Abstract
Background and Objective:
Early life adverse events may influence susceptibility/resistance to chronic inflammatory diseases later in life by permanently dysregulating brain-controlled immune-regulatory systems. We have investigated the impact of infant-mother separation during early postnatal life on the severity of experimental periodontitis, as well as systemic stress and immune responses, in adulthood.
Material and Methods:
Pups of periodontitis resistant Lewis rats were separated from their mothers for 3 h daily during postnatal days 2-14 (termed maternal deprivation; MD), separated for 15 min daily during the same time period (termed handling; HD), or left undisturbed. As adults, their behaviour was tested in a novel stressful situation, and ligature-induced periodontitis applied for 21 days. Two h before sacrifice all rats were exposed to a gram-negative bacterial lipopolysaccharide (LPS) challenge to induce a robust immune and stress response.
Results:
Compared to undisturbed controls, MD rats developed significantly more periodontal bone loss as adults, whereas HD rats showed a tendency to less disease. MD and HD rats exhibited depression-like behaviour in a novel open field test, while MD rats showed higher glucocorticoid receptor (Gr) expression in the hippocampus, and HD rats had altered methylation of genes involved in the expression of hippocampal Gr. LPS provoked a significantly lower increase in circulating levels of the cytokine TGF-1β in MD and HD rats, but there were no significant differences in levels of the stress hormone corticosterone.
Conclusion:
Stressful environmental exposures in very early life may alter immune responses in a manner that influences susceptibility/resistance to periodontitis.
Keywords: Postnatal stress, epigenetics, behaviour, LPS, cytokines, periodontitis, rats.
INTRODUCTION
Periodontitis is a tissue-destructive inflammatory condition of the tooth-supporting tissues commonly considered to be initiated by increased colonization of pathogenic microorganisms in subgingival dental plaque biofilms [1]. They readily activate cells belonging to the innate immune system, and the subsequent release of mediators is probably responsible for the periodontal tissue destruction [2]. Periodontitis is also associated with ageing and genetic and environmental factors such as smoking, diabetes and stress-related diseases [3-7]. The details of how these multifarious disorders influence the development and progression of the disease has been poorly understood.
The pathogenesis indicates that immune system responses vital for clearing pathogens are inappropriately regulated in people with periodontitis. We have previously studied the impact of different immune aberrations in a rat model. Our studies support recent findings indicating that agents that drive adaptive immunity, while inhibiting innate immunity, are protective [2, 8-11]. They also showed that the immune system responses are under brain control via autonomic nervous and neuroendocrine (hormonal) systems, and that rats with differential reactivity or responsiveness of these overarching brain-controlled pathways, including the hypothalamic-pituitary-adrenal (HPA) axis reactivity to environmental stressful challenges, whether of emotional or immunological origin, also differ in their susceptibility to periodontitis [12-19]. As we have demonstrated in two rat models with extreme differences in stress reactivity, the diversity in neural and hormonal reactivity can be genetically determined [12, 14, 20]. In rats with environmentally induced differences in stress reactivity like experimental depression [18] and diabetes [21], as well as induced postnatal stress [22-25], similar mechanisms are evident.
Adverse early postnatal life events have been found to alter brain development, and in adulthood to alter behavioural responses to stressful situations, stress hormone responses to stressors, immune responses to pathogens, and susceptibility to infectious and inflammatory diseases [26-29]. Very early life experiences, in particular, can permanently change the reactivity of immune-regulatory systems controlled by the brain, including the HPA axis [26, 27]. People who have experienced severe life stressors early in life (e. g., abuse, neglect, family conflict, low socioeconomic conditions, infections) are at greater risk for a variety of diseases later in life regardless of their subsequent health behaviours [30, 31], and emerging evidence suggests that immune dysregulation, i.e., misguided immune system responses, may be one potential pathway linking stressful early life events to increased susceptibility to later disease onset [31, 32]. These adjustments in the normal course of development may result from epigenetic programming, i.e. the phenomena that environmental stimuli in early life can lead to changes in the expression of genes involved in stress and immune system regulation, and that these changes are potentially responsive to similar stimuli throughout life [26-32].
In the present study, we used the maternal separation model, which is a widely used postnatal psychological stress paradigm that can permanently change the reactivity to stressors, including immune system responses to pathogens [26-29]. This model is also commonly used to study the development of psychiatric disorders such as anxiety and depression [33, 34], and it is well suited for investigating the effects of postnatal experiences on immune system responses and the susceptibility to various diseases in adulthood [29, 34]. New-born rats that are separated from their mothers for an extended time every day develop persistent changes in brain proteins and neurotransmitter systems involved in immune system regulation [34]. Since the adult effects of maternal separation also depend on the genetic background and gender [23, 35], and maternal separation may influence immunity and disease susceptibility through brain-controlled immunoregulatory pathways other than the HPA axis [36], we used male and female offspring of Lewis rats in this experiment. Lewis rats generally show a low HPA axis response, and thus lower release of immunoregulatory glucocorticoid hormones (predominantly cortisol in humans and corticosterone in rodents), to all kinds of imposed stressors, including emotional stress and immunological stress induced by gram-negative bacterial lipopolysaccharides [LPS; 37-39]. Furthermore, they are relatively resistant to periodontitis when compared to histocompatible and HPA axis high-responding Fischer 344 rats [12, 14], whereas the opposite is the case in animal models of pro-inflammatory T helper (Th) 1- mediated autoimmune diseases such as rheumatoid arthritis and multiple sclerosis [40]. To investigate biological mechanisms responsible for the putative relationship between periodontitis and early postnatal psychosocial stressors, we stimulated the animals with LPS just before sacrifice, and measured the effect of maternal separation on the HPA axis derived stress hormone corticosterone and selected cytokines, as well as the methylation of genes involved in the expression of glucocorticoid receptors (Gr) within the hippocampus. The expression of Gr within this brain structure has been found to be important for determining the HPA axis set-point and controlling stress reactivity [26].
MATERIAL AND METHODS
Animals
Pregnant female Lewis rats (from Möllegaard Breeding Center, Ejby, Denmark) were individually housed in standard polypropylene cages containing about 2.5 cm of wood chip bedding material. The animals had free access to standard rat pellets and tap water, and they were maintained under a 12-24 h light/dark cycle (light on 7.00 a.m. to 7.00 p.m.) with temperature and humidity at 22°C and 40-60%, respectively. The experiments were registered and approved by the Norwegian Experimental Animal Board (NEAB).
Maternal Separation Procedure
The present experiments were performed according to a previously described method (38). In short, the rat pups were removed from their mothers for 15 min daily from life day 2 to 14 (handling; HD), for 3 h over the same time period (maternal deprivation; MD) and kept singly in temperature-controlled cages at 31°C. From the same rearing litter, one or two rats were used in each experimental group. The separation was implemented daily between 9:00 to 12:00. The remaining third of the pups were left undisturbed and served as controls. From day 15 to 21 all pups (HD, MD and Controls) were left with their mothers. The pups were then sex-matched, and males and females were placed in standard cages with 4 to 5 animals in each.
The undisturbed Controls (n=12) included 5 males and 7 females. The HD group (n=13) comprised 6 males and 7 females. The MD group (n=18) comprised 7 males and 11 females. At the age of 13 weeks the animals were behaviourally tested and experimental periodontitis was induced 7 days later. Three weeks thereafter, all animals received LPS and were decapitated 2 h later.
Behavioural Testing
At the age of 13 weeks emotional behavioural responses to a novel stressful situation were estimated with the open field test (18). Locomotor activity and explorative behaviour were evaluated. The apparatus consisted of a 100 cm x 100 cm square-shaped box with the floor divided into 10 cm2 squares. The walls surrounding the base were black and 40 cm high. The only illumination was provided by a 15 W bulb, positioned centrally and 50 cm above the floor. Each animal was gently placed in the centre of the apparatus and allowed to explore the arena over a 5-min period. An observer manually recorded movements and rearing behaviour between 9:00 and 12:00 hours. Each animal was tested once, and after each testing the apparatus was cleaned with water and bleach solution and dried.
The Following Parameters were Registered
1. Movements across square boundaries. For each animal the number of squares crossed was added up and used as an indicator of locomotor activity in a novel environment. Depending on factors like genetic background, age, sex, type, time and length of maternal separation, maternal care, and type and length of stressors, locomotor activity is a behavioural parameter that has been found to be affected in animals that are postnatally separated from their mothers [41, 42]. Decreased locomotor activity has been described in depressed patients [43], and maternal deprivation has been used as a model for postnatal stress-induced development of adult depression-related behaviour [33, 37]. Low locomotor activity also appears to be a main fear response of animals exposed to novelty [44]. Therefore, the locomotor activity was used as an indicator of maternal deprivation-induced anxiety.
2. Distance moved in the centre of the illuminated open field apparatus, defined as the inner middle of the arena (60 x 60 cm; 360 cm2). This test is commonly employed to measure anxiety, and “depressed-like” rats usually spend less time in the illuminated centre of the open field apparatus than their sham-operated controls, and some antidepressants attenuate this effect [44].
3. Vertical activity (rearing). The extent of rearing in a new environment reflects exploratory behaviour. In adolescence, this activity has been found to be significantly decreased in MD rats [41].
Experimental Periodontal Disease Model
Fourteen weeks after birth, the animals were anaesthetised with a subcutaneous injection in the neck with Hypnorm-Dormicum (fentanyl/fluanizone, midazolam), 0.2 ml/100 g body weight. A sterile silk ligature (Ethicon Perma-hand® seide, Norderstedt, Germany) was tied around the neck of the maxillary right 2nd molar tooth. The ligatures were left in the same position during the entire experiment and served as a retention device for oral microorganisms. At the end of the experiment all animals were injected intraperitoneally with LPS, and killed by decapitation. Sacrifice was carried out in the morning between 10.00 and 12.00. Trunk blood samples were collected in chilled heparinised tubes for analysis of serum concentration of corticosterone. The maxillae were excised and fixed in 4% formaldehyde.
LPS Challenge
To assess whether the treatment regimen had any effects on corticosterone and selective cytokine responses to a pathogen associated molecular pattern (PAMP), the animals were injected with LPS (E.coli serotype 0111:B4, Sigma Chemicals, MO, USA) i.p. (150 microg/kg) 2 h before decapitation. After decapitation, blood samples were collected (6-10 ml from each animal) in vacutainer tubes (10 ml without additives) and allowed to clot on ice for 1 h. Thereafter, the samples were centrifuged for 20 min at 2000 x g. The serum samples were removed, liquated and stored at -20◦C prior to analysis of corticosterone and cytokines.
Analysis of Serum Corticosterone, TNF-α, IL-10, and TGF-β
Serum corticosterone was measured with a radioimmunoassay (RIA) Coat-A-Count kit from Diagnostic Products Corporation, Los Angeles, CA, USA, catalogue Number TKRC1. The limit of detection was 5.7 ng/ml. The antibody is highly specific with the highest cross reactivity with 11-deoxycorticosterone of less than 2%. The serum levels of TNF-α, IL-10, and TGF-β were measured by means of enzyme-linked immunosorbant assays (ELISA) kits from R&D Systems, Inc., Minneapolis, MN, USA, with catalogue numbers RAT00 for TNF-α, MB100 for TGF-1β, and R1000 for IL-10, respectively. The minimum detectable level/concentration for TNF-α is less than 12.5 pg/mL, less than 31.2 pg/mL for IL-10 and TGF-1β, and less than 62,5 pg/mL for IL-6.
Radiographic Examination of Periodontal Bone Loss
The rat maxillary jaws were placed and stabilised with dental wax on a Sidexis digital x-ray sensor, oriented with the axis of the teeth parallel to the sensor surface. The distance between the cemento-enamel junction (CEJ) and the alveolar bone (B) on mesial and distal surfaces of the 2nd molars was displayed digitally. The examination was done blinded. Bone loss as measured with digital X-rays was chosen as an indicator of the severity of periodontitis because our previous studies have shown that this measurement is significantly more reliable than measuring periodontal fibre loss and bone loss on histological sections [12-24].
Quantative RT-PCR Assay of mRNA for the Glucocorticoid Receptor Within the Hippocampus
Genomic DNA and RNA were isolated from hippocampal tissue using the AllPrep® DNA/RNA Mini kit (Qiagen, Venlo, Netherlands) following the manufacturer’s instructions. First-strand (cDNA) synthesis was performed in a 40 μl reaction containing 375 mM KCl, 250 mM Tris–HCl,15 mM MgCl2, 10mM dithiothreitol and 500 μM deoxynucleoside triphosphates (dNTPs)at 42 °C for 50 min using 200 U SuperScript II Reverse Transcriptase (Invitrogen) and 2.5 μM dT16 primer.
PCR amplifications of cDNA were performed in 25μl reactions containing 50mM KCl, 20mM Tris–HCl (pH 8.4), 2mM MgCl2, 2.5U Platinum Taq DNA polymerase (Invitrogen), 200mM deoxynucleoside triphosphates (dNTP), SYBR Green (Cambrex, Verviers, Belgium) and primers. Thermal cycling, conditions were: 95°C, 2 min; with 45 amplification cycles of 95°C, 20 s; 60°C, 20 s; 72°C, 30 s. All PCR products were separated on a 2% agarose gel and visualized under illumination with SYBR Safe (Invitrogen). Primer sequences used were: β-actin sense: 5’-aga-aga-gct-atg-agc-tgc-ctg-acg, antisense: 5’-tac-ttg-cgt-cag-gag-gag-caa-tg (1µM, 301bp); Total Gr sense:5’ctt-agc-tcc-ccc-tgg-tag-aga-cga, antisense: 5-tgc-tgc-ttg-gaa-tct-gcc-tg (0.5µM, 162bp). All primers were synthesised by Eurogentec (Seraing, Belgium). Data was analysed using the 2-ddCt method [45].
Methylation Analysis
Genomic DNA was bisulphite modified using the EpiTect kit (Qiagen), promoter 17 was amplified, and all amplicons were pyrosequenced using a Pyromark ID with Pyrogold reagents (Biotage) as previously described [46-48]. The bisulphite conversion efficiency was >96% allowing quantitative analysis of DNA methylation patterns in throughout the amplicon.
Statistical Methods
The effects of postnatal separation on behavioural, neuroendocrine, immunological, and clinical responses were assessed with two-way analyses of variance (AVOVA), with treatment (MD, HD, or Controls) and sex as between-group factors. These analyses were followed by post hoc Fisher´s LSD test if appropriate. DNA methylation data were obtained from female rats only. These were analysed with a one-way analysis of variance with treatment (MD, HD, or Controls) as the between group factor. Data are presented as mean ± SD. Level of statistical significance was set at p<0.05.
RESULTS
Effect of Maternal Separation on Body Weight at Disease Induction
The MD rats displayed lower body weight at disease induction compared with undisturbed controls as well as HD rats. At disease induction, the male controls and male MD animals weighed 309.2 ± 7.1 g and 297.1 ± 8.6 g respectively (p<.05), and the female controls 197.1 ± 11.3 g and female MD rats 181.9 ± 8.5 g respectively (p < 0.01). No significant difference was found between the weight of male HD rats (303.8 ± 9.7 g) or female HD rats (188.4 ± 8.8 g) and their respective undisturbed controls.
Effect of Maternal Separation on Periodontal Tissue Destruction
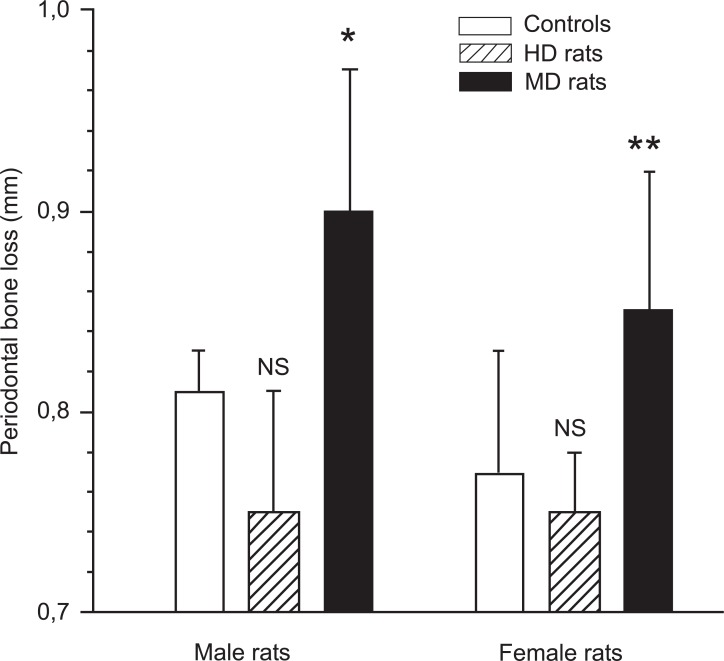
The animals were sacrificed 21 days after application of the ligature. The severity of periodontitis was evaluated by radiographic measurement of alveolar bone loss, i.e.: the distance between the cemento-enamel junction (CEJ) and the most coronal bone (CEJ-B). The alveolar bone loss in the MD animals was significantly more severe in both males and females compared with the undisturbed controls (Fig. 1). In male controls the distance between CEJ and CEJ-B was 0.81 ± 0.02 mm, and in male MD rats the same distance was 0.90 ± 0.07 mm (p < 0.05; Fig. 1). In female controls this distance was 0.77 ± 0.06 mm, and 0.85 ± 0.07 mm in female MD rats (p < 0.01).
Fig. (1).
The mean distance from the cemento-enamel junction to the alveolar bone crest in male and female control rats, and male and female maternal deprived (MD) rats as measured on digital radiographs. p < 0.05 between controls and MD rats, in both males and females, demonstrating that rats exposed to long-term separation (360 min) from their mother during the first 14 days of life developed significant more severe periodontitis in adulthood. In contrast, male and female rats exposed to short-term separation from their mother (10 min) during the same time period (handled rats; HD rats) tended to develop less periodontitis (NS).
In contrast to MD rats, HD rats did not differ from controls with respect to alveolar bone loss in either males (0.75 ± 0.06 mm) or females (0.75 ± 0.03 mm).. When MD rats and HD rats were compared, the p-value was less than 0.01 in both males and females, with HD rats showing less bone loss than MD rats.
Effect of Maternal Separation on Cytokine Serum Levels to LPS Challenge
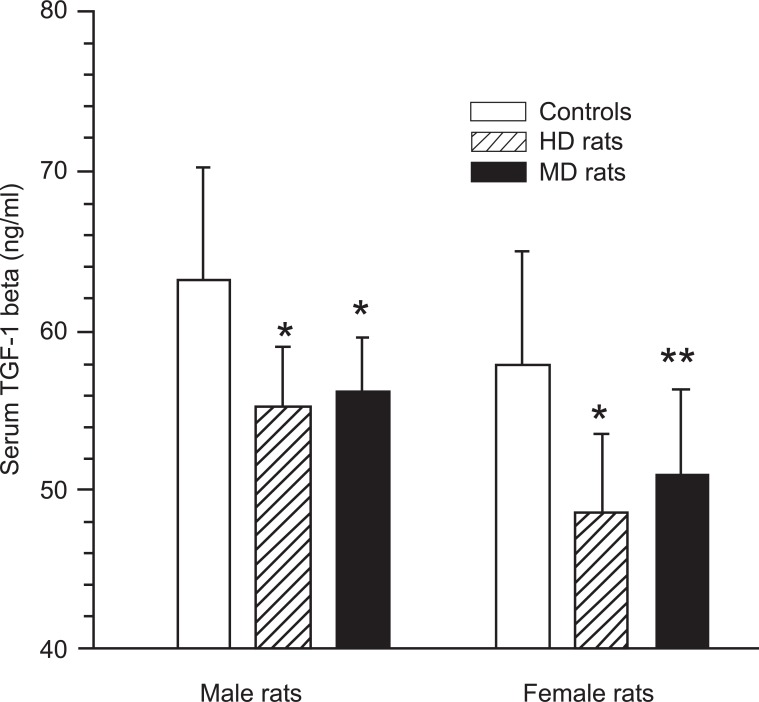
Overall, serum TGF-1β levels were higher in males than in females rats, p < 0.05. The MD rats had significantly lower serum TGF-1β levels 2 h after LPS stimulation compared with controls in both males and females (Fig. 2). In MD male rats the serum TGF-1β levels were 56.3 ± 3.3 pg/ml versus 63.2 ± 7.0 pg/ml in male controls (p < 0.05), while the values for female rats were 51.0 ± 5.2 pg/ml versus 57.9 ± 7.1 pg/ml (p < 0.05).
Fig. (2).
Levels of transforming growth factor (TGF)-1beta in serum 2 h after intraperitoneal injection of LPS (150 microg/kg) in male and female control rats, handled (HD) rats, and maternal deprived (MD) rats. p < 0.05 between controls and HD rats as well as MD rats in both males and females.
Also male and female HD rats had lower TGF-1β levels than the undisturbed control animals. In HD male rats the serum TGF-1β levels were 55.2 ± 3.7 pg/ml versus 63.2 ± 7.0 pg/ml in male controls (p < 0.05). The values for female rats were 48.5 ± 4.8 pg/ml versus 57.9 ± 7.1 pg/ml (p < 0.05). The TGF-1β levels in MD and HD rats were not significantly different, either in males or females.
Compared with controls, there was also a tendency to lower IL-10 serum levels in both male and female MD rats, but the difference was not statistically different, neither were the serum levels of TNF-α (data not shown).
Effects of Maternal Separation on Corticosterone Plasma Levels after LPS Challenge
The serum corticosterone levels 2 h after LPS injection did not differ between male MD rats (1234.4 ± 101.1 nmol/l) and male undisturbed controls (1057.2 ± 389.4 nmol/l). Neither in females was there any significant difference between the MD and control groups: 2007.1 ± 147.5 nmol/l in MD rats versus 1999.7 ± 183.0 nmol/l in controls. When females and males were compared, the serum corticosterone levels were significantly higher in females in both MD and control rats (p < 0.001).
Male and female HD rats did not differ from undisturbed controls after LPS stimulation. In male HD rats the serum levels were 1229.0 ± 88.7 nm/litre versus 1057.2 ± 389.4 nm/litre in male controls, while the values for female rats were 1927.9 ± 180 nm/litre versus 1999.7 ± 183.0 nm/litre in female controls. Serum corticosterone levels in HD rats were significantly higher in females than in males (p < 0.001; data not shown).
Effects of Maternal Separation on Open Field Behaviour
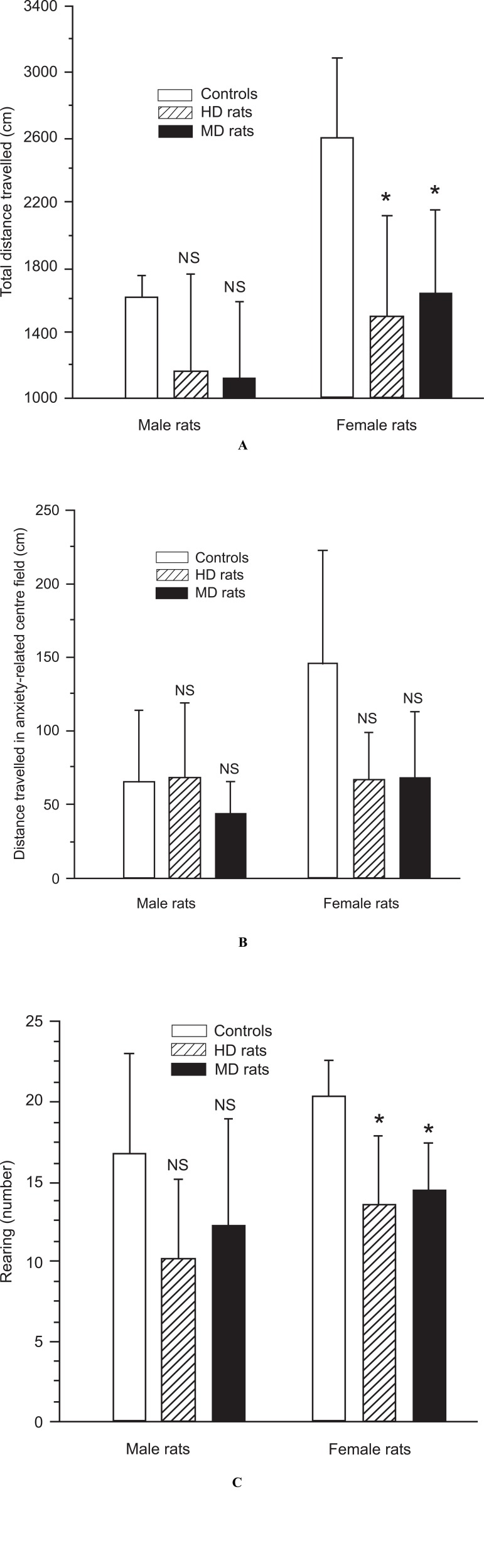
In keeping with the findings from other studies (30, 31), MD rats were less active in a new environment (the open field apparatus) than the controls, although this difference failed to reach statistical significance in males. Mean distance travelled was 1128.6 ± 464.5 cm for male MDs, and 1620.0 ± 130.4 cm for male controls (p = 0.1; Fig. 3A). In females the same distance was 1645.5 ± 508.6 cm, and 2592.9 ± 493.8 cm for controls (p = 0.001; Fig. 3A). In addition, male rats were significantly less active than female rats, both in the MD (p < 0.05), and control group (p < 0.01).
Fig. (3).
A,B,C. Analysis of emotionality- and anxiety-related behaviour in the open field test of male and female control rats, handled (HD) rats, and maternal deprived (MD) rats. (A) Female and male HD and MD rats demonstrated decreased locomotor activity compared to control rats in the novel environment, but the difference was significant in females, only (p = 0.001). (B) Female and male MD rats as well female HD rats tended to spent less time in in the illuminated anxiety-related centre field of the open field apparatus (NS). (C) HD and MD rats reared less than controls, but this behavioural effects was significant in females, only (p < 0.001).
Also HD rats moved less than controls in the open field apparatus, but again this difference was significant in females only. Mean distance travelled was 1166.7 ± 592.2 cm for male HD rats, and 1620.0 ± 130.4 cm for male controls. In females the same distance was 1500.0 ± 624.5 cm, and 2592.9 ± 493.8 cm for controls (p = 0.001; Fig. 3A).
As demonstrated in other studies (40, 42), MD rats moved less than controls in the illuminated anxiety-related centre field of the open field apparatus, but in the present experiment the difference was significant in females only. Mean distance travelled in the centre field was 44.3 ± 21.5 cm for male MD rats, and 66.0 ± 48.3 cm for male controls. In females the same distance was 68.2 ± 45.6 cm for MD rats, and 145.7 ± 77.2 cm for female controls (p < 0.01;
Male HD rats showed no difference in distance moved in the illuminated anxiety-related centre field of the open field apparatus compared to male controls. The distance was 68.3 ± 50.4 cm for male HD rats, and 66.0 ± 48.3 cm for male controls. In contrast, female HD rats moved less than female control rats. The distance was 67.1 ± 32.0 cm for female HD rats, and 145.7 ± 77.2 cm for female controls (p < 0.01). There was no significant difference between male and female HD rats.
Also the reported general decrease of explorative behaviour in MD rats, as measured by reduced rearing (39), was supported in this experiment, but the difference was significant in females only. In male MD rats the rearing activity was 12.3 ± 6.7 versus 16.8 ± 6.2 in male controls (NS), and in females MD rats 14.5 ± 2.9 versus 20.3 ± 2.3 in female controls (p < 0.05).
Male and female HD rats showed less explorative behaviour as demonstrated with their rearing activity in this experiment. The rearing activity in male HD rats was 10.2 ± 5.0 versus 16.8 ± 6.2 in male controls (p < 0.05), and in female HD rats 13.6 ± 4.3 versus 20.3 ± 2.3 in female controls (p < 0.01). There were no differences between male HD and female HD rats.
Effects of Maternal Separation on Hippocampal DNA Methylation of Exon 17 Gr Promotor Region and Gr Expression
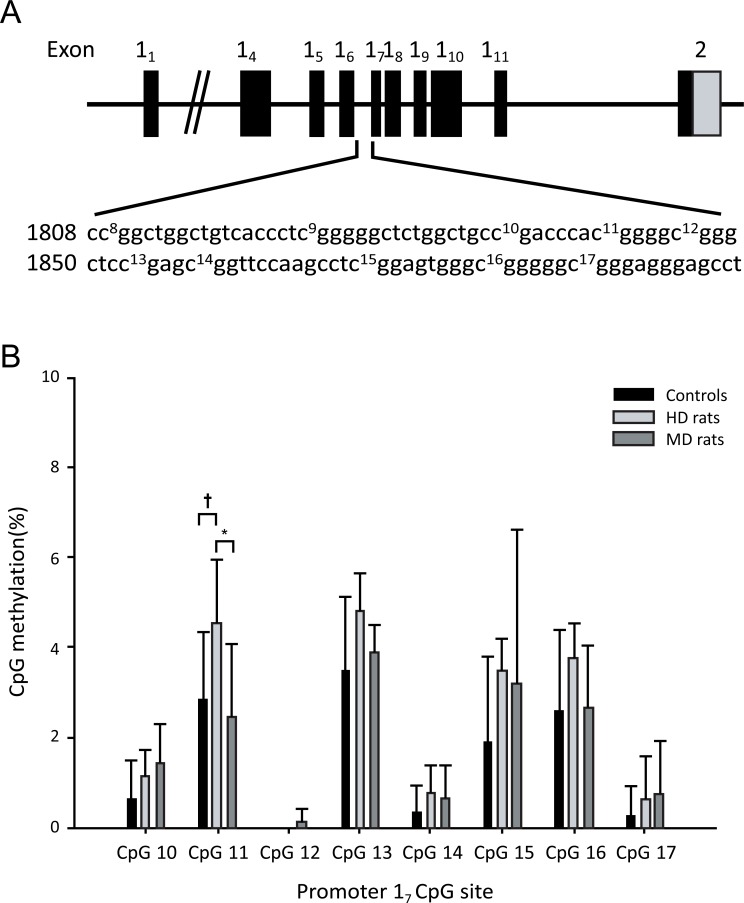
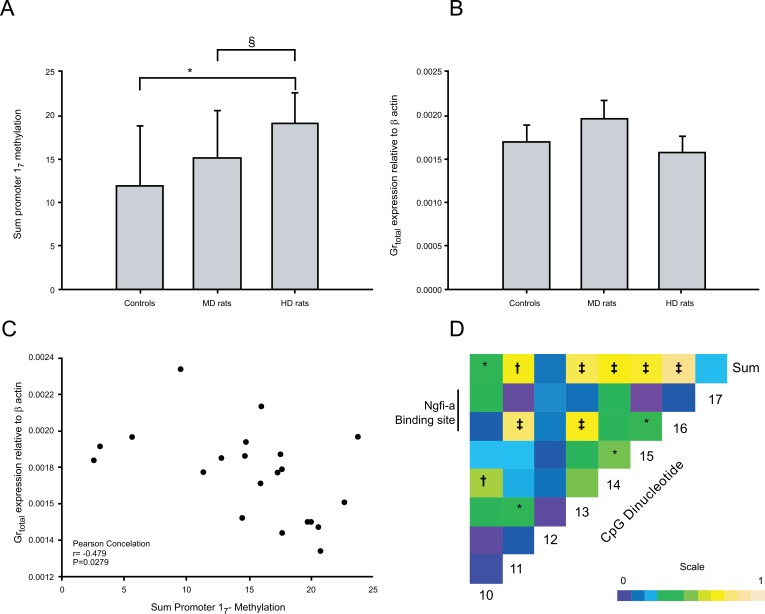
Methylation data were obtained from female rats; 6 HD rats, 8 MD rats and 7 Controls (Fig 4). Pyrosequencing was performed on the hippocampal Gr 17 promoter. Analysis of the individual CpGs showed that HD rats showed significantly higher levels of methylation than MD rats (p < 0.05) only for CpG11, and a trend for higher levels of methylation than Controls (p = 0.06). Analysis of variance of total methylation over all CpGs (Fig. 5A) showed that HD rats had higher total methylation than Controls (p = 0.05). MD animals did not differ significantly from either HD rats or Controls. Gr expression data were obtained from female rats (7 HD rats, 8 MD rats and 7 Controls rats; Fig. 5B). MD animals had higher Gr expression than both Control rats (p < 0.05) and HD rats (p = 0.001).
Fig. (4).
(A) Schematic representation of the Gr gene structure covering exons 11 to 111 and the sequence of the 17 promoter including the CpGs 10-17 analysed. (B) Pyrosequencing analysis of the promoter 17 CpG dinucleotides from control, handled (HD), and maternally deprived (MD) rats. * p < 0.05, † p<0.1. Data are mean +/-SD.
Fig. (5).
Sum methylation levels throughout the Gr 17 promoter (A), the relative expression of total Gr levels (B) and the correlation of promoter 17 sum methylation and total Gr transcript levels (C) in the hippocampus after exposure to control, handling (HD) or maternal deprivation (MD) conditions. (D) Pearson correlation heatmap for individual CpGs in the hippocampus and the sum methylation level throughout the Gr 17 promoter. Pearson correlation coefficients are expressed by colour (0 (blue) to 1 (yellow)) as per scale. § p=0.06, * p < 0.05, † p<0.01, ‡ p<0.001. Panels A and B are mean +/-SD.
After examining the group differences in individual and sum methylation levels we aggregated the data from the three groups and investigated the link between CpG proximity and methylation levels. Pairwise Pearson correlations were calculated between the modification levels of the 8 promoter 17 CpG sites (Fig. 5D). The individual CpG dinucleotide showed very strong correlations in their CpG methylation levels, particularly between all pairwise combinations of CpGs 11, 13, 15 and 16, and individually with the sum methylation levels, suggesting that these CpG dinucleotides are co-regulated. Across all three groups, Gr expression correlated negatively with total methylation, r = - 0.48, p = 0.028 (Fig. 5C), with methylation at CpG11 (r = - 0.58, p = 0.006), and with methylation at CpG16 (r = - .47, p = 0.031).
DISCUSSION
Previously, we have demonstrated that exposure of rat pups to various very early life stressors profoundly alters the susceptibility/resistance to periodontitis in adulthood [21-24]. These experiments included postnatal over-stimulation of the glutaminergic system [21], postnatal exposure to gram-negative bacterial LPS [22], cross-fostering (where rat pups from mothers that were selected as genetically stress high responders were raised by genetically stress low responding mothers [23]), and postnatal administration of the synthetic stress hormone and Gr agonist dexamathasone [24].
In the present study, we have extended our findings by using a maternal separation model that is known to permanently change an individual’s reactivity to stressors and various diseases later in life [26]. First, we compared the severity of ligature-induced periodontitis in adult periodontitis resistant Lewis rats that as new-born were daily long term (3 h) separated from their mothers from life day 2 to 14 (maternal deprivation; MD) with rats that were separated for 15 min daily over the same time period (handling; HD), or left undisturbed by their mothers (Controls). We also compared behaviour during a stressful situation (the open field test), as well as selected immune and stress responses to systemic gram-negative bacterial LPS stimulation. Immune responses were compared by measuring the serum levels of the pro-inflammatory cytokines TNF-α and the anti-inflammatory cytokines IL-10 and TGF-1β. The stress responses were compared by measuring the serum levels of corticosterone, which is an end-effector hormone, and an index of the HPA axis response. In addition, we compared the expression of hippocampal Gr expression, and methylation of genes involved in hippocampal Gr expression, which is known to be an integral part of the regulatory mechanism controlling stress and HPA axis reactivity [47].
A key finding in the present study is that adult MD rats developed significantly more severe experimental periodontitis than HD and undisturbed Control rats in both males and females. Male and female MD rats also gained less weight and female MD rats displayed increased emotionality and anxiety-related behaviour to novelty. Short-term (15 min) daily postnatal separation (HD) had more limited effects. In HD rats, the severity of periodontitis was unaffected, as was weight gain, and only emotionality and anxiety-related behaviour to novelty was significantly altered. In male and female MD rats, as well as male and female HD rats,
LPS provoked significantly lower increase in circulating levels of the T-regulatory cytokine TGF-1β, but LPS did not induce significant differences in other systemic cytokines measured (TNF-α and IL-10), or the HPA axis derived stress hormone corticosterone. In addition, our data showed that MD rats had higher Gr expression in the hippocampus compared to both HD and Control rats, while HD rats had a higher degree of methylation at CpG11.
The results are in line with reports from other recent investigations. For example, Milde et al. showed that MD enhanced the susceptibility to adult experimentally-induced ulcerative colitis in male Wistar rats when exposed to uncontrollable foot shock stress [49], while Vig et. al. found altered numbers of inflammatory cells and cytokine levels in an experimental model of airway inflammation in mice [50]. In a female rat model of nematode Nippostrongylus brasilensis infection, MD increased the number of jejunal adult worms and faecal eggs and larvae output [51]. In a murine model of influenza A/PR8 virus infection, MD altered several cytokine responses and host resistance throughout life [52].
The altered behavioural responses to stress are likewise consistent with studies from other investigators. For example, MD rats exhibit more anxiety-like behaviour and less exploratory responses in adulthood, while HD causes increased anxiolytic-like behaviour and increased exploratory responses in both Lewis and Fischer 344 rats [53]. Moreover, when exposed to maternal deprivation, adult Lewis rats have decreased anxiety-like behaviour compared to F344 rats. In addition, females of both strains exhibit more anxiety-like behaviour than males (53). It is well documented that maternal deprivation and handling differently alter behavioural and neuroendocrine responses to various challenges in adulthood, and that these effects are highly determined by genetic background as well as gender [24, 35, 53]. As demonstrated in this experiment, MD rats also often exhibit reduced weight gain in both males and females [54]. Interestingly, people born with a low birth weight are at increased risk of chronic adult diseases, including coronary heart disease, type 2 diabetes, cognitive decline and depression, and prenatal stressors have significant impact on birth weight, anxiety, and depression of the offspring [55-57]. Furthermore, there is evidence suggesting that early life programming of stress reactivity, including HPA axis and autonomic nervous system reactivity, are among the likely mechanism that link low birth weight and late onset diseases [56].
Immune function parameters are modified in depressed humans [58], including altered cytokine levels in gingival cervicular fluid [59]. In the present study, we found that “depressed” MD rats released significantly lower amounts of the T-regulatory cytokine TGF-1β in response to LPS stimulation. Interestingly, a recent study has shown a significant negative correlation between systemic TFG-1β and depressive mood in humans [58], and reduced T-regulatory reactivity may play an important role in depression [60]. Moreover, we have found that vaccination with heat-killed Mycobacterim vaccae, which is a potent T-regulatory cytokine inducer, was protective in experimental periodontitis [8, 9]. Thus, the present data support the suggestion that adaptive immunity plays a protective role in periodontitis, and that decreased T-regulatory activity and weaker TFG-1β response may play a role in depression and periodontitis.
The relationship between depressive mood and periodontitis has been confirmed by earlier animal experiments [18], and humans with severe depression have been found to show significantly more severe periodontitis [61]. In addition, we have found that manipulation of the neurotransmitter systems involved in anxiety, depression, and immune system regulation in rats, significantly influenced the development and severity of periodontitis [13, 15, 16-19, 21, 24]. Moreover, enhanced susceptibility to experimental periodontitis in a rat model of depression could be abolished with pre-treatment with the antidepressant drug tianeptine [18]. The antidepressant treatment also normalised behavioural responses, as well as the reduction of TNF-α cytokine response to LPS stimulation [18].
Maternal separation also has differential effects on adult stress coping behaviours, which in turn are dependent on the genetic background [62]. Interestingly, we and others have shown that humans with a poorly developed coping style, which usually results in increased vulnerability to stress-related diseases, are significantly more susceptible to periodontitis than those with a an active coping style in response to stress [63, 64]. These findings support recent studies demonstrating relationships between different coping strategies for social stress, neuroendocrine and immune activity and susceptibility to infectious diseases [65].
The MD model in rats, as used in this experiment, is a well-established model for postnatal stress-induced development of adult anxiety and depression-related behaviour [66]. It has been found to produce anxiety and depression-like behaviour in rat mothers, and the depression-induced reduction in maternal behaviour may account for depression-like behaviour found in the offspring [67, 68]. In humans, early life adverse events are known to be major risk factors for developing severe anxiety and major depressive disorders in adult life [69]. Moreover, chronic HPA axis hyper-activity is considered to be a consistent biological finding in major depression, and it is looked upon as one of the major pathophysiological mechanism leading to this disorder, and not a consequence of depression [70].
Earlier experiments have revealed that chronic HPA axis hyper-reactivity, whether genetically determined or environmentally induced, including experimental depression and diabetes, is associated with increased susceptibility to periodontitis [8-14, 18, 21]. Hyper-reactivity of the HPA axis is a typical feature of MD in rodents [37, 43]. HPA axis hyper-reactivity is also a typical feature in humans with major depression of the melancholic type [66, 70], and is also associated with increased susceptibility to periodontitis in rats [12-14, 18]. The HPA axis is a major brain-controlled immunoregulatory system, which regulates immune system responses mainly by the release of endogenous glucocorticoid hormones [71]. These hormones act via binding to two types of intracellular receptors, i.e., the mineralocorticoid receptor (Mr), and the Gr. It has been shown that during low basal glucocorticoid levels Mr are occupied, while Grs are activated additionally when glucocorticoid hormone levels are high, such as during emotional stressful conditions and infections [72]. Upon binding of the glucocorticoid hormones to these receptors, the hormone-receptor complex translocates to the nucleus where it acts as a transcription factor regulating the activity of genes, including those involved in the production and release of immune mediators, including cytokines [59]. Therefore, changes in the regulation of the HPA axis response to a bacterial challenge are likely to result in alterations in immune system responses and subsequent host susceptibility to infectious and inflammatory diseases, including periodontitis. However, in the present experiments with Lewis rats, there was no increase in HPA axis reactivity in the MD rats to an in vivo challenge with LPS, as measured by circulating levels of corticosterone, although we found a clear effect of MD on anxiety and depression-like behaviour as well as ligature-induced periodontitis. This may suggest that the alterations in immune responses and disease susceptibility may not be mediated by higher circulating glucocorticoid hormone levels per se.
Immune system responses are, however, not only controlled by the concentrations of glucocorticoids hormones, but also the sensitivity of glucocorticoid receptors is a major factor in determining the final immune response [73]. For example, in traumatised veterans with post-traumatic stress disorder, a state associated with HPA axis hypo-responsive-ness, which is also a typical feature in Lewis rats, no significant differences in plasma cortisol levels were found, but these people showed lower leukocyte glucocorticosteroid receptor density and alteration in immune system responses compared to healthy controls [74]. It is also possible that other overarching immunoregulatory mechanisms than the HPA axis are involved. Thus, although HPA axis activation regulates immune and inflammatory responses by releasing glucocorticoid hormones, and maternal deprivation is associated with increased HPA activity axis and altered immune responses, HPA axis hyper-reactivity is not necessarily the major determinant factor inducing altered immunity and susceptibility to periodontitis in this experiment with stress and HPA axis low responding Lewis rats. Other tentative explanations include MD-induced alterations of the reactivity of the sympathetic, parasympathetic and sensory peptidergic nervous systems, which are all found to be altered by maternal deprivation [55, 75-77], and which reactivity we have found to play a significant role in the susceptibility/resistance to periodontitis [15, 17, 19].
The reactivity of the stress response system, which includes the HPA axis and the sympathetic nervous systems, is highly determined by the Gr level within the brain, including the hippocampus [72]. When increased amounts of glucocorticoids are released from the cortex of the adrenal glands during a stressful situation, some of these hormones rapidly enter the brain via the general blood stream where they bind to Mr and Gr, and by so doing, the further release of glucocortiocoids is down-regulated via negative feedback regulation [72]. It has been found that high expression or levels of hippocampal Gr are associated with strong negative feedback regulation and subsequent weak HPA axis reactivity to various stressors, whereas low Gr levels are associated with the opposite effect, i.e., weak negative feedback regulation and subsequent HPA axis hyper-reactivity [72]. Thus, the amount of Gr within the brain, and particularly the hippocampus, seems to be one of the main determinants of HPA axis reactivity, and thus disease susceptibility [72]. Such a relationship has also been found in our earlier periodontitis experiments in rats [18].
One of the ways by which environmental stress like maternal separation may change the Gr expression and thus the HPA axis reactivity and susceptibility to diseases is by DNA methylation, which inhibits transcriptional regulation of genes [47]. Actually, DNA methylation may represent an important link between very early stress, brain Gr expression, stress reactivity, immune system regulation, and susceptibility/resistance to diseases [47]. The present study confirmed that there is a direct link between the methylation of the Gr promoter 17 and total Gr transcript levels. Gr transcripts have previously been correlated with the sum of methylation throughout the 17 promoter, but not with single CpG dinucleotides [78, 79]. Here, both CpG 11 and 16 as well as the total methylation level correlated negatively with Gr transcript levels. However, the strong proximity based link in methylation levels, particularly between CpGs 11, 13, 15, 16 suggests that expression of the Gr is controlled by the overall methylation level, rather than any one particular CpG dinucleotide, as we have previously suggested [78].
Maternal deprivation did not, however, have the anticipated effect on DNA methylation levels at the group level. Elevated Gr transcript levels in the MD group were not accompanied by lower methylation levels, but methylation levels remained comparable to control animals. However, the limited statistical power of the study may have masked the link between methylation and expression at the group level that was seen on the aggregated data. Interestingly, we are not the only ones to fail to find effects of 3 hours maternal separation on methylation of 17 CpG sites [80].
Based on this and previous findings, we suggest that the augmented colonisation of periodontopathogens, which are regarded as initiating agents of periodontitis [1], may be a result of a dysregulated or misguided brain–neuroendocrine-immune balance. The reduced ability of adaptive immunity to respond optimally thus impedes clearance of pathogens. Chronic over-activity of the innate immune system, which may be responsible for tissue destruction [2], we have suggested to be a compensatory response that protects the gingival connective tissues from being infected by these pathogens. Accordingly, we have hypothesized that periodontitis is the result of a hidden benefit that protects against infection in individuals with reduced ability to respond with an optimal adaptive immune response to pathogens [2, 19].
CONCLUSION
The present study shows that a very early maternal separation procedure had a significant impact on the Gr expression in the hippocampus, behavioural responses to novelty, systemic immune system responses to an immunological challenge, and the severity of experimental periodontitis in rats, although it did not produce the anticipated changes in Gr methylation. The study expands previous findings by investigating maternal separation as a stressor in stress low-responding and periodontitis resistant Lewis rats. As discussed above, there are several biological mechanisms that can explain our findings. The findings may also help to understand how psychological factors may contribute to individual differences in the susceptibility/resistance to periodontitis. With the reservation that results from animal studies never can be directly transferred to humans, the results from this study may be considered to have clinical relevance in humans. First, they prompt close consideration of the relationship between postnatal adversity, depression, and disease severity in patients with periodontitis. Second, apart from the present restricted procedures for treating periodontitis,, patients may in the future be offered treatments capable of normalising misguided brain-neuroendocrine-immune regulatory pathways, including those induced by very early adverse life stress and predisposing for major depression in later life.
ACKNOWLEDGEMENTS
We are grateful to the Norwegian Defence Research Establishment, Division of Protection, Kjeller, Norway for supporting this work. The authors thank Laila Drogseth and Ingjerd Thrane at the same institute for their skilful technical assistance and help with cytokine and corticosterone analysis. We would also like to thank Sophie Mériaux and Carlos Leija from the CRP-Sante, Luxembourg, for their technical assistance.
CONFLICT OF INTEREST
The authors report no financial relationship related to any products involved in this study and declare that no funding has been available other than from the Norwegian Defence Research Establishment, Division of Protection, Kjeller, Norway.
REFERENCES
- 1.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol. 2000;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 2.Breivik T, Gundersen Y, Gjermo P, Taylor SM, Woodruff TM, Opstad PK. Oral treatment with complement factor C5a receptor (CD88):antagonists inhibits experimental periodontitis in rats. J Periodontal Res. 2011;46:643–7. doi: 10.1111/j.1600-0765.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 3.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–9. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 4.Preshaw PM, Seymour RA, Heasman PA. Current concepts in periodontal pathogenesis. Dent Update. 2004;31:570–4. doi: 10.12968/denu.2004.31.10.570. [DOI] [PubMed] [Google Scholar]
- 5.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Kornman KS. Mapping the pathogenesis of periodontitis a new look. J Periodontol. 2008;79:1560–8. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 7.Peruzzo DC, Benatti BB, Ambrosano GM, Nogueira-Filho GR, Sallum EA, Casati MZ, Nociti FH ., Jr A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78:1491–504. doi: 10.1902/jop.2007.060371. [DOI] [PubMed] [Google Scholar]
- 8.Breivik T, Rook GA. Prevaccination with SRL172 (heat-killed Mycobacterium vaccae) inhibits experimental periodontal disease in Wistar rats. Clin Exp Immunol. 2000;120:463–7. doi: 10.1046/j.1365-2249.2000.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breivik T, Rook GA. Oral treatment with SR299 (killed Mycobacterium vaccae) inhibits experimental periodontal disease in Wistar rats. J Clin Periodontol. 2003;30:931–6. doi: 10.1034/j.1600-051x.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 10.Breivik T, Opstad PK, Engstad R, Gundersen G, Gjermo P, Preus H. Soluble b-1,3/1,6-glucan from yeast inhibits experimental periodontal disease in Wistar rats. J Clin Periodontol. 2005;32:347–52. doi: 10.1111/j.1600-051X.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Hashizume T, Kurita-Ochiai T, Fujihashi K, Yamamoto M. Oral immunization with porphyromonas gingivalis outer membrane protein and CpGoligodeoxynucleotides elicits T helper 1 and 2 cytokines for enhanced protective immunity. Mol Oral Microbiol. 2010;25:178–89. doi: 10.1111/j.2041-1014.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breivik T, Opstad PK, Gjermo P, Thrane PS. Effects of hypothalamic-pituitary-adrenal axis reactivity on periodontal tissue destruction in rats. Eur J Oral Sci. 2000;108:115–22. doi: 10.1034/j.1600-0722.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- 13.Breivik T, Thrane PS, Gjermo P, Opstad PK. Glucocorticoid receptor antagonist RU-486 treatment reduces periodontitis in Fischer 344 rats. J Periodont Res. 2000;35:285–90. doi: 10.1034/j.1600-0765.2000.035005285.x. [DOI] [PubMed] [Google Scholar]
- 14.Breivik T, Thrane PS, Gjermo P, Opstad PK, Pabst R, von Horsten S. Hypothalamic-pituitary-adrenal axis activation by experimental periodontal disease in rats. J Periodont Res. 2001;36:295–300. doi: 10.1034/j.1600-0765.2001.360504.x. [DOI] [PubMed] [Google Scholar]
- 15.Breivik T, Gundersen Y, Opstad PK, Fonnum F. Chemical sympathectomy inhibits periodontal disease in Fischer 344 rats. J Periodont Res. 2005;40:325–30. doi: 10.1111/j.1600-0765.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 16.Breivik T, Gundersen Y, Osmundsen H, Opstad PK, Fonnum F. Chronic treatment with the glutamate receptor antagonist MK-801 alters periodontal disease susceptibility. J Periodont Res. 2005;40:28–35. doi: 10.1111/j.1600-0765.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- 17.Breivik T, Gundersen Y, Gjermo P, von Hörsten S Opstad PK. Nicotinic acetylcholine receptor activation mediates nicotine-induced enhancement of experimental periodontitis. J Periodont Res. 2009;44:297–303. doi: 10.1111/j.1600-0765.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- 18.Breivik T, Gundersen Y, Myhrer T , et al. Enhanced susceptibility to periodontitis in an animal model of depression reversed by chronic treatment with the antidepressant tianeptine. J Clin Periodontol. 2006;33:469–77. doi: 10.1111/j.1600-051X.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- 19.Breivik T, Gundersen Y, Gjermo P, von Hörsten S, Fristad I, Opstad PK. Systemic chemical desensitization of peptidergic sensory neurons with resiniferatoxin inhibits experimental periodontitis. Open Dent J. 2011;5:1–6. doi: 10.2174/1874210601105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breivik T, Sluyter F, Hof M, Cools A. Differential susceptibility to periodontitis in genetically selected Wistar rat lines that differ in their behavioral and endocrinological response to stressors. Behav Genet. 2000;30:123–30. doi: 10.1023/a:1001903221046. [DOI] [PubMed] [Google Scholar]
- 21.Breivik T, Gundersen Y, Gjermo P, Opstad PK. Chronic treatment with the glucocorticoid receptor antagonist RU486 inhibits diabetes-induced enhancement of experimental periodontitis. J Periodontol Res. 2013;[Epub ahead of print] doi: 10.1111/jre.12076. [DOI] [PubMed] [Google Scholar]
- 22.Breivik T, Thrane PS, Gjermo P, Fonnum F. Postnatal glutamate-induced central nervous system lesions alter periodontal disease susceptibility in adult Wistar rats. J Clin Periodontol. 2001;28:904–9. doi: 10.1034/j.1600-051x.2001.028010904.x. [DOI] [PubMed] [Google Scholar]
- 23.Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Hörsten S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;16:421–38. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- 24.Sluyter F, Breivik T, Cools A. Manipulations in maternal environment reverse periodontitis in genetically predisposed rats. Clin Diagn Lab Immunol. 2002;9:931–2. doi: 10.1128/CDLI.9.4.931-932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breivik T, Gundersen Y, Osmundsen H, Fonnum F, Opstad PK. Neonatal dexamethasone and chronic tianeptine treatment inhibit ligature-induced periodontitis in adult rats. J Periodontol Res. 2006;41:23–32. doi: 10.1111/j.1600-0765.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murgatroyd C, Spengler D. Epigenetic programming of the HPA axis early life decides. Stress. 2011;14:581–9. doi: 10.3109/10253890.2011.602146. [DOI] [PubMed] [Google Scholar]
- 28.Weaver IC. Shaping adult phenotypes through early life environments. Birth Defects Res C Embryo Today. 2009;87:314–26. doi: 10.1002/bdrc.20164. [DOI] [PubMed] [Google Scholar]
- 29.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer SJ, Galic MA, Pittman QJ. Neonatal programming of innate immune function. Am J Physiol Endocrinol Metab. 2011;300:11–8. doi: 10.1152/ajpendo.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newport DJ, Stowe ZN, Nemeroff CB. Parental depression animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–83. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- 34.Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function relevance to immunotoxicology. J Immunotoxicol. 2008;5:419–44. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- 35.Ellenbroek BA, Cools AR. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology. 2000;23:99–106. doi: 10.1016/S0893-133X(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Lee JH, Choi SH, Lee YS, Jahng JW. Fasting-induced increases of arcuate NPY mRNA and plasma corticosterone are blunted in the rat experienced neonatal maternal separation. Neuropeptides. 2005;39:587–94. doi: 10.1016/j.npep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Hennessy MB, Deak T, Schiml-Webb PA. Early attachment-figure separation and increased risk for later depression potential mediation by proinflammatory processes. Neurosci Biobehav Rev. 2010;34:782–90. doi: 10.1016/j.neubiorev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lariviere WR, Sattar MA, Melzack R. Inflammation-susceptible Lewis rats show less sensitivity than resistant Fischer rats in the formalin inflammatory pain test and with repeated thermal testing. J Neurophysiol. 2006;95:2889–97. doi: 10.1152/jn.00608.2005. [DOI] [PubMed] [Google Scholar]
- 39.Calogero AE, Sternberg EM, Bagdy G , et al. Neurotransmitter-induced hypothalamic-pituitary-adrenal axis responsiveness is defective in inflammatory disease-susceptible Lewis rats in vivo and in vitro studies suggesting globally defective hypothalamic secretion of corticotropin-releasing hormone. Neuroendocrinology. 1992;55:600–8. doi: 10.1159/000126173. [DOI] [PubMed] [Google Scholar]
- 40.Cizza G, Sternberg EM. The role of the hypothalamic-pituitary-adrenal axis in susceptibility to autoimmune/inflammatory disease. Immunomethods. 1994;5:73–8. doi: 10.1006/immu.1994.1039. [DOI] [PubMed] [Google Scholar]
- 41.Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav Processes. 2006;71:51–8. doi: 10.1016/j.beproc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–8. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 43.Lucas M, Mekary R, Pan A , et al. Relation between clinical depression risk and physical activity and time spent watching television in older women a 10-year prospective follow-up study. Am J Epidemiol. 2011;174:1017–27. doi: 10.1093/aje/kwr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephan M, Straub RH, Breivik T, Pabst R, von Hörsten S. Postnatal maternal deprivation aggravates experimental autoimmune encephalomyelitis in adult Lewis rats reversal by chronic imipramine treatment. Int J Dev Neurosci. 2002;20:125–32. doi: 10.1016/s0736-5748(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Alt SR, Turner JD, Klok MD , et al. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–56. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Turner JD, Pelascini LP, Macedo JA, Muller CP. Highly individual methylation patterns of alternative glucocorticoid receptor promoters suggest individualized epigenetic regulatory mechanisms. Nucleic Acids Res. 2008;36:7207–18. doi: 10.1093/nar/gkn897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witzmann SR, Turner JD, Meriaux SB, Meijer OC, Muller CP. Epigenetic regulation of the glucocorticoid receptor promoter 1(7):in adult rats. Epigenetics. 2012;7:1290–301. doi: 10.4161/epi.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milde AM, Enger Ø , Murison R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiol Behav. 2004;81:71–84. doi: 10.1016/j.physbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Vig R, Gordon Jr, Thébaud B, Befus AD, Vliagoftis H. The effect of early-life stress on airway inflammation in adult mice. Neuroimmunomodulation. 2010;17:229–39. doi: 10.1159/000290039. [DOI] [PubMed] [Google Scholar]
- 51.Barreau F, de Lahitte JD, Ferrier L, Frexinos J, Bueno L, Fio-ramonti J. Neonatal maternal deprivation promotes nippostrongylus brasiliensis infection in adult rats. Brain Behav Immun. 2006;20:254–60. doi: 10.1016/j.bbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–48. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A. Early social isolation alters behavioral and physiological responses to an endotoxin challenge in piglets. Horm Behav. 2006;50:753–61. doi: 10.1016/j.yhbeh.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 54.Teunis MA, Heijnen CJ, Sluyter F , et al. Maternal deprivation of rat pups increases clinical symptoms of experimental autoimmune encephalomyelitis at adult age. J Neuroimmunol. 2002;133:30–8. doi: 10.1016/s0165-5728(02)00351-x. [DOI] [PubMed] [Google Scholar]
- 55.Xiong F, Zhang L. Role of the hypothalamus-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2010;34:27–46. doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kajantie E, Räikkönen K. Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25:141–8. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. 2005;88:167–73. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Johannsen A, Rylander G, Söder B, Asberg M. Dental plaque, gingival inflammation, and elevated levels of interleukin-6 and cortisol in gingival crevicular fluid from women with stress-related depression and exhaustion. J Periodontol. 2006;77:1403–9. doi: 10.1902/jop.2006.050411. [DOI] [PubMed] [Google Scholar]
- 60.Miller AH. Depression and immunity a role for T cellsκ. Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johannsen A, Rydmark I, Söder B, Asberg M. Gingival inflammation, increased periodontal pocket depth and elevated interleukin-6 in gingival crevicular fluid of depressed women on long-term sick leave. J Periodontal Res. 2007;42:546–52. doi: 10.1111/j.1600-0765.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 62.Vegas O, Fano E, Brain PF, Alonso A, Azpiroz A. Social stress, coping strategies and tumor development in male mice behavioral, neuroendocrine and immunological implications. Psychoneuroendocrinology. 2006;31:69–79. doi: 10.1016/j.psyneuen.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 63.Hugoson A, Ljungquist Breivik T. The relationship of some negative events and psychological factors to periodontal disease in an adult Swedish population 50 to 80 years of age. J Clinical Periodontol. 2002;29:247–53. doi: 10.1034/j.1600-051x.2002.290311.x. [DOI] [PubMed] [Google Scholar]
- 64.Wimmer G, Janda M, Wieselmann-Penkner K, Jakse N, Polansky R, Pertl C. Coping with stress its influence on periodontal disease. J Periodontol. 2002;73:1343–51. doi: 10.1902/jop.2002.73.11.1343. [DOI] [PubMed] [Google Scholar]
- 65.Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav. 2002;77:711–6. doi: 10.1016/s0031-9384(02)00923-x. [DOI] [PubMed] [Google Scholar]
- 66.Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB. Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology (Berl). 2004;176:248–55. doi: 10.1007/s00213-004-1883-x. [DOI] [PubMed] [Google Scholar]
- 67.Boccia ML, Razzoli M, Vadlamudi SP, Trumbull W, Caleffie C, Pedersen CA. Repeated long separations from pups produce depression-like behavior in rat mothers. Psychoneuroendocrinology. 2007;32:65–71. doi: 10.1016/j.psyneuen.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldman R, Eidelman AI. Maternal postpartum behavior and the emergence of infant-mother and infant-father synchrony in preterm and full-term infants the role of neonatal vagal tone. Dev Psychobiol. 2007;49:290–302. doi: 10.1002/dev.20220. [DOI] [PubMed] [Google Scholar]
- 69.Widom CS, White HR, Czaja SJ, Marmorstein NR. Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. J Stud Alcohol Drugs. 2007;68:317–26. doi: 10.15288/jsad.2007.68.317. [DOI] [PubMed] [Google Scholar]
- 70.Pariante CM, Lightman SL. The HPA axis in major depression classical theories and new developments. Trends Neurosci. 2008;31:464–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Liberman AC, Druker J, Perone MJ, Arzt E. Glucocorticoids in the regulation of transcription factors that control cytokine synthesis. Cytokine Growth Factor Rev. 2007;18:45–56. doi: 10.1016/j.cytogfr.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 72.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocrine Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 73.Heijnen CJ. Receptor regulation in neuroendocrine-immune communication current knowledge and future perspectives. Brain Behav Immun. 2007;21:1–8. doi: 10.1016/j.bbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 74.de Kloet CS, Vermetten E, Bikker A , et al. Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Mol Psychiatry. 2007;12:443–53. doi: 10.1038/sj.mp.4001934. [DOI] [PubMed] [Google Scholar]
- 75.Esquivel BB, Levin G, Rivarola MA, Suárez MM. Maternal separation and lesion of pups alters anxiety and adrenal activity in male rats. Int J Neurosci. 2009;119:9–36. doi: 10.1080/00207450902931847. [DOI] [PubMed] [Google Scholar]
- 76.Stephan M, Helfritz F, Pabst R, von Hörsten S. Postnatally induced differences in adult pain sensitivity depend on genetics, gender and specific experiences reversal of maternal deprivation effects by additional postnatal tactile stimulation or chronic imipramine treatment. Behav Brain Res. 2002;133:149–58. doi: 10.1016/s0166-4328(01)00468-5. [DOI] [PubMed] [Google Scholar]
- 77.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–18. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simone RW, Jonathan DT, Sophie BM, Onno CM, Claude PM. Epigenetic regulation of the glucocorticoid receptor promoter 17 in adult rats. Epigenetics. 2012;7:1290–301. doi: 10.4161/epi.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao-Lei L, Suwansirikul S, Jutavijittum P, Mériaux SB, Turner JD, Muller CP. Glucocorticoid receptor gene expression and promoter CpG modifications throughout the human brain. J Psychiatr Res. 2013;47:1597–60. doi: 10.1016/j.jpsychires.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 80.Daniels WMU, Fairbairn LR, Tilburg G , et al. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 17 glucocorticoid receptor promoter region. Metabolic Brain Disease. 2009;24:615–27. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]