Abstract
Background:
Oral health is an integral and important component of general health. Infectious diseases such as caries, periodontal, and gingivitis indicate the onset of imbalance in homeostasis between oral micro biota and host. The present day medicaments used in oral health care have numerous side effects. The uses of herbal plants as an alternative have gained popularity due to side effects of antibiotics and emergence of multidrug resistant strains. Anacardium occidentale (cashew) and Mangifera indica (mango) have been used as traditional oral health care measures in India since time immemorial.
Materials and Methods:
The ethanol extracts of cashew and mango leaves were obtained by maceration method. The antimicrobial activity was evaluated by clear zone produced by these plant extracts against Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, Escherichia coli, and Candida albicans in agar plate method, determination of minimum inhibitory concentration (MIC), minimum bactericidal/fungicidal concentration (MBC/MFC), and suppression of biofilm. The cytotoxic effects of plants extract was determined by microculture tetrazolium assay on human gingival fibroblast and Chinese hamster lung fibroblast (V79) cell lines.
Results:
Cashew and mango leaf extract significantly (P < 0.05) produced larger zone of inhibition against test pathogens when compared to povidone-iodine-based mouth rinses. Although the MIC and MBC/MFC values of mouth rinses were effective in lower concentrations; plant extracts significantly (P < 0.001) suppressed the biofilms of oral pathogens. The leaf extracts were less cytotoxic (P < 0.001) compared to mouth rinses.
Conclusions:
Plant extracts are superior to the mouth rinses and have a promising role in future oral health care.
KEY WORDS: Anacardium occidentale, antimicrobial, cytotoxicity, Mangifera indica, oral care
Although modern medicine may exist side-by-side with traditional practice, herbal medicines have often maintained their popularity for historical and cultural reasons. In many developing countries, a large proportion of the population relies on traditional practitioners and their armamentarium of medicinal plants in order to meet healthcare needs.[1] Medicinal plants represent a rich source of antimicrobial agents. Plants and herbs have attained a significant role not only as therapeutic agent, but also in maintaining oral health care.[2]
Oral diseases such as dental caries, periodontal disease, tooth loss, and oral mucosal lesions play a major role in oral health problems. Poor oral health has profound effect on general health of a person.[3] Antimicrobial agents such as chlorhexidine, povidone-iodine (PI) have been employed extensively as an adjunct to mechanical cleaning. They have unpleasant side effects and alter the oral micro flora.[4]
The normal commensals in oral cavity such as Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, Escherichia coli, and Candida albicans become opportunistic pathogens and are considered to be the most resistant species in the oral cavity. The increasing prevalence of multidrug resistant strains raises the search for new infection fighting strategies. Microbial reduction is one of the primary objectives which consecutively promote normal healing process of the periodontal tissues.[5]
Development in alternative medicine research has led to many mouth rinses and tooth pastes based on plant extracts. Anacardium occidentale and Magnifera indica belonging to Anacardiaceae family have great economic and medicinal value.[6] An infusion of the leaves of the cashew plant is used as a remedy for tooth ache and sore gums.[7] In vitro tests done with tannin-containing extract have demonstrated that such compounds have diverse antibacterial and antifungal activities. The pharmacological activities of tannins are likely to be the result of three factors: Complexation with metal ions, antioxidant activity, and free-radical scavenger. Tannins are also capable of combining with other types of molecules including macromolecules such as proteins and polysaccharides. The anticarcinogenic and antimutagenic potentials of tannins may be related to their antioxidative property, which is important in protecting the cellular oxidative damage including lipid peroxidaton.[8]
In this study, ethanol extracts of mango and cashew leaves were being screened for their antimicrobial activities against selected bacterial and fungal strains and cytotoxicity on human gingival fibroblast (HGF) and V79 Chinese hamster lung fibroblast cell lines.
Materials and Methods
Collection of plant leaves and processing
The fresh leaves of cashew and mango, free from insect infestation and infection were collected from Dakshina Kannada, Karnataka, India. The specimen leaf samples were examined and identified in the Department of Pharmacognosy, Sri Dharmasthala Manjunatheshwara Center for Research in Ayurveda and Allied Sciences, Udupi, India. The collected leaves were washed in tap water followed by distilled water and shade-dried. The dried samples were powdered using a blender followed by ethanol extraction of leaves by maceration method.[9] A known weight of the powder was kept immersed in ethanol for 24 h with occasional shaking. The supernatant is filtered through the muslin cloth and whatman filter paper. The collected filtrate is kept in water bath for evaporation of solvent. Then extract is dried at 105°C for 6 h. Thus, obtained extract was stored in air tight screw cap tubes at −4°C till use.
Control groups and chemicals
Commercially available mouth rinses rexidin (0.2% chlorhexidine gluconate [CHX]) and betadine (0.2% Povidone Iodine, PI) were used as positive control groups (standards) in the study. The microbial culture media Mueller Hinton agar (MHA), Sabouraud Dextrose agar (SDA), Mueller Hinton Broth (MHB), Tryptone Soya Broth (TSB), Sabouraud Dextrose Broth (SDB) chemicals like resazurin indicator, crystal violet, and 96_well microtiter plates were purchased from Himedia, Mumbai. The cell culture media and supplements, culture flasks, 96_well microtiter plates were also purchased from Himedia, Mumbai. Acetic acid and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich and ethanol from Hayman.
Microorganisms and growth conditions
The microorganisms employed in the current study were E. faecalis (ATCC 29212), S. aureus (ATCC 25923), S. mutans (MTCC 890), E. coli (ATCC 25922), and C. albicans (ATCC 90028). The E. faecalis, E. coli and S. aureus were subcultured in Mueller Hinton Broth (MHB). S. mutans was subcultured in Tryptone Soya Broth (TSB). C. albicans was subcultured in Sabouraud Dextrose Broth (SDB) and incubated aerobically at 37°C for 18 to 24 h.
Cell lines and culture conditions
Human gingival fibroblast [Figure 1] and V79 Chinese hamster lung fibroblast [Figure 2] were obtained from Nitte University Center for Science Education and Research. The cell lines were maintained in Dulbeccos modified Eagle medium (DMEM), supplemented with 15% fetal bovine serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 0.25 mg/ml of amphotericin B at 37°C with 5% CO2 and relative humidity in an incubator.
Figure 1.
Confluent monolayer of human gingival fibroblast cells
Figure 2.
Confluent monolayer of V79 cells
Antimicrobial assays
Determination of zone of inhibition
The susceptibility of oral pathogens to plant extracts and to control mouth rinses were determined by well diffusion method.[10] The planktonic cultures of bacteria and fungus were adjusted to 0.5 McFarland constant. The aliquots of the microbial cultures uniformly spread on the surface of solidified MHA and SDA using a sterile swab. With the help of a sterile cork borer, wells of 6 mm were made in the agar plates. The cashew and mango extracts were reconstituted in 99.9% ethanol, and wells were filled with 80 μl (20 mg/ml) of the extract. Subsequently, the plates were placed in an incubator at 37°C. After overnight incubation, the diameter of clear zone produced was measured in mm. The control mouth rinses were also evaluated for antimicrobial activity. Along with the extracts and mouth rinses, ethanol (negative control) also inoculated into the wells to see the possible antimicrobial effects of ethanol.
Determination of minimum inhibitory concentration and minimum bactericidal/fungicidal concentration
The minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) of extracts, mouth rinses, and ethanol were evaluated by microdilution method with slight modifications.[11] Hundred and sixty microliters of the extracts, standards, and ethanol were pipetted into the first row of the plate. Rest of the wells was added with 80 μl of sterile normal saline. The serial dilution of the extracts, positive controls, and ethanol were done in normal saline. 10 μl of resazurin indicator (prepared by dissolving 270 mg in 40 ml of sterile distilled water) was added to all wells. A volume of 30 μl of MHB, TSB, or SDB was pipetted into the wells with respect to the organisms to be inoculated. Then 10 μl of bacterial or fungal suspensions were added to each well. The plates were placed in an incubator at 37°C for 24 h. The highest concentration of extracts taken was 10,000 μg/ml and positive control was 160 μg/ml. The color change from purple to pink or colorless was recorded as the MIC value. In order to find the MBC/MFC value subculturing till MIC value was done on MHA and SDA plates, respectively. After 24 h of incubation, MBC/MFC values were recorded.
Biofilm suppression
The bacterial and fungal biofilm were grown in 96-well microtiter plate following O’Toole method.[12] The overnight culture of the microorganisms was diluted to 1:100 in the respective broth and known volume of 100 μl was seeded into each well followed by 24 h of incubation at 37°C in an incubator. The unattached or floating cells were removed by giving two gentle wash in sterile double distilled water. The reconstituted extracts were diluted in respective broth. A volume of 100 μl of extract at concentration of 200 μg/ml was added into the wells and incubated for 4 h at 37°C. Simultaneously, 100 μl of positive standards was also evaluated for the efficacy of biofilm suppression. The control wells were treated with 100 μl of broth. After 4 h of incubation, contents of the wells were discarded. Subsequently, staining of biofilm was done by incubating the biofilm with 100 μl of 0.1% crystal violet stain at room temperature for 15 min. The excess stain was removed by gentle washing of the wells with distilled water for three times. The plates were air-dried. The destaining of the microbial cells was done using 125 μl of 30% acetic acid for 15 min. The contents were transferred to another microtiter plate and quantification of biofilm was done by taking the optical density readings at 600 nm using Lisa Chem spectrophotometer.
Cytotoxicity assay
The cytotoxicity of plant extracts and mouth rinses were determined using microculture tetrazolium (MTT) assay[13,14] with minor modifications. The confluent monolayer of cells was detached with 0.05% trypsin and 0.001% ethylenediaminetetraacetic acid solution to make single cell suspension of HGF and V79 cells. The viable cells were counted by Tryphan blue (0.4%) assay using a hemocytometer. The cells were diluted in DMEM media to a final density of 1 × 104 cells for HGF and 2 × 104 for V79 cells in 100 μl/well. After seeding with cells, the 96-well plate was incubated to allow the cells attachment at 37°C, 5% CO2, and with relative humidity.
The extract initially reconstituted in ethanol was diluted in serum free media. In diluted extracts, the concentration of ethanol was not more than 10%. The 100 μl of the extracts (200 μg/ml) was added to the wells and incubated for 4 h. The same was followed for the positive control groups. A 10% of ethanol in serum free media was also evaluated for the possible cytotoxicity of ethanol. On completion of the incubation, the contents of the plates were gently removed and 50 μl of 0.05% MTT was added. Followed by 2 h of incubation, MTT solution was removed and 100 μl of DMSO was added to each well and incubated for 15 min. The viability of cells was quantified by taking the optical density at 545 nm using Lisa Chem spectrophotometer.
Statistical analysis
Each parameter was repeated twice in triplicates. The statistical analysis of the data was done using GraphPad Prism (version 3.02, San Diego, California). The level of significance was determined by one-way analysis (ANOVA), Bonferroni multiple comparison test. P <0.05 was taken as level of significance.
Percentage inhibition of biofilm was determined using the formula,
Percentage inhibition = 100−(abs sample)/(abs control) ×100
Percentage viability of cells was determined using the formula,
Percentage viability = (abs test − abs blank)/(abs control–abs blank) ×100
Results
The antimicrobial activity (mean ± standard deviation [SD]) of cashew and mango leaf extracts is shown in Table 1. S. mutans and E. coli showed the highest susceptibility to cashew and mango leaf extracts, respectively [Figure 3]. The zone of inhibition of cashew and mango against E. faecalis was highly significant (P < 0.001) compared to PI-based mouth rinse [Figure 4a and b]. Although, the extracts produced a greater zone of inhibition against E. faecalis; compared to CHX mouth rinse, was found to be not significant (P > 0.05). There was no difference in the antimicrobial action of cashew leaf extract against S. aureus, S. mutans, E. coli, and C. albicans compared to CHX-based mouth rinse (P > 0.05). But compared to PI mouth rinse, the cashew leaf extract showed significantly (P < 0.001) greater zone of inhibition against S. aureus, S. mutans, and E. coli. The antimicrobial activity of mango extract against S. aureus [Figure 4c and d] and S. mutans was significant (P < 0.05) as compared to PI mouth rinse. Against E. coli, mango leaf extract was highly significant (P < 0.001) compared to both the mouth rinses. The antifungal activity by the cashew leaf extract [Figure 5] was comparatively more than the CHX based mouth rinse although no significant difference was noted (P > 0.05). No zone of inhibition was observed around the ethanol against the tested pathogens.
Table 1.
Zone of inhibition by extracts and mouth rinses against oral pathogens in millimeter
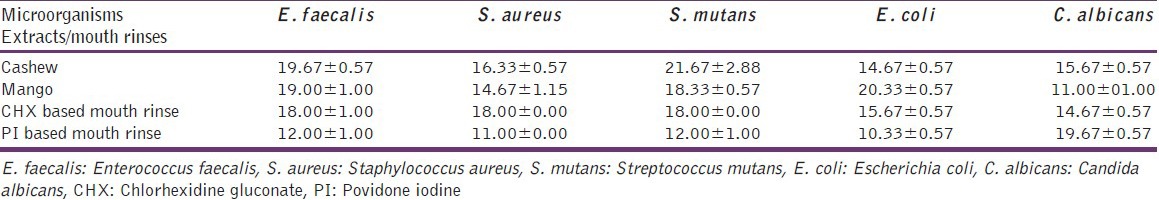
Figure 3.
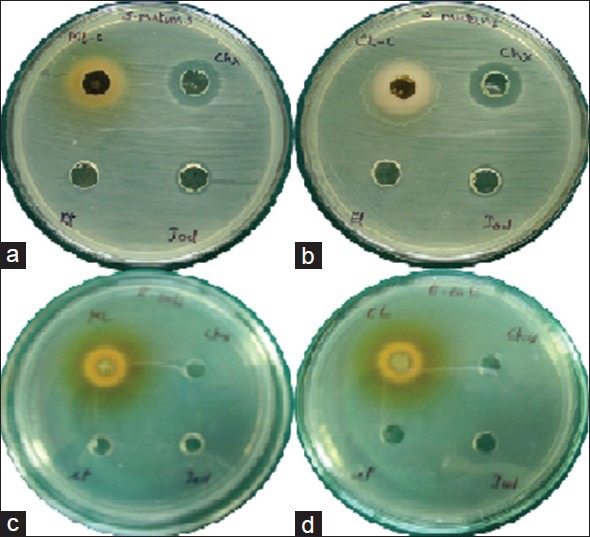
(a and b) Zone of inhibition by mango and cashew extract against Streptococcus mutans respectively. (c and d) Zone of inhibition by mango and cashew extract against Escherichia coli, respectively. In each plate from upper left extract, chlorhexidine gluconate based mouth rinse, povidone-iodine-based mouth rinse and ethanol are placed in clockwise direction
Figure 4.
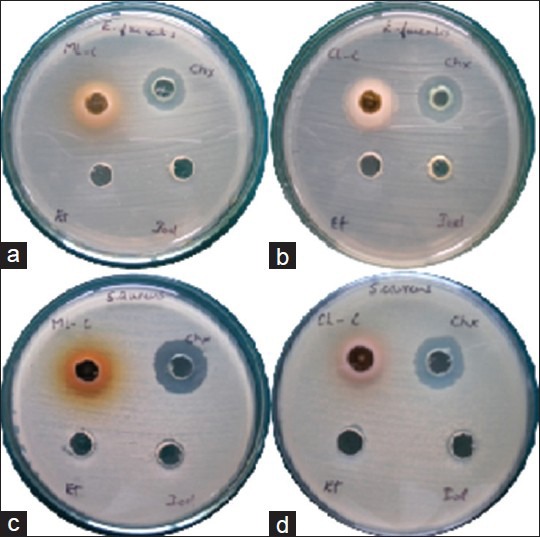
(a and b) Zone of inhibition by mango and cashew extract against Enterococcus faecalis respectively. (c and d) Zone of inhibition by mango and cashew extract against Staphylococcus aureus, respectively. In each plate from upper left extract, chlorhexidine gluconate-based mouth rinse, povidone-iodine-based mouth rinse and ethanol are placed in clockwise direction
Figure 5.
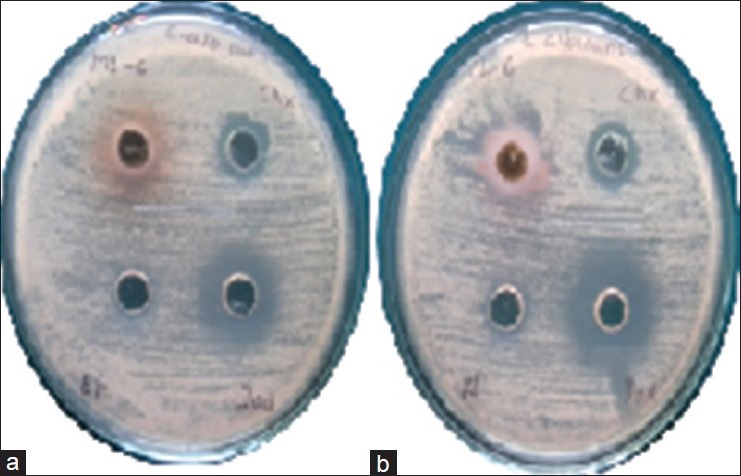
(a and b) Zone of inhibition by mango and cashew extract against Candida albicans, respectively. In each plate from upper left extract, chlorhexidine gluconate-based mouth rinse, povidone-iodine-based mouth rinse and ethanol are placed in clockwise direction
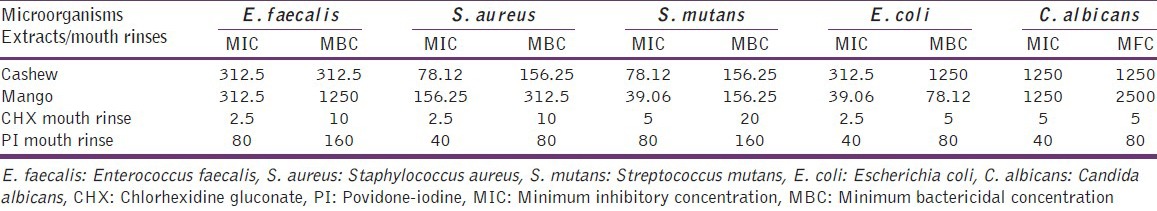
The MIC and MBC/MFC of extracts and mouth rinses are presented in Table 2. The MIC of cashew leaf found in the range of 78.12-1250 μg/ml and MBC/MFC concentration was varied from 156.25 μg/ml to 1250 μg/ml. The MIC of mango leaf varied from 39.06 μg/ml to 1250 μg/ml and MBC/MFC was seen between 78.12 μg/ml and 2500 μg/ml. The CHX mouth rinses MIC value varied from 2.5 to 5 μg/ml, whereas MBC/MFC value varied from 5 to 10 μg/ml. The range of MIC values of PI was found to be between 40 and 80 μg/ml and MBC/MFC 80 to 160 μg/ml. The possible antimicrobial effect of ethanol was ruled out as growth was observed in microtiter plate for all the microbes.
Table 2.
MIC and MBC/MFC of extracts and mouth rinses on oral pathogens in microgram per milliliter
The optical density (mean ± SD) of biofilm study is presented in Table 3. CHX mouth rinse was significant (P < 0.001) over PI in suppression of biofilm. Both cashew and mango leaf extracts were highly significant (P < 0.001) and more effective in suppressing the oral pathogens compared to PI mouth rinse. There was no significant difference (P > 0.05) in the antibiofilm efficacy of both plant extracts with CHX-based mouth rinse.
Table 3.
Biofilm suppression of oral pathogens by plant extracts and mouth rinses
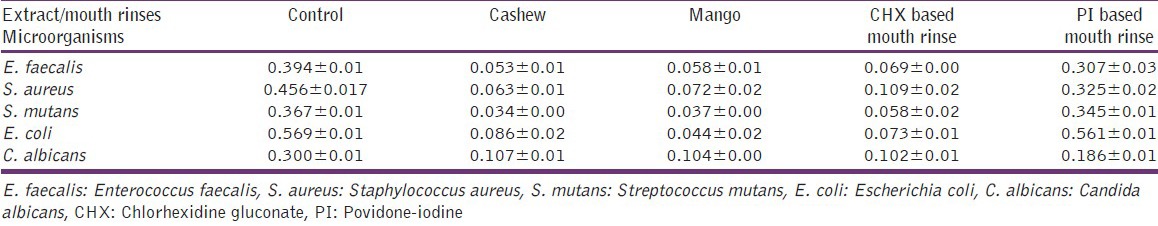
The percentage viability of HGF cells and V79 Chinese hamster lung fibroblast cells is presented in Figure 6. Cashew leaf extract significantly (P < 0.001) reduced the viability of HGF and V79 cell lines. Mango leaf reduced the cell viability of HGF and V79 with a significance level of P < 0.05 and P < 0.001, respectively. The lowest survival rate was observed for PI-based mouth rinses in both the cell lines. The mouth rinses found to be more toxic to the cells compared to the plant extracts with lower survival rates. There was significantly (P < 0.01) higher viability of HGF cells and V79 cells treated with plant extracts, as compared to CHX and PI-based mouth rinses. 10% ethanol in serum free media was not cytotoxic to both the cell lines.
Figure 6.
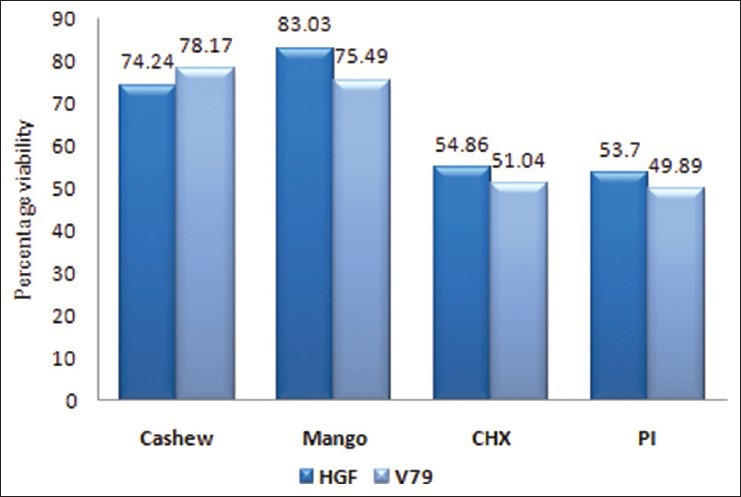
Percentage viability of human gingival fibroblast and V79 cells
Discussion
Dental plaque in the oral cavity is recognized as the most complex oral biofilm.[15] Microorganisms such as E. faecalis, S aureus, S. mutans, E. coli, and C. albicans are known to cause wide range of oral infections such as dental caries, periodontal diseases, and peri-implant diseases due to the formation of biofilm. The structural organization of cells and thick exopolysaccharide matrix reduces the efficacy of topically applied agents and results in emergence of multidrug resistant microbes.[16]
Chlorhexidine gluconate is proven to be the gold standard in oral care; certain undesirable effects such as taste disturbance, tooth discoloration, and mucosal erosions do exist.[17] PI used in mouth rinses also stains teeth, burning sensation of mouth, drying of oral mucosa, and has possible potential for carcinogenic effects.[18] The emergence of multidrug resistant strains and potential side effects of conventional mouth rinse have shifted the focus of oral health care to plant-based products.
Mango and Cashew leaves were used in indigenous system of oral care in India. Ethanol extract of A. occidentale Linn. Leaves exhibited significant antimicrobial and antifungal activity.[19] The strong in vitro antibacterial activity of the separated compound against methicillin resistant S. aureus suggests the wide pharmaceutical applications.[2] According to ayurveda, varied medicinal properties are attributed to different parts of mango tree. Pharmacologically and medicinally, important chemical like mangiferin, being a polyphenolic antioxidant and a glucosyl xanthone, it has strong antioxidant, antilipid peroxidation, immunomodulation, cardiotonic, hypotensive, wound healing, antidegenerative, and antidiabetic activities. Various effects such as antibacterial, antifungal, anthelmintic, and antiparasitic have been studied.[20]
In vitro agar diffusion technique was done to evaluate the antimicrobial properties of mango and cashew plant extracts. The results of this study confirmed the antimicrobial efficacy of these plant extracts in par with the CHX and superior to PI-based mouth rinses on all test microbes. The results of our study was in accordance with Dahake et al., Parasa et al.,[19,2] and Stoilova et al.,[21] whose reports confirmed the antibacterial and antifungal efficacy of plant extracts.
Periodontal disease is a common, slow, progressive inflammatory disease resulting from a complex biofilm of resident commensal and pathogenic bacteria and their products. Along with the environment and host-related factors, the microbes also form an integral part of the disease. Cashew contains high concentration of tannins (poly phenols) which bind and precipitate proteins. Mango contains Mangiferin (polyphenol); with potent antioxidant, antilipid peroxidation activities. Thus, the presence of polyphenolic compound of plant extracts may have caused disruption of microbial biofilm. The Study results were in accordance with Varghese et al.,[7] proving that these plant extracts be utilized for chemical plaque control formulations.
Minimum inhibitory concentration is a sensitive and quantitative approach to distinguish between bacteriostatic and bactericidal effects. Test results of our study showed that CHX- and PI-based mouth rinses were better than the plant extracts. The logical reasoning for this may be that mouth rinses are miscible in saline and exerted their antimicrobial activity effectively. The ethanol extracts of the plants was sparingly soluble in saline to exert their antimicrobial spectrum. The study results were not in accordance with Dahake et al.,[19] who reported ethanolic extracts and petroleum ether extracts performed well on test microorganisms at a concentration of 15 μg/ml. Parasa et al., stated that pure and 70% acetone extracts of Cashew nut shell exerted lower MIC than methanol extract.[2]
Microculture tetrazolium assay is a sensitive, quantitative, and reliable method to assess the cellular metabolic activity, where methyl trizolyl tetazolium is converted into a dark purple colored formazan by cellular mitochondrial dehydrogenase enzyme.[22] In our study, the percentage survival rate of HGF cells and V79 cells treated with cashew and mango was found to be significantly more than CHX-based and PI-based mouth rinses. This indicates less toxicity and long time usage of active components of these plants as an alternative to commercial mouth rinses.
Conclusion
The ethanol extracts of cashew and mango displayed antimicrobial activity on all test microorganisms. The inhibitory effect could be attributed to the presence of bioactive components present in extracts. Plant extracts or phytochemicals inhibit the growth of oral pathogens, reduce the development of biofilms. In addition, they found to be biocompatible. Further bioactive component isolated from these plant extracts should be evaluated for their antimicrobial and cytotoxicity with the goal of developing effective adjuvant for treatment of gingival and periodontal diseases with lesser side effects.
Footnotes
Source of Support: Board of Research in Nuclear Sciences, Department of Atomic Energy, Mumbai
Conflict of Interest: None declared.
References
- 1.Vol. 82. France: IARC Press; 2002. WHO. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthaline and Styrene. [PMC free article] [PubMed] [Google Scholar]
- 2.Parasa LS, Tumati SR, Kumar LC, Chigurupati SP, Rao GS. In vitro – Antimicrobial activity of cashew (Annacardium occidentale, L.) nut shell liquid against methicillin resistant Staphylococcus aureus (MRSA) clinical isolates. Int J Pharm Pharm Sci. 2011;3:436–40. [Google Scholar]
- 3.Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/nep067. 680354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solmaz G, Ozen F, Ekinci Y, Bird PS, Korachi M. Inhibitory and disruptive effects of shiitake mushroom (Lentinula edodes) essential oil extract on oral biofilms. Jundishapur J Microbiol. 2013;6:E9058. [Google Scholar]
- 5.Aspalli S, Shetty VS, Devarathnamma MV, Nagappa G, Archana D, Parab P. Evaluation of antiplaque and antigingivitis effect of herbal mouthwash in treatment of plaque induced gingivitis: A randomized, clinical trial. J Indian Soc Periodontol. 2014;18:48–52. doi: 10.4103/0972-124X.128208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande RR, Dolas A, Jadhav M, Desphande NR, Devas S. Screening of antimicrobial activity of herbal extract of "Morinda pubescence", chlorhexidine and amoxicillin against salivary microflora of mixed dentition age group. Asian J Pharm Clin. 2013;6:125–7. [Google Scholar]
- 7.Varghese J, Tumkar VJ, Ballal V, Bhat GS. Antimicrobial effect of Anacardium occidentale leaf extract against pathogens causing periodontal disease. Adv Biosci Biotechnol. 2013;4:15–8. [Google Scholar]
- 8.Ayepola OO, Ishola RO. Evaluation of antimicrobial activity of Anacardium occidentale (Linn. ) Adv Med Dent Sci. 2009;3:1–3. [Google Scholar]
- 9.Geneva: World Health Organisation; 1998. WHO. Quality Control Methods for Medicinal Plant Materials. [Google Scholar]
- 10.Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–23. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 11.Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–4. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargent JM, Taylor CG. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer. 1989;60:206–10. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar DJ, Santhi RJ. Antioxidant and cytotoxic effects of hexane extract of Morinda pubescens leaves in human liver cancer cell line. Asian Pac J Trop Med. 2012;5:362–6. doi: 10.1016/S1995-7645(12)60060-1. [DOI] [PubMed] [Google Scholar]
- 15.Marsh PD. Dental plaque as a biofilm and a microbial community-implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filoche S, Wong L, Sissons CH. Oral biofilms: Emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 17.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 18.Elmore JG, Horwitz RI. Oral cancer and mouthwash use: Evaluation of the epidemiologic evidence. Otolaryngol Head Neck Surg. 1995;113:253–61. doi: 10.1016/S0194-5998(95)70114-1. [DOI] [PubMed] [Google Scholar]
- 19.Dahake AP, Joshi VD, Joshi AB. Antimicrobial screening of different extract of Anacardium occidentale Linn. Leaves. Int J Chem Tech Res. 2009;1:856–8. [Google Scholar]
- 20.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (mango) Pharmacogn Rev. 2010;4:42–8. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoilova I, Gargova S, Stoyanova A, Ho L. Antimicrobial and antioxidant activity of the polyphenol mangiferin. Herb Pol. 2005;51:37–44. [Google Scholar]
- 22.Avila EV, Pugsley MK. An overview of calorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–4. [PubMed] [Google Scholar]


