultured for 24 h. Color images available online at www.liebertpub.com/tea
For equilibrium Young's modulus of acelluar hydrogels, mechanical properties of CS-based hydrogels were statistically higher compared with other hydrogels. There was no significant difference between PEG-based and HA-based hydrogels. Negatively charged sulfate groups in CS may have increased in accumulation of water content. Further, compressive modulus of CS-based hydrogels may have increased due to electrostatic repulsive forces of charged CS at physiological pH (Fig. 3C).20
We further examined whether ECM-based hydrogels can degrade by ECM-specific enzymes. HA-based hydrogels and CS-based hydrogels were incubated in PBS with hyaluronidase and chondroitinase, respectively. HA-based hydrogels were degraded over time and 100 U/mL of hyaluronidase resulted in 12.91% mass loss in HA-RGD and 13.61% of mass reduction in HA-RDG after 4 days of incubation, respectively. The degradation of CS-RGD and CS-RDG hydrogels was comparable as 28.12% and 27.54% mass remained, respectively, in the presence of 1 U/mL chondroitinase over 4-days period (Fig. 3D). These results showed that ECM-based hydrogels have comparable swelling ratio characteristics, although CS-based hydrogels displayed higher young's modulus and faster enzyme-dependent degradation.
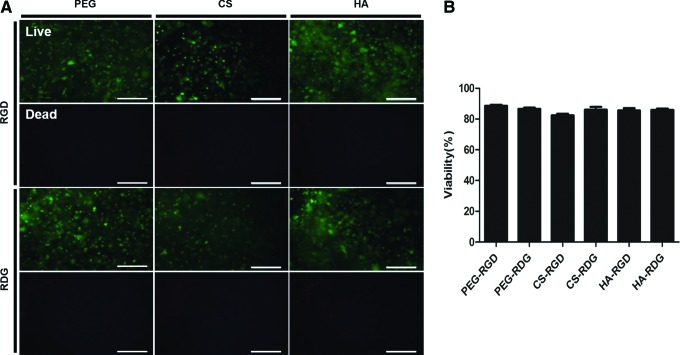
The cytotoxicity of the hydrogels was examined by a viability assay (Fig. 4A). All scaffold compositions supported cell viability over 2 days of cultivation. Quantitative analysis of cell viability showed that 86% of encapsulated cells were live, and the presence of RGD did not enhance the chondrocyte viability (Fig. 4B). These results indicate that ECM-based hydrogels can support the cell survival regardless of integrin engagement.
FIG. 4.
Analysis of chondrocyte viability in RGD/RDG-modified ECM hydrogels. (A) Live/dead viability cytotoxicity kit was used for viability test. Live cell was stained by Calcein AM and dead cell was stained by Ethd-1. Scale bar=200 μm. (B) Viability was measured by calculating the ratio of live cell and total cell number. Error bars represent the standard deviation on the mean for n=3. Color images available online at www.liebertpub.com/tea
Effect of ECM-based hydrogels on chondrocyte morphology, proliferation, and ECM synthesis
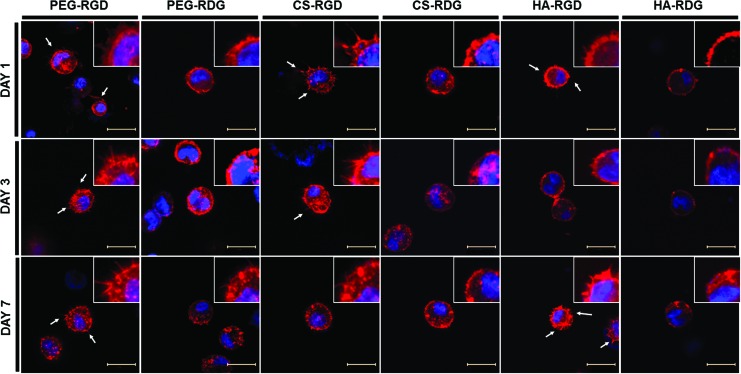
We further examined the ECM-hydrogel-dependent cellular morphology. Bovine chondrocytes were encapsulated in RGD/RDG-modified ECM-based hydrogels and maintained in chondrocyte culture medium for 7 days. Cell morphologies were evaluated by actin staining. F-actin staining revealed few microsize protrusions of the cells into the surrounding matrix (white arrows in Fig. 5A). All ECM-based hydrogels presented uniformly distributed viable cells that displayed a rounded morphology. However, some cellular extended feature morphologies were only present in RGD-modified groups. This extension was followed by interaction between cell and RGD peptide, which was incorporated within hydrogel. From the result, we confirmed that cell adhesion receptors (RGD) are involved in modulation of cellular phenotype by stress fiber formation within hydrogel independent of ECM microenvironment.
FIG. 5.
Morphological analysis of chondrocytes in RGD/RDG-modified ECM hydrogels. Each group of hydrogel was stained with phalloidin and DAPI to observe actin cytoskeleton and nucleus. White arrows are representing cellular sprouting within hydrogel. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
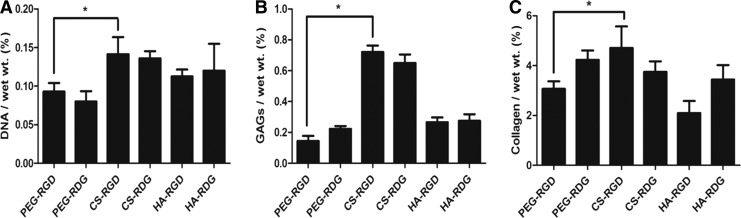
We next examined the proliferation of bovine chondrocytes within ECM-based hydrogels by quantifying the DNA content after 3 weeks of culture. All groups showed a trend of increasing DNA content over the 3-week period as compared with day 0 (data not shown). DNA content after 3 weeks of time point is depicted in Figure 6A. A significant increase in DNA content was seen in CS-RGD hydrogel compared with PEG-RGD and PEG-RDG. In addition, HA-based hydrogels were able to significantly improve proliferation of cells compared with PEG-based hydrogels. Interestingly, cellular proliferation was not affected by the presence of RGD peptide. Quantitative analysis of GAG indicated that chondrocytes in CS-RGD hydrogels produced the highest levels of GAG accumulation (0.72087% w/w). It was four times higher than GAG content observed in PEG-RGD hydrogels. Similar to DNA measurement result, greater amounts of sulfated GAGs were detected in CS-RGD hydrogels (Fig. 6B). Production of collagen was also quantified after 3 weeks of in vitro culture. Measured collagen content in CS-RGD hydrogels was 4.71% (w/w), which is statistically higher compared with 3.07% of PEG-RGD hydrogel. Interestingly, HA-RGD resulted in lowest collagen accumulation (Fig. 6C).
FIG. 6.
Biochemical analysis of chondrocytes in RGD/RDG-modified ECM hydrogels. (A) Quantification of cell proliferation (DNA) and matrix production of GAGs (B) and collagen (C) was determined by biochemical assays. Error bars represent the standard deviation on the mean for n=3: *p<0.05.
Histological and immunostaining analyses of engineered cartilage tissues
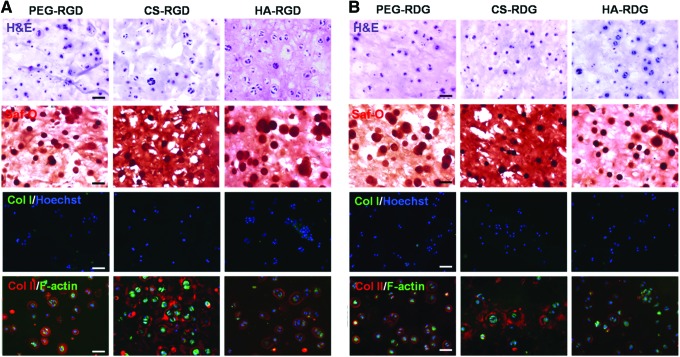
Histological evaluation of chondrocytes after 3 weeks of culture indicated that PEG-RGD, CS-RGD, and HA-RGD groups maintained higher GAG accumulation in the hydrogel (Fig. 7A). In addition, number of pericellular matrix visualized with H&E staining was higher in the RGD group compared with the RDG group (Fig. 7A, B). Moreover, the diameter of pericellular matrix visualized by Safranin-O staining (red) was larger and vivid in the PEG-RGD, CS-RGD, and HA-RGD groups of hydrogel, which demonstrates that accumulation of cell-secreted proteoglycan was more apparent in RGD-modified groups compared with RDG-modified groups of hydrogel. Even though CS-RGD and RDG hydrogels stained positive for Safranin-O due to CS content in the hydrogel, GAG contents around the cell appeared much intense in CS-RGD group. To determine the extent of background staining, acellular CS-RGD hydrogel was stained with Safranin-O (Supplementary Fig. S2). Immunostaining analysis for collagen types I and II demonstrated significant differences between RGD-modified hydrogels and RDG-modified hydrogels. Cells encapsulated in RGD-modified hydrogels produced concentrated and well-defined cartilaginous ECM in the periphery of the cells as indicated by staining with type II collagen antibody. Especially, CS-RGD group produced strong type II collagen network throughout the hydrogel, which closely mimics the natural tissue. Type I collagen, indicative of dedifferentiated chondrocytes, was not detected throughout the RGD/RDG-modified ECM-based hydrogels.
FIG. 7.
Chemical and immunostaining of RGD/RDG-modified ECM-based hydrogels encapsulated with chondrocytes at 3 weeks. Hematoxylin and eosin (H&E) staining demonstrates cellular morphology, Safranin-O staining (Saf-O) demonstrates deposition of GAGs, and collagen I staining (green) demonstrates dedifferentiation with Hoechst DNA staining (blue), and F-actin (green) and collagen type II (red) expression of RGD group (A) and RDG group (B). Scale bar=50 μm (20×). Color images available online at www.liebertpub.com/tea
Gene expression analysis of chondrocytes in RGD/RDG-modified ECM-based hydrogels
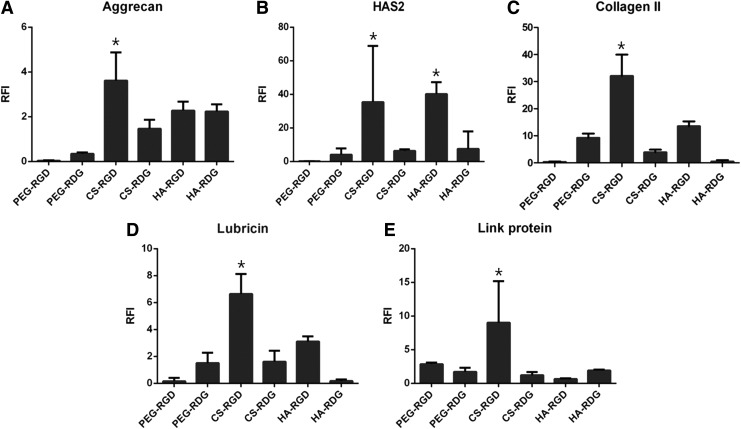
To determine the biological effects of ECM-based hydrogel with RGD, we investigated cartilage-specific gene expression, such as type II collagen, aggrecan, hyaluronan synthase 2 (HAS2), lubricin, and Link protein, in each hydrogel cultured for 3 weeks (Fig. 8A–E). All gene expression was normalized to PEG-RDG hydrogel for day-1 culture. The results showed that the expression of aggrecan, HAS2, and type II collagen genes was significantly upregulated in PEG-RDG compared with the PEG-RGD. On the contrary to this, incorporation of RGD into CS and HA, all three of chondrogenic (or cartilage-specific) markers were upregulated compared with its RDG groups. Thus, ECM production due to cell adhesion was activated only in the hydrogels containing cartilage-specific ECM molecules. Real-time PCR analysis of aggrecan also matches with GAG biochemical assays in Figure 6B. Also, other two markers, lubricin and link protein, are upregulated compared with other groups. These results propose that RGD modification of biomaterial will enhance the chondrocyte behavior in cartilage regeneration.
FIG. 8.
Analysis of gene expression level for aggrecan (A), HAS2 (B), collagen II (C), lubricin (D) and link protein (E) after cultured for 3 weeks. Gene expression was normalized by PEG-RDG hydrogel for 1-day culture and GAPDH was used for housekeeping gene: *p-value<0.05.
Discussion
Matrix-dependent chondrocyte phenotypes have been studied extensively in hydrogels, primarily via encapsulating cells in three-dimensional (3D) scaffolds.21 In the case of cartilage, both ECM composition and integrin engagement have been shown to influence chondrocyte phenotype.22 To define whether ECM components can influence stem cell behavior, we have previously investigated the effects of ECM components in 3D microenvironment by creating PEG-based hydrogels with exogenous type I collagen, type II collagen, CS, and HA.23,24 Incorporation of ECM in PEG-based hydrogel resulted in ECM-dependent modulation of stem cell fate, indicating the importance of ECM microenvironment.25
Designing a proper scaffold, which could provide the necessary biological environmental to the encapsulated cells, is a pivotal issue in cartilage tissue engineering. PEG-based photopolymerizing hydrogels have been widely utilized for cartilage tissue engineering applications by providing bio-inert space in which the encapsulated cells function and produce matrix; however, they lack moieties that promote cell–matrix interactions. In this study, we outlined a method for peptide (RGD/RDG) incorporation into ECM-modified hydrogels to permit engineered biospecific cell adhesion in ECM microenvironment. A finding of this study has yielded ECM-based hydrogels with notable cytocompatibility and ability to provide a suitable microenvironment to produce cartilage-like tissues. In particular, we demonstrated here that the incorporation of host tissue-mimetic biomolecules along with covalently linked RGD peptide sequences significantly resulted in GAG-rich as well as collagen-rich tissues. Chondrocytes surrounded by their ECM maintain round morphology. Cell shape or actin organization plays an important role in cellular proliferation, cell differentiation, and matrix synthesis.26,27
RGD binds to αv and β1 integrins of chondrocytes and integrin activation leads to intracellular signaling pathway regulating cell–matrix interaction. These adhesion peptides and their receptors mediated inside-out signaling and outside-in signaling, resulting in cell anchorage, differentiation, motility, migration, and ECM remodeling in homeostasis.28–30 However, in regards to chondrocytes in RGD microenvironment, previous studies showed conflicting results. Study by Levenston and coworkers showed that chondrogenesis of mesenchymal stem cells was inhibited by RGD-containing hydrogel. They found that cell-adhesive ligands inhibit stimulation of chondrogenic markers.31 Similarly, chondrocyte spreading due to high RGD concentration has shown to decrease cell size and reduce ECM synthesis overtime in polyethylene glycol dimethacrylate (PEGDM) hydrogel.32,33 Accordingly, in our study, slight decrease in GAG and collagen accumulation was observed in PEG-RGD hydrogel compared with that in PEG-RDG hydrogels. Conversely, ECM-based hydrogels exhibited biodegradation properties upon exposure to their respective degradable enzymes.34,35 From the experiment, we conclude that RGD peptide response in ECM-based hydrogels represented a significant contrast to RGD peptide response in PEG-based hydrogels. A recent study by Kloxin et al. has demonstrated that temporal RGD presentation resulted in increased GAG and collagen contents compared with persistent RGD presentation on cells.36 This study was performed utilizing dynamic removal of RGD moieties in PEG-based hydrogels by photolytic degradation. Similarly, we hypothesize that our CS-RGD hydrogels may have provided temporal RGD presentation on encapsulated chondrocytes due to cell-responsive degradation of CS components. Early phase of temporal RGD exposure may have stimulated the encapsulated cell in ECM-based hydrogels.
Although many factors, such as degree of methacrylation and concentration of functionalized macromolecules (CS and HA), can influence the swelling ratio and mechanical properties of acellular and cellular hydrogels, in the current study composition concentrations of MeCS and MeHA were adjusted to have similar swelling ratio and mechanical properties to the PEG-based hydrogels. When compared with the acellular PEG-RGD and PEG-RDG hydrogels, cellular hydrogels resulted in slight increase in mechanical properties after 3 weeks of culture (Supplementary Fig. S3), indicating that cell-secreted ECM has contributed to the increased mechanical properties of engineered tissues. Interestingly, mechanical properties of cellular CS-based hydrogels were not increased when compared with acellular CS-based hydrogels. This is probably due to the faster rate of CS-based hydrogel degradation, which is unmatched by ECM secretion. Likewise, CS content in CS-RGD or CS-RDG was 5% while that of HA in HA-RGD or HA-RDG was 1%. These discrepancies of ECM content may have caused faster enzyme-dependent degradation in CS-based hydrogels compared with HA-based hydrogels. Of the ECM-based hydrogels examined, CS-RGD hydrogel exhibited the greatest extent of DNA and GAG contents. Immunostaining for type II collagen indicated that collagen accumulation in RGD-CS hydrogels was not limited to cellular periphery but detected throughout the hydrogel. Further, interconnected collagen networks were detected throughout CS-RGD hydrogel (Supplementary Fig. S4). In contrast, HA-based hydrogels resulted in collagen accumulation concentrated near the cell periphery. Dynamic cell-responsive degradation in CS-RGD hydrogels probably may have contributed to increased GAG and collagen accumulation compared with other hydrogels.
The production of functional proteins is still remained as the challenge for cartilage tissue engineering. In particular, among many functional proteins, lubricin, a mucinous glycoprotein encoded by the PRG4 gene, provides boundary lubrication in articular joints. Lubricating property plays an important role in integration with surrounding tissue but optimal cell scaffold regulating this property has not been established. It has been demonstrated that lubricin-secreting phenotype of chondrocytes is retained in the monolayer culture, whereas 3D alginate hydrogel culture inhibited this phenotype.37 Additionally recent study indicated that ECM, such as CS and HA, added to alginate hydrogel induced lubricin expression.38 Our findings confirmed that incorporation of these ECMs along with cell-adhesions moieties enhanced lubricin secretion of encapsulated chondrocytes. ECM molecule included hydrogel with cell-adhesive characteristic enhanced lubricin secretion. CS microenvironment with RGD significantly enhanced lubricin and HAS gene expression. Strategies to engineer articular cartilage tissues should, therefore, focus on improving the expression of the lubricin and HAS genes, in analogy to functionality of articular cartilage. In addition, optimizing ECM–cell interactions may provide enhanced microenvironment for cartilage tissue engineering.
Supplementary Material
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF grant No. 2012M3A9C6050102), the Ministry of Health and Welfare (grant No. HI13C0451020013) and the Seoul National University Brain Fusion Research Grant (0458-20140023).
Disclosure Statement
No competing financial interests exist.
References
- 1.Karageorgiou V., and Kaplan D.Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26,5474, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Tibbitt M.W., and Anseth K.S.Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103,655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt T.A., Gastelum N.S., Nguyen Q.T., Schumacher B.L., and Sah R.L.Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum 56,882, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Tibbitt M.W., and Anseth K.S.Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103,655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang N.S., Varghese S., Zhang Z., and Elisseeff J.Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng 12,2695, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hoffman A.S.Hydrogels for biomedical applications. Ann N Y Acad Sci 944,62, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., and Elisseeff J.In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9,679, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Zhu J.Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31,4639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zustiak S.P., and Leach J.B.Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 11,1348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuttelman C.R., Tripodi M.C., and Anseth K.S.Synthetic hydrogel niches that promote hMSC viability. Matrix Biol 24,208, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hwang N.S., Kim M.S., Sampattavanich S., Baek J.H., Zhang Z., and Elisseeff J.Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells 24,284, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Smith D.K.Supramolecular gels: building bridges. Nat Chem 2,162, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Hwang N.S., Varghese S., Lee H.J., Theprungsirikul P., Canver A., Sharma B., and Elisseeff J.Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett 581,4172, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang N.S., Varghese S., and Elisseeff J.Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PloS One 3,e2498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q., Williams C.G., Sun D.D., Wang J., Leong K., and Elisseeff J.H.Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res Part A 68,28, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Bryant S.J., and Anseth K.S.Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res 59,63, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Farndale R.W., Buttle D.J., and Barrett A.J.Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883,173, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Woessner J.F., Jr.The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93,440, 1961 [DOI] [PubMed] [Google Scholar]
- 19.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Bryant S.J., Davis-Arehart K.A., Luo N., Shoemaker R.K., Arthur J.A., and Anseth K.S.Synthesis and characterization of photopolymerized multifunctional hydrogels: Water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules 37,6726, 2004 [Google Scholar]
- 21.Li Z., and Cui Z.Three-dimensional perfused cell culture. Biotechnol Adv 32,243, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Kim I.L., Khetan S., Baker B.M., Chen C.S., and Burdick J.A.Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 34,5571, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varghese S., Hwang N.S., Canver A.C., Theprungsirikul P., Lin D.W., and Elisseeff J.Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 27,12, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Hwang N.S., Varghese S., Li H., and Elisseeff J.Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res 344,499, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Zhu J., He P., Lin L., Jones D.R., and Marchant R.E.Biomimetic poly(ethylene glycol)-based hydrogels as scaffolds for inducing endothelial adhesion and capillary-like network formation. Biomacromolecules 13,706, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glowacki J., Trepman E., and Folkman J.Cell-Shape and Phenotypic-Expression in Chondrocytes. P Soc Exp Biol Med 172,93, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Brown P.D., and Benya P.D.Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin-B induced reexpression. J Cell Biol 106,171, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meighan C.M., and Schwarzbauer J.E.Temporal and spatial regulation of integrins during development. Curr Opin Cell Biol 20,520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giancotti F.G., and Ruoslahti E.Transduction—Integrin signaling. Science 285,1028, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Hood J.D., and Cheresh D.A.Role of integrins in cell invasion and migration. Nat Rev Cancer 2,91, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Connelly J.T., Garcia A.J., and Levenston M.E.Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J Cell Physiol 217,145, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Vonwil D., Schuler M., Barbero A., Strobel S., Wendt D., Textor M., Aebi U., and Martin I.An Rgd-restricted substrate interface is sufficient for the adhesion, growth and cartilage forming capacity of human chondrocytes. Eur Cells Mater 20,316, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Smith Callahan L.A., Childers E.P., Bernard S.L., Weiner S.D., and Becker M.L.Maximizing phenotype constraint and extracellular matrix production in primary human chondrocytes using arginine-glycine-aspartate concentration gradient hydrogels. Acta Biomater 9,7420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leach J.B., Bivens K.A., Patrick C.W., and Schmidt C.E.Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng 82,578, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kirker K.R., and Prestwich G.D.Physical properties of glycosaminoglycan hydrogels. J Polym Sci Pol Phys 42,4344, 2004 [Google Scholar]
- 36.Kloxin A.M., Kasko A.M., Salinas C.N., and Anseth K.S.Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324,59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein T.J., Schumacher B.L., Blewis M.E., Schmidt T.A., Voegtline M.S., Thonar E.J., Masuda K., and Sah R.L.Tailoring secretion of proteoglycan 4 (PRG4) in tissue-engineered cartilage. Tissue Eng 12,1429, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Coates E.E., Riggin C.N., and Fisher J.P.Matrix molecule influence on chondrocyte phenotype and proteoglycan 4 expression by alginate-embedded zonal chondrocytes and mesenchymal stem cells. J Orthop Res 30,1886, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.