Abstract
Stem cell-derived erythroid cells hold great potential for the treatment of blood-loss anemia and for erythropoiesis research; however, cultures using conventional flat plates or bioreactors have failed to show promising results. By mimicking the in vivo bone marrow (BM) environment in which most erythroid cells are physically aggregated, we show that a three-dimensional (3D) aggregate culture system facilitates erythroid cell maturation and red blood cell (RBC) production more effectively than two-dimensional high-density cell cultivation. Late erythroblasts (polychromatic or orthochromatic erythroblasts) were differentiated from cord blood CD34+ cells over 15 days and then allowed to form tight aggregates at a minimum density of 1×107 cells/mL for 2–3 days. To scale up the cell culture and to make the media supply efficient throughout the cell aggregates, several macroporous microcarriers and porous scaffolds were applied to the 3D culture system. In comparison to control culture conditions, erythroid cells in 3D aggregates were significantly more differentiated toward RBCs with significantly reduced nuclear dysplasia. When 3D culture was performed inside macroporous microcarriers, the cell culture scale was increased and cells exhibited enhanced differentiation and enucleation. Microcarriers with a pore diameter of approximately 400 μm produced more mature cells than those with a smaller pore diameter. In addition, this aggregate culture method minimized the culture space and media volume required. In conclusion, a 3D aggregate culture system can be used to generate transfusable human erythrocytes at the terminal maturation stage, mimicking the in vivo BM microenvironment. Porous structures can efficiently maximize the culture scale, enabling large-scale production of RBCs. These results enhance our understanding of the importance of physical contact among late erythroblasts for their final maturation into RBCs.
Introduction
Recently, there has been an increasing demand for in vitro red blood cell (RBC) production to generate a safe and consistent blood supply. The possibility of producing RBCs from many stem cell sources has received great research interest; however, the amount of blood required is much larger than the amount of stem cell products available and, thus, revolutionary techniques are necessary for efficient RBC production. There has been much progress with regard to the biology and associated technologies of other cell products, such as cell lines and stem cells.
However, such technologies are not applicable to erythroid cells owing to their distinct characteristics. For example, the characteristics of erythroid cells change every cell cycle as they differentiate toward mature RBCs, and, therefore, the cell culture conditions should be constantly changed. In addition, although erythroid progenitor cells have surface adhesion molecules, these cells progressively lose their adhesiveness. Moreover, the surfaces of mature RBCs are strongly negatively charged, meaning these cells repel each other. More importantly, erythroid cells have mostly been studied in relation to macrophages, which control the maturation, apoptosis, and metabolism of erythroid cells,1 However, stromal cells, such as macrophages, cannot be used for mass production of RBCs. Based on previous models of adhesive cells and established cell lines, it seems difficult to generate RBCs with a high efficiency.
A major obstacle to RBC generation is the limited understanding of the effects of physical parameters on erythroid maturation. Bioreactors provide controlled environments to improve the quality of the generated cells and to study erythropoiesis. Among the few reports on RBC generation, one study using a stirring bioreactor generated RBCs that lacked an intact morphology, probably owing to shear stress induced by media flow, and did not report any potential means to overcome this.2 Another report used a three-dimensional (3D) perfusion bioreactor to increase the cell density and to reduce the media volume3; however, the RBCs generated lacked an intact morphology.
In bone marrow (BM), which is the most efficient blood-producing system, erythroid cells are in direct physical contact with each other. However, most studies of in vitro erythropoiesis have focused on the relationship between macrophages and erythroid cells.4 We recently showed that even in two-dimensional (2D) culture plates, such as Petri dishes and T-flasks, physical contact among erythroid progenitor cells accelerates the maturation of terminally differentiated erythroblasts (poly-/ortho-chromatic erythroblasts; late erythroblasts) and enucleation and increases adhesion-related mRNA signaling.5 Furthermore, these culture conditions significantly reduce the dysplastic features of cells. In a previous report, the effects of 3D culture could not be evaluated, because nonadhered cells to the plates floated in the culture media and did not contact the adhered cells. In addition, the 3D culture of erythroid cells using bony structures has not been reported.
Therefore, in this study, we used 3D culture models, in which cells were tightly packed as aggregates, to evaluate whether 3D physical contact among late erythroblasts facilitates their maturation. As a control, we cultured cells in a 2D system at a high density. The optimal maturation stages of cells before their transfer to 3D culture and the optimal agitation speed of stirring systems were also assessed for future bioreactor applications. Thereafter, to maximize the 3D culture scale and to permit erythroid cell contacts, porous microcarriers and scaffolds were used.
For the first time, we report the effective cultivation of erythroblasts as 3D aggregates. This culture condition enhances the differentiation of late erythroblasts into mature RBCs, mimicking the BM niche. Furthermore, 3D aggregate culture in macroporous microcarriers or porous scaffolds enhances cell–cell contacts and leads to the formation of erythroblastic islands to foster cell maturation. These contacts also markedly promote the expression of adhesion- and maturation-related signals, as is observed in the BM, and could minimize the amount of space and culture media required for mass RBC production.
Materials and Methods
Erythroblast maturation from cord blood CD34+ cells before 2D and 3D culture
Cord blood (CB) was collected from healthy pregnant women after obtaining written consent. After isolating mononuclear cells by density gradient centrifugation using Ficoll–Paque (GE Healthcare Bio-Science AB, Pittsburgh, PA), CD34+ cells were isolated from the CB using the EasySep CD34 isolation kit (StemCell Technologies, Vancouver, Canada). Then, CD34+ cells were cultured in 2D culture plates for 15–17 days in serum/plasma-free medium as described in previous reports.5
To generate 3D cell aggregates at days 13–17, cells were seeded at a minimum density of 1×107 cells/mL and left to settle. The culture conditions were sustained for 1–3 days and supplemented with 2 IU/mL Erythropoietin (EPO; Calbiochem, La Jolla, CA) and 5% CB plasma-derived serum6 at 37°C and in a 5% CO2 humidified atmosphere. Half the medium was replaced every 24 h. Cells were cytocentrifuged onto slides and stained with Wright–Giemsa stain to observe their maturation status and integrity.7 Analysis of cell morphology was performed by two hematology experts in a blinded manner by counting more than 200 cells in five different areas per slide or condition.
The effect of 3D culture on the maturation of terminal erythroid cells
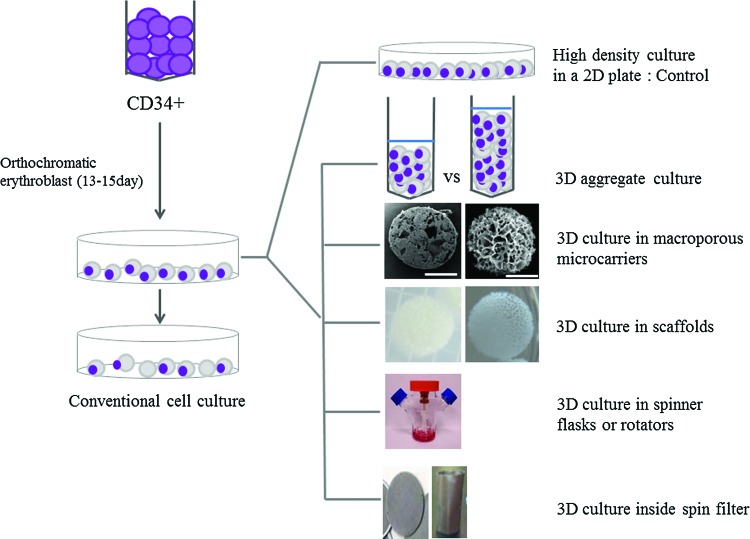
At days 15–17, cells in 2D culture conditions were maintained at a high density (1×106 cells/mL in six-well plates) to encourage the formation of cell–cell contacts. Cells in 3D culture conditions (1×107 cells/mL) were maintained in narrow tubes with V-shaped bottoms, and cells were, therefore, accumulated and aggregated. The culture medium used was the same for the 2D and 3D conditions. In the 3D cultures, either 1×107 cells/1 mL or 2×107 cells/2 mL were placed into narrow V-shaped tubes to evaluate the possible size of cell aggregates. To supply nutrients and oxygen, cells were gently pipetted up and down more than 10 times every 2 h (Fig. 1).
FIG. 1.
Cell culture. Conventional two-dimensional (2D) cell culture in plates, high-density 2D cell culture (used as the control in this study), aggregate erythroid cell culture, and cell culture in porous materials are shown. The effects of sheer stress owing to media flow were also evaluated by culture in spinner flasks and on a rocking rotator, with or without a spin filter. Color images available online at www.liebertpub.com/tea
Three-dimensional culture with macroporous microcarriers in a narrow conical tube
To expand the cell culture volume and to minimize the pressure of the cell aggregates, macroporous microcarriers (Cytopore and Cytoline™ 1) with various pore diameters were applied to the 3D aggregate cultures (Table 1). The structural characteristics of Cytoline 1 were more similar than those of Cytopore to the bony trabeculae structure within human BM. Poly-/ortho-chromatic erythroblasts at day 15–17 were seeded (1×107 cells/mL) into narrow conical tubes containing Cytopore or Cytoline 1, which had been previously hydrated, sterilized by autoclaving, and equilibrated in prewarmed phosphate-buffered saline (PBS). Cells were cultured for 1–2 days and compared with cells cultured at a high density in a monolayer. Half the medium was replaced every 24 h.
Table 1.
Characterization of Biomimetic Materials
| Type | Component | Pore size (μm) | Diameter (mm) | Density (g/mL) | |
|---|---|---|---|---|---|
| Macroporous–microcarrier | Cytopore | 100% Cellulose matrix | 30 | 0.23 | 1.03 |
| Cytoline I | Polyethylene, silica | 10–400 | 2–2.5 | 1.3 | |
| Scaffold | Honeycomb | 100% Bovine collagen | 200–400 | 6×2 mm | |
| Biomerix 3D | Poly-urethane | 250–500 | 5×2 mm |
Three-dimensional culture with microcarriers in a spinner flask or on a gyro-rocker
To study the effect of medium flow-induced shear stress in a bioreactor, erythroid cells were cultivated in 125 mL spinner flasks at an agitation rate of 100, 60, or 30 rpm (Fig. 1). The flasks had a diameter of 65 mm at the base and a working volume of 25–125 mL. The impeller had 90° paddles and a magnetic stir bar, and its widest diameter was 40 mm. Erythroid cells are extremely weakened by shear stress induced by medium flow; therefore, the lowest agitation speed of 30 rpm with cells at a density of 1×107 cells/mL was routinely used. The culture was sustained for 4 days, and half the medium was replaced every day. To maintain the adhesion of cells and to reduce shear stress induced by media flow, 3.8×108 cells in 38 mL media were seeded onto Cytopore and Cytoline 1. Thereafter, the cell-seeded scaffolds were cultured in a 125 mL spinner flask. After 6 h of intermittent stirring (60 min of stirring at 30 rpm, followed by 10 min of no stirring), cells were continuously cultured at 30 rpm.
To prevent cells leaking from pores, microcarriers containing cells were placed inside spin filters, which were fixed in 24 well plates. Thereafter, the plates were rotated on a gyro-rocker at 30 rpm. At each culture time, samples of the supernatant were collected and the pH and glucose and lactate concentrations were determined using a RAPID Systems blood gas analyzer (Siemens, Medfield, MA).
Three-dimensional culture with Cytoline 1, a collagen Honeycomb disc, or a Biomerix 3D scaffold inside a spin filter
BM-mimicking scaffolds with adequate pore sizes were identified and from these, the collagen Honeycomb disc (KOKEN, Tokyo, Japan) and the Biomerix 3D (Biomerix, Fremont, CA) scaffold were evaluated (Table 1). To trap cells within microcarriers and to enable media circulation, a cylindrical spin filter fixed in 24 well plates was used. Cells were seeded at a density of 1×107 cells/mL, and cultures were agitated at 30 rpm or were not agitated. Half the medium was replaced every 24 h, and the number of viable erythroid cells was counted in a blinded manner by Trypan blue staining.
Analysis of cell surface marker expansion by flow cytometry
After 1–2 days of cultivation, 1×105 cells were washed and labeled with anti-human antibodies (Ab) by incubating with Ab at 4°C for 20 min in the dark. The antibodies used in experiments were glycophorin A (GPA)-PE (BD Pharmingen, San Diego, CA); CD71-FITC (BD Pharmingen); CD11B-Alexa 488 (Biolegend, San Diego, CA); and CD13-APC (BD Pharmingen). Suitable IgG isotype controls were used per experiment. Then, cells were washed twice, resuspended in 1% bovine serum albumin and stained cells were analyzed by flow cytometry using Accuri C6 Personal Flow Cytometer (BD Biosciences, Franklin Lakes, NJ).
Quantitative real-time PCR for adhesion- and maturation-related molecules
Total RNA was isolated using TRIzol Reagent (Ambion, Austin, TX) according to the manufacturer's instructions. The concentration of RNA samples was quantified using a Nanodrop (BioSpec Nano Spectrophotometer, Shimadzu, Japan). Total RNA was reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). mRNA levels were measured in duplicate by quantitative real-time polymerase chain reaction (qPCR) using SYBR TOPreal qPCR 2X PreMIX (Enzynomics, Daejeon, South Korea). The sequences of the primer were as follows (forward/reverse), and other primer sequences (DLC-1, GATA-1, ICAM-4, Hemoglobin [Hb]-β, and Hb-γ) were previously described.5,8
DLC-1, 5′-AGTGCGTGCAACAAGCGGGT-3′/5′-TCCGGGTAGCTCTCGCGGTT-3′;
ICAM-4, 5′-CCGGACTAAGCGGGCGCAAA-3′/5′-AGCCACGAACTCCGGGCTCA-3′;
GATA-1, 5′-CCAAGCTTCGTGGAACTCTC-3′/5′-CCTGCCCGTTTACTGACAAT-3′;
Hb-β, 5′-GAAGGCTCACAAGAAAG-3′/5′-CACTGGTGGGGTGAATTCTT-3′;
Hb-γ, 5′-GCTGACTTCCTTGGGAGATG-3′/5′-GAATTCTTTGCCGAAATGGA-3′;
GAPDH, 5′-GAAGGTGAAGGTCGGAGT-3′/5′-GACAAGCTTCCCGTTCTCAG-3′.
Hemoglobin analysis
To evaluate the hemoglobin subtypes, we collected the mature erythroid cells cultured with Honeycomb scaffolds for 24 h. Then, the cells were washed with PBS and stained with CD235a-FITC (BD Bioscience), β-globin-PE (BD Bioscience), and γ-globin-FITC (BD Bioscience). The stained cells were analyzed using Accuri C6 flow cytometer (BD Biosciences).
To evaluate the oxygen-binding capacity, oxygen equilibrium curves were measured by a Hemox analyzer, Model B (TCS Scientific, New Hope, PA) according to the manufacturer's recommendations.9
Statistical analysis
The quantitative data are reported as mean±standard error of the mean. Significant differences in experiment conditions were analyzed using Wilcoxon-signed rank test. Statistical significance was denoted by p<0.05 using GraphPad InStat version 3 (GraphPad, San Diego, CA).
Results
Effect of 3D aggregate culture on erythroid maturation
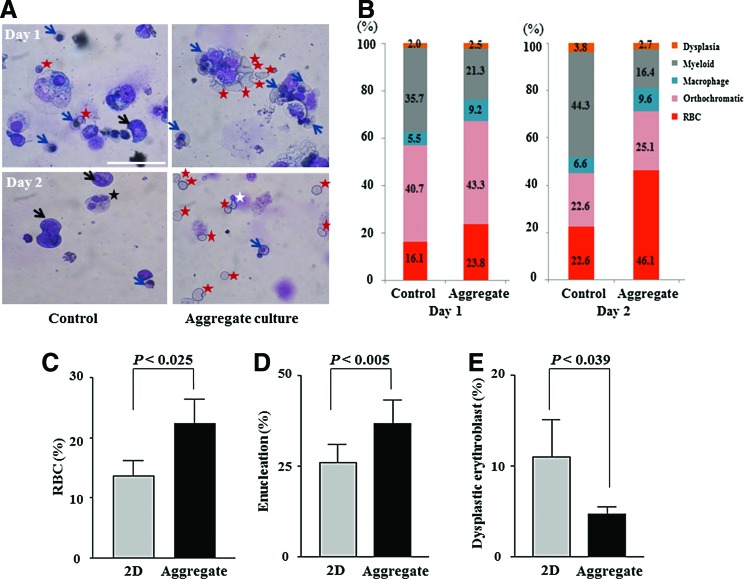
Late erythroblasts were differentiated from CB CD34+ cells in a 2D monolayer for 13–15 days. The purity of the erythroid lineage was 50–100%. At day 13 of culture, the number of cells had increased by about 2200-fold and erythroid cells had mainly matured into polychromatic erythroblasts (>50%), which subsequently matured into poly-/ortho-chromatic erythroblasts until day 17 (>50%). These cells were cultured in 3D conditions to study RBC production. The cells were allowed to form aggregates at a minimum density of 1×107 cells/mL in narrow conical tubes. As a control, erythroid cells were cultured in 2D plates at a high density to encourage the formation of cell–cell contacts (Fig. 1). Cell–cell contacts were well maintained, similar to those in BM erythroblastic islands, in 3D culture conditions (Fig. 2A). Intact erythroid cells were rarely observed in 2D cultures, whereas there were high numbers of orthochromatic erythroblasts and enucleated RBCs in 3D cultures (Fig. 2A). At day 2, the percentages of orthochromatic erythroblasts (25.1%) and RBCs (46.1%) were the highest in 3D cultures (Fig. 2B). The percentage of orthochromatic erythroblasts was lower at day 2 of the 3D culture than at day 1, which seems to be owing to the increased enucleation of orthochromatic erythroblasts to form RBCs. In addition, at day 2, the percentage of cells with nuclear dysplasia, such as bi-/multi-nucleation, was lower in 3D cultures (2.7%) than in 2D cultures (3.8%) (Fig. 2B).
FIG. 2.
Effects of three-dimensional (3D) culture on the maturation of erythroid cells. (A) A representative image of late erythroblasts at day 1 of 3D culture. Blue arrows, mature orthochromatic erythroblasts; white star, cells undergoing enucleation; and red star, red blood cells (RBCs). At day 2 of aggregate 3D culture, the number of RBCs was increased and the numbers of bi- or multi-nucleated dysplastic cells (black star) and myeloid cells (black arrow) were decreased. (B) The percentages of erythroid cells at various maturation stages were calculated by two hematology experts. (C) The percentage of RBCs among total cells (n=8, p< 0.025) (D) and the enucleation rates among erythroid cells (n=8, p<0.005) in 2D and 3D cultures are shown. (E) The percentage of dysplastic erythroblasts is shown (n=8, p<0.039). p-Values were calculated using a two-tailed t-test. Color images available online at www.liebertpub.com/tea
When we evaluated the optimal size of cell aggregates by doubling the number of seeded cells, cells in larger size aggregates showed lower viability probably due to inadequate gas and nutrient supply (data not shown). Therefore, to scale up the culture, the culture space needed to be divided into small compartments. We compared the 2D and 3D culture conditions in terms of the percentages of enucleated and dysplastic cells. In 3D cultures, the percentage of enucleated RBCs was increased to 36.7% and the percentage of dysplastic erythroblasts was significantly decreased to 4.7% compared with 11.0% in 2D cultures (n=8) (Fig. 2C–E). In summary, the 3D culture conditions facilitated erythroid cell maturation and enucleation and reduced dysplastic cytokinesis.
Cell culture with macroporous microcarriers
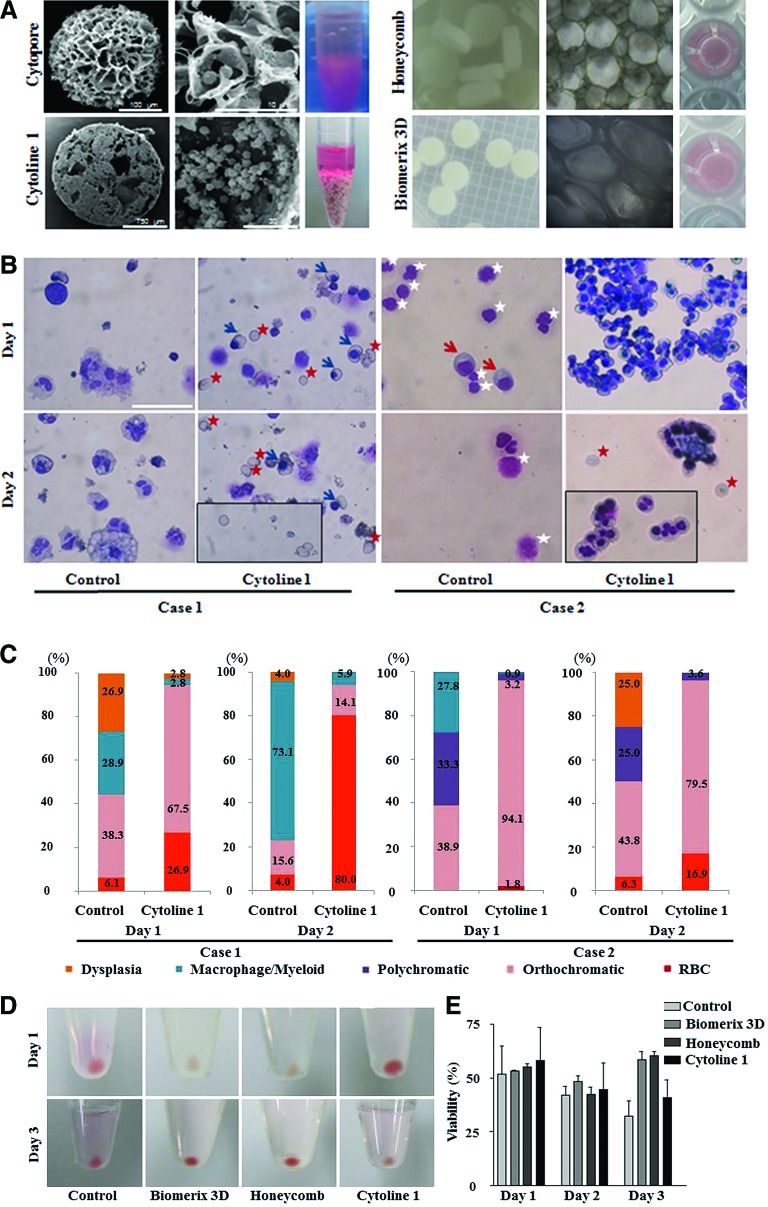
To simulate the compartmental microenvironment of the BM, in which the space is divided by bony trabeculae, commercial biomaterials that mimic the architecture of the trabecular BM were identified and four porous materials were tested (Fig. 3A). The characteristics of these materials are summarized in Table 1. Cell–cell contacts were observed inside porous microcarriers by scanning electron microscopy (Fig. 3A, left panel, middle line), mimicking BM erythroblastic islands. However, in the case of Cytopore, which has small compartmental spaces of 30 μm in diameter, only several cells were in contact.
FIG. 3.
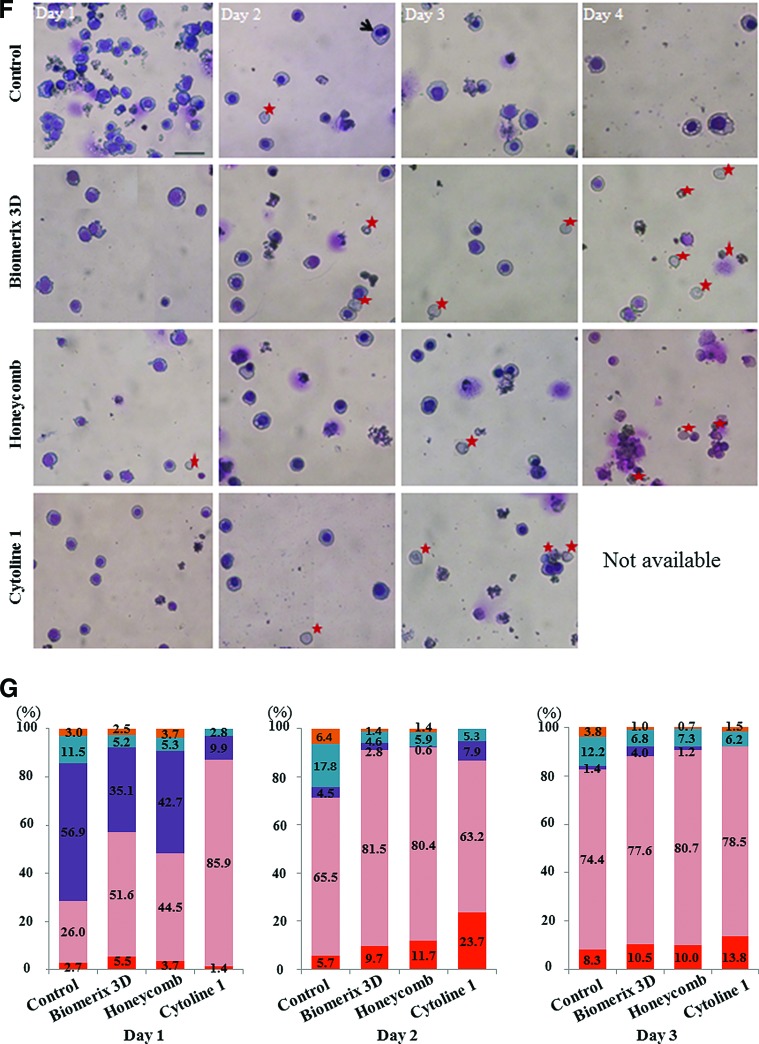
Culture with porous microcarriers and scaffolds. (A) The left and middle columns in each box show porous microcarriers and scaffolds visualized by scanning electron microscopy and phase-contrast microscopy, respectively. The right columns in each box show macroporous microcarriers in conical tubes and porous scaffolds in 96-well plates. (B, C) At day 2 of culture with Cytoline 1, the percentage of enucleated RBCs was greatly increased. Blue arrows, orthochromatic erythroblasts; red star, RBCs; white star, dead cells. Wright–Giemsa stain, 200×magnification, scale bar: 50 μm; (D) Cultures with Cytoline 1 had the fastest maturation rate, followed by cultures with a Biomerix 3D scaffold, as determined by the day on which the cell pellets appeared red. (E) At day 4, the number of RBCs and cell viability were higher in cultures with scaffolds than in those with Cytoline 1. (F, G) The maturation stages of cultured late erythroblasts were evaluated (n=2). Color images available online at www.liebertpub.com/tea
At day 1, 2, and 3, the percentage of viable cells grown with Cytopore was substantially decreased to 23.3%, 10.6%, and 8.5%, respectively, in comparison to 45.2%, 32.6%, and 31.5%, respectively, in control conditions. In addition, more myeloid cells and macrophages were observed in cells grown with Cytopore than in control conditions (n=2, data not shown). The pores of Cytopore seemed too small for sufficient erythroid cell contacts to form. In contrast, cells grown with Cytoline 1 maintained cell–cell contacts when cytocentrifuged onto slides (Fig. 3B). In this condition, the percentage of orthochromatic erythroblast rate was increased to 67.5% and the percentage of enucleated cells was increased to 80.0% at day 1 and 2 of culture, respectively (n=2). In addition, cultivation with Cytoline 1 reduced the percentage of cells with nuclear dysplasia to 2.8% and 0.0% at days 1 and 2, respectively (compared with 26.9% and 4.0% at days 1 and 2, respectively, among control samples) (Fig. 3C). Microcarriers with a pore size of approximately 400 μm could produce many more mature cells than those with a smaller pore space, confirming the importance of sufficient contacts among late erythroid cells. The increased cell maturation and enucleation rates suggest that 3D conditions promote efficient erythroid terminal maturation.
Three-dimensional culture with scaffolds
Erythroid cells are suspension cells that adhere weakly; therefore, a cylindrical spin filter was introduced to keep cells inside microcarriers. Although enucleated reticulocytes and orthochromatic erythroblasts were larger than 10 μm in diameter, spin filters with a pore size of 8 μm were inadequate owing to the leakage of cultured erythroblasts (data not shown). Therefore, we used a spin filter with a pore size of 3 μm and examined whether these small pores became clogged with cell debris, thereby blocking exchange of the culture medium. Erythroblasts grew well inside the spin filter, showing that cell debris did not block the pores.
Next, 3D culture was performed using two types of scaffolds (Honeycomb disc scaffold and Biomerix 3D scaffold) and Cytoline 1 inside the cylindrical spin filter. At day 3 of culture, the pellets of cells grown on the Biomerix 3D and Honeycomb disc scaffolds were red, indicative of the accumulation of hemoglobin (Hb) (Fig. 3D). Although the cell pellets grown in Cytoline 1 were red at day 1, cell viability was decreased at day 2–3 and the pellets were no longer red, probably owing to Hb leakage (Fig. 3D). Cell viability at day 3 was enhanced among cells grown on either of the two scaffolds (Fig. 3E).
After 4 days of culture, cell morphology was monitored by Wright–Giemsa staining (Fig. 3F), which showed that the percentage of enucleated cells was the highest among cells grown with Cytoline 1; however, the number of cells was not maintained at day 4, probably owing to fragmentation of the microcarriers. The second highest percentage of RBCs was among cells grown on a Honeycomb disc scaffold (Fig. 3F, G). The percentage of enucleated cells in Biomerix 3D scaffold was 21.4% at day 4, showing that erythroblast maturation is effective using either type of scaffold. These results show that macroporous microcarriers and scaffolds maintained cell aggregates and enhanced terminal erythropoiesis. In addition, a spin filter with a small pore size helped efficiently retain cells inside microcarriers and scaffolds without blocking media or gas exchange.
Cell culture without microcarriers in a spinner flask
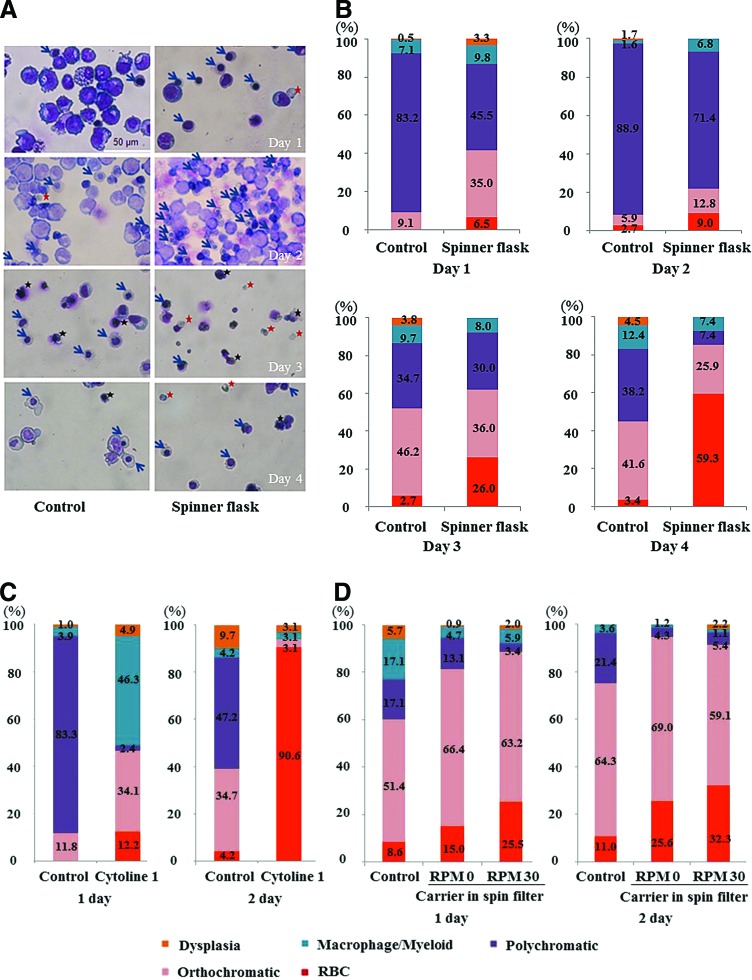
To evaluate the feasibility of stirring bioreactor cultivation, we assessed the effect of shear stress induced by media flow on late erythroid cells cultured at various stirring speeds (Fig. 4A). Cell viability was lower when cells were cultured at a high agitation rate (100 or 60 rpm) than when they were cultured at a low agitation rate (30 rpm), showing that late erythroblasts are extremely weak owing to shear stress induced by media flow. Therefore, erythroblasts were cultured for 4 days at 30 rpm. In 2D cultures, the percentage of mature orthochromatic erythroblasts gradually increased to 41.6% by day 4, with a few RBCs (Fig. 4A, B). In the stirring condition without microcarriers, erythroid cell maturation was markedly enhanced and the percentage of enucleated cells was increased to 59.3% at day 4 (Fig. 4B). However, orthochromatic erythroblasts and RBCs had irregular morphologies and damaged cytoplasm, and the percentage of viable cells was reduced to 35.5%, in comparison to 65.4% among control cells. These data suggest that reducing shear stress induced by media flow is essential and that cells should be trapped inside porous materials.
FIG. 4.
Assessment of the feasibility of 3D culture in spinner flasks or on a gyro-rocker. (A) Enuleated RBCs (red star) stained with Wright–Giemsa at day 3–4 of culture in a spinner flask without microcarriers. Blue star: orthochromatic erythroblasts, black star: dead cells. Magnification: 200×, scale bar: 50 μm. (B) Percentages of cultured cells at various maturation stages (>200 cells scored in three independent fields). (C) The maturation status of terminal erythroid cells was evaluated in cultures in a spinner flask with Cytoline 1 (>200 cells scored in three independent fields). (D) Maturation status of terminal erythroid cells cultured on a gyro-rocker at various agitation rates (>250 cells scored in five independent fields). Representative images are shown. The maturation status was evaluated by two experts. Color images available online at www.liebertpub.com/tea
Cell culture with Cytopore and Cytoline 1 in a spinner flask
To scale up the cell culture, the macroporous microcarriers Cytopore and Cytoline 1 were used. When cultured with Cytopore in a spinner flask, late erythroid cells were damaged, similar to the results shown in Figure 3A, confirming that Cytopore is not suitable for the culture of erythroid cells.
When cultured with Cytoline 1 in a spinner flask, the percentage of orthochromatic erythroblasts at day 1 was increased to 34.1%, in comparison to 11.8% in the control (Fig. 4C). The next day, the percentage of orthochromatic erythroblasts was markedly decreased to 3.1% and the percentage of enucleated RBCs was markedly increased to 90.6% in cultures with Cytoline 1, in contrast to 4.2% in 2D cultures (Fig. 4C). The percentage of dys-erythropoietic cells was markedly decreased in cultures with Cytoline 1 (3.1% vs. 9.7% in control cultures), suggesting that cell–cell contacts were maintained inside Cytoline 1, even with the media flow in the spinner flask (Fig. 4C). Given the increased enucleation rate and the increased percentage of orthochromatic erythroblasts, these data show that cell aggregate culture within porous microcarriers facilitates terminal erythropoiesis and reduces damage from media flow. However, when compared with cells in 2D cultures, enucleated RBCs have a flaccid cytoplasm and an irregular membrane, confirming that they were extremely weak owing to their exposure to shear stress.
To evaluate whether there are differences in the levels of gas or waste between the culture conditions, the conditioned media at day 2 was analyzed. The pH, gas (O2 and CO2) levels, and lactate and glucose concentrations did not differ between the 2D and 3D cultures (n=2; data not shown).
Media flow effects in 3D cultures with scaffolds inside a spin filter
We examined the maturation status of cells grown under various levels of sheer stress. At a high agitation speed (60 or 100 rpm), late erythroblasts were damaged and fewer than 20% were viable. Therefore, we compared 2D and 3D cultures that were not agitated or were agitated at a speed of 30 rpm. When cultured with Cytopore 1, orthochromatic erythroblasts at day 2 were better maintained in 3D cultures that were not agitated (69.0%) than in 2D cultures (64.3%) and in 3D cultures which were agitated at 30 rpm (59.1%) (Fig. 4D). However, the enucleation rate was the highest when cultures were agitated at 30 rpm, probably because media flow facilitated the separation of nuclei from enucleated cells (Fig. 4D).10 These data indicate that erythroid cells are extremely vulnerable to media flow-induced shear stress and that they should be cultured without agitation, at least during the enucleation phase. Therefore, we confirmed that 3D culture with scaffolds can be achieved using a rotating plate or a stirring spinner flask, but shear stress needs to be minimized.
Effects of 3D culture on cell lineage and maturation markers
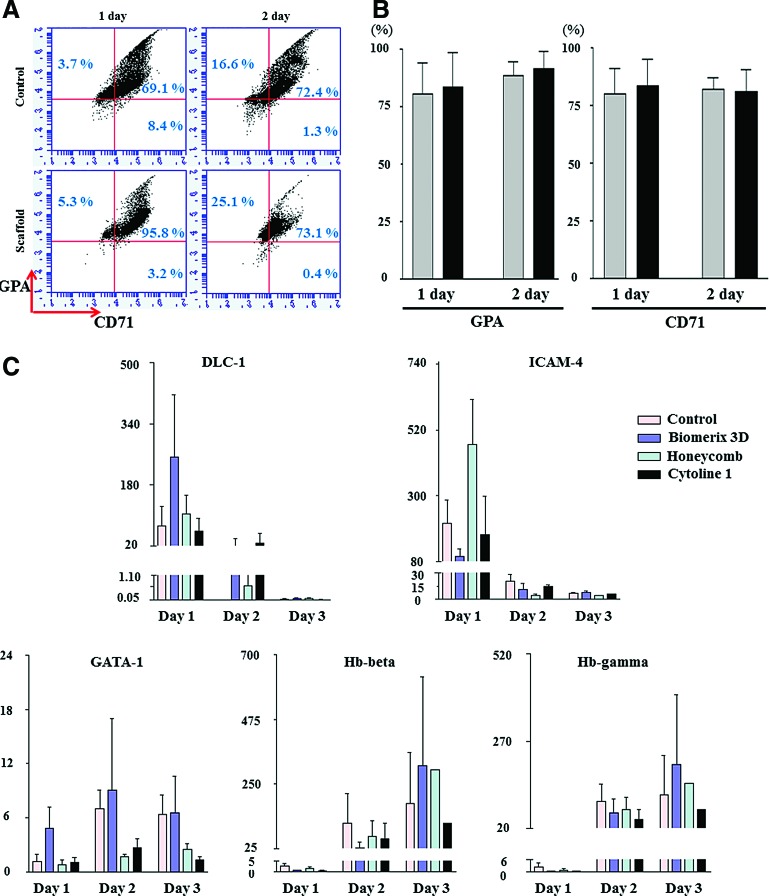
To monitor terminal erythroid maturation, we stained erythroid cells for erythroid lineage-specific markers (CD71 and GPA), a monocyte/macrophage-specific marker (CD11B), and an early/late myeloid cell marker (CD13) (Fig. 5A). GPA and CD71 were detected in approximately 100% of cells (mean: GPA, 91.3%; CD71, 81.2%, n=2) at day 2, showing that erythroid-specific markers were adequately expressed (Fig. 5B). Other cells, such as macrophages and myeloid cells, were rarely found, with CD11B and CD13 detected in 1.0% and 1.9% of cells, respectively.
FIG. 5.
Erythropoiesis markers and adhesion-related signals in 3D cultures. (A, B) At day 1–2 of 3D culture, glycophorin A (GPA) and CD71 were highly expressed, indicative of erythroid cells, in cultures with a Honeycomb disc, as analyzed by flow cytometry. Representative images are shown. Values are the mean±standard error of the mean (SEM) by two independent experiments. (C) mRNA expression of genes was analyzed by quantitative polymerase chain reaction, normalized to that of GAPDH, and compared with that at 0 h. mRNA expression of the erythroblast adhesion-related markers deleted in liver cancer-1 (DLC-1) and intercellular adhesion molecule-4 (ICAM-4) was markedly increased at day 1 of culture, with the highest expression in cultures with scaffolds. mRNA expression of the erythropoiesis markers GATA-1, hemoglobin (Hb)-β, and Hb-γ increased over 3 days of culture, with the highest expression in cultures with a Biomerix 3D scaffold. The mRNA expression values indicate mean±SEM from three independent experiments. Color images available online at www.liebertpub.com/tea
mRNA changes in cells grown in 3D aggregate culture
We previously reported that deletion in liver cancer-1 (DLC-1) and intercellular adhesion molecule-4 (ICAM-4) might play a role in contact among erythroid cells; therefore, we evaluated their mRNA expression in 3D cultures. As expected, DLC-1 and ICAM-4 mRNA expression levels were markedly higher at day 1 of the 3D culture than at 0 h (increased by 253.2-fold in cultures with a Biomerix 3D scaffold and increased by 104.9-fold in cultures with a Honeycomb disc). These data indicate that cell–cell contacts induced by cell aggregation dramatically evoke terminal maturation signals. The mRNA levels of the transcription factor GATA-1 and the erythroid cell maturation markers Hb-β and Hb-γ were evaluated during 3D culture. In cultures with a Biomerix 3D scaffold, the mRNA level of GATA-1 was 9.1-fold higher at day 2 than at 0 h. The Hb-β mRNA level was increased by 313.0- and 298.2-fold in cultures with a Biomerix 3D scaffold and a Honeycomb disc, respectively. In addition, the mRNA level of Hb-γ was increased by 203.3-fold at day 3 of culture with a Biomerix 3D scaffold (Fig. 5C) These data indicate that cell–cell contacts in 3D cultures induce erythroid cell adhesion and maturation signals, accompanied by the accumulation of Hb (Fig. 3D).
Function analysis of hemoglobin in cultured RBCs
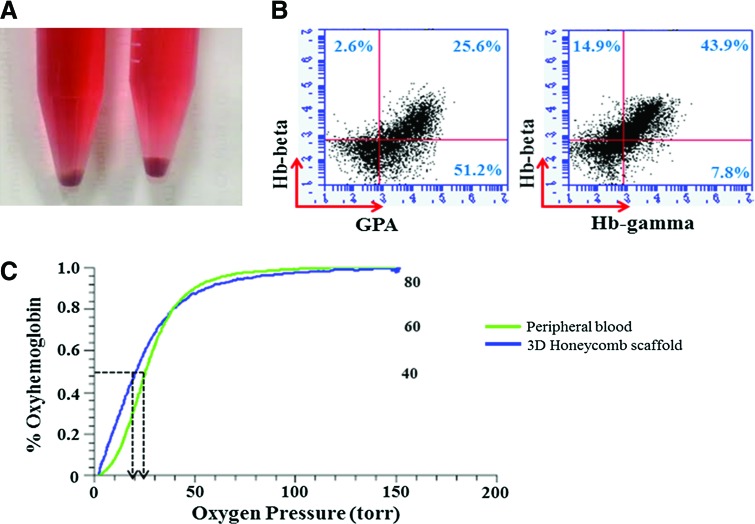
To evaluate the subtypes of hemoglobins in cultured RBCs derived from CB CD34+ cells, we stained mature erythroid cells for fetal hemoglobin (Hb-γ) and adult hemoglobin (Hb-β). Erythroid cells cultured with a Honeycomb scaffold showed GPA positivity in about 76%. Hb-β and Hb-γ were detected in 58.8% and 51.7%, respectively, showing that most erythroid cells contained adult- and fetal-type hemoglobin (Fig. 6B).
FIG. 6.
Functional characterization of hemoglobin in cultured erythroid cells. (A) At day 17, cells grown in scaffolds for 24 h were red, indicative of sufficient hemoglobin accumulation. (B) The erythroid cells also expressed erythroid cell markers, GPA, β-globin, and γ-globin. (C) Oxygen equilibrium curves were measured by a Hemox analyzer. The oxygen-binding capacity of mature erythroid cells cultured in a scaffold showed similar oxygen-binding capacity compared with the fresh adult peripheral blood. Color images available online at www.liebertpub.com/tea
To study the functionality of the generated RBCs, we calculated the oxygen equilibrium curves of erythroid cells cultured with a Honeycomb scaffold and a spin filter and compared them with that of fresh peripheral blood from a healthy donor using a Hemox analyzer. Cultured RBCs (p50=20.8) had a high affinity for oxygen, similar to RBCs in peripheral blood (p50=25.8). Therefore, the oxygen equilibrium curves of cells grown in 3D culture conditions were similar or slightly shifted to the left compared with adult peripheral blood, confirming the oxygen-carrying capacity of cultured RBCs (Fig. 6C).
Discussion
Many protocols have been developed to differentiate human stem cells into RBCs. The main barriers to this process seem to be low cell viability, inefficient maturation, and low enucleation at the terminal stage of erythropoiesis. Although early erythroblasts proliferate well and mature into polychromatic erythroblasts, they do not readily differentiate into orthochromatic erythroblasts and reticulocytes, and the generated erythroid cells do not have a healthy morphology. For other cell types, 3D culture methods have been designed to mimic in vivo microenvironments. However, conventional 3D culture conditions are unlikely to be helpful for erythroid cell culture, because these cells are not adherent and their characteristics continuously change as they become enucleated. In addition, the membrane surfaces of late erythroblasts and reticulocytes are negatively charged.
A few reports of RBC generation utilized a 3D cultivation method incorporating stirring, packed bed bioreactors, or hollow fiber bioreactors and showed promising results with high expansion rates and high cell densities.3 However, the expansion was due to the proliferation of early erythroblasts, and the problems of low cell viability and low enucleation rates were not solved. Moreover, precise data on the conditions or functions of cells at the terminal maturation phase are lacking and the generated cells do not have an intact morphology. Furthermore, in the stirring bioreactor model, the morphology of the final RBCs was not intact, probably owing to damage from media flow.2 Importantly, there is no report regarding the effects of contact among erythroid cells in 3D cultures.
We previously found that contacts among late erythroblasts are important for their terminal maturation in 2D culture conditions; therefore, we attempted to maximize cell–cell contacts by seeding a sufficient number of cells into narrow tubes. With this method, we found clusters of mature erythroblasts, reminiscent of erythroblastic islands in the BM (Fig. 2A).4 As a result, the maturation status and enucleation rate were increased.
To scale up the culture and to mimic the porous environment of the BM, highly porous structures were used. These biomaterials are commercially available and relatively simple to use. With the exception of Cytopore, these porous materials have a large pore diameter of approximately 400 μm, enabling sufficient cell–cell contacts. These contacts were observed by the staining of slides onto which cells had been cytocentrifuged and by electron microscopy. Maturation of mature erythroblasts into erythrocytes was accelerated by 3D culture, in comparison with the 2D culture of cells at high density. This was accompanied by changes in mRNA levels. From our previous study, the mRNA levels of DLC-1 and ICAM-4 were elevated in late erythroblasts cultured at a high density in 2D.5 In this study, their mRNA levels were markedly increased in 3D cultures, confirming that the level of cell–cell contact was higher in these conditions than in high-density 2D cell cultures. The expression of several erythropoiesis-related genes, GATA-1 and Hb-β/-γ, was markedly increased throughout the culture period.
Interestingly, the maturation status of cells differed among the 3D culture methods. Cultured cell pellets became red after 1 day of culture with Cytoline 1, whereas they became red after 3 days of culture with scaffold materials (Fig. 3D). Consistent with this, after 1 day of culture, the percentage of orthochromatic erythroblasts with Cytoline 1 was 34–41% higher than that with scaffolds (Fig. 3G). These results indicate that erythroid cells were not fully packed in the scaffold materials, while cells in Cytoline 1 were more tightly packed with a high level of cell–cell contacts as shown in Figure 3A.
Erythroid-specific cell surface markers were highly expressed in cells before 3D culture, and there was little change in the expression of CD markers during 3D culture. Nonetheless, CD71 expression was slightly decreased and GPA expression was maintained during 3D culture, reflecting the presence of mature RBCs.
To choose a suitable bioreactor model, we incorporated spinner flasks and rotator plates into the 3D culture system. Collins et al. achieved a high density of cells (6.0×106 cells/mL) in a stirred tank bioreactor; however, the generated RBCs were not viable.11 Boehm et al. cultivated erythroblasts at low agitation speeds; however, the cell density was low, and cells were not cultured in a 3D system.12 In our method, terminal erythroid maturation was achieved by 3D culture in a spinner flask; however, erythroid cells were extremely weak owing to their exposure to shear stress, and some cells leaked from the microcarriers. Therefore, cells were cultured with porous materials inside a cylindrical spin filter. A universally used spin filter with a pore size of 8 μm was inadequate, because cells continued to leak. A spin filter with a pore size of 3 μm allowed gas and media exchange, as evaluated by the analysis of gases and lactate in the media and cell morphology. Using static culture flasks, the production of a single unit of RBCs requires a culture area of 200 m2, equivalent to ∼11,430 T175 culture flasks.13 However, mimicking the BM microenvironment using a bioreactor reduced the volume of medium and the culture space required. Our data may provide a basis for cost-efficient RBC generation in a bioreactor by confirming the importance of contacts among late erythroblasts.
This study has some limitations. There are insufficient CB sample numbers per experiment. In addition, the data for each CB sample showed a high level of variability. For example, in one experiment in Figure 4, 27% of cells were RBCs at day 1 of 3D culture. By contrast, in another experiment using another CB, only 1.1% were RBCs. However, maturation was consistently accelerated and cells consistently had a good morphology in all 3D cultures. Finally, we did not confirm that cells could be cultured on a large scale using automatic bioreactors. This cell contact system is only applicable to late erythroblasts; therefore, a culture system for the entire differentiation process (from hematopoietic stem cells) should be studied in the future.
Conclusions
Our data demonstrate, for the first time, that cell–cell contacts in 3D cultures promote erythroid cell maturation till the enucleation stage by inducing the formation of an erythroblastic niche. These gross changes were accompanied by markedly increased levels of mRNAs related to cell adhesion and maturation. The space required for aggregate cell culture limits mass production, and, thus, cells were inserted into small spaces that were subdivided using porous materials. Although media exchange is essential in a bioreactor, sheer stress should be minimized. These 3D conditions with porous materials could minimize the cultivation space and volume of medium required, enabling mass production. In the future, cells cultured in vitro could replace a proportion of, or all, donated RBCs.
Acknowledgments
The authors thank Dr. Wang Joon Yoon for providing cord blood samples. This study was supported by grants of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI10C1740 and HI12C0202).
Authors' Contributions
E.M.L. substantially contributed to most of the experiments and the analysis of data, and wrote the article. S.Y.H. and H.S.C. did some experiments. B.C. and B.H. contributed to the design of the experiments and helped with interpretation of data. E.J.B. substantially contributed to design of the experiments, interpretation of data, and wrote and revised the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chow A., Huggins M., Ahmed J., Hashimoto D., Lucas D., Kunisaki Y., Pinho S., Leboeuf M., Noizat C., van Rooijen N., Tanaka M., Zhao Z.J., Bergman A., Merad M., and Frenette P.S.CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med 19,429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmins N.E., Athanasas S., Gunther M., Buntine P., and Nielsen L.K.Ultra-high-yield manufacture of red blood cells from hematopoietic stem cells. Tissue Eng Part C Methods 17,1131, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Housler G.J., Miki T., Schmelzer E., Pekor C., Zhang X., Kang L., Voskinarian-Berse V., Abbot S., Zeilinger K., and Gerlach J.C.Compartmental hollow fiber capillary membrane-based bioreactor technology for in vitro studies on red blood cell lineage direction of hematopoietic stem cells. Tissue Eng Part C Methods 18,133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasis J.A., and Mohandas N.Erythroblastic islands: niches for erythropoiesis. Blood 112,470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi H.S., Lee E.M., Kim H.O., Park M.I., and Baek E.J.Autonomous control of terminal erythropoiesis via physical interactions among erythroid cells. Stem Cell Res 10,442, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Baek E.J., Kim H.S., Kim J.H., Kim N.J., and Kim H.O.Stroma-free mass production of clinical grade red blood cells by using poloxamer 188 as a RBC survival enhancer. Transfusion 49,2285, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Mathur S.C., S.K.I. , and Hutchison R.E.Hematopoiesis. Henry's Clinical Diagnosis and Management by Laboratory Methods. 21st ed., Philadelphia, PA: Saunders Elsevier, 2007, pp. 487–488 [Google Scholar]
- 8.Baek E.J., Y.J. , Kim M.S., Lee S.Y., Cho S.J., Kim E., and Kim H.O.Enhanced production of red blood cells in suspension by electrostatic interactions with culture plates. Tissue Eng Part C Methods 16,1325, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Kurita R., Suda N., Sudo K., Miharada K., Hiroyama T., Miyoshi H., Tani K., and Nakamura Y.Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS One 8,e59890, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebiguchi M., Hirokawa M., Guo Y.M., Saito K., Wakui H., Komatsuda A., Fujishima N., Takahashi N., Takahashi T., Sasaki T., Nunomura W., Takakuwa Y., and Sawada K.Dynamics of human erythroblast enucleation. Int J Hematol 88,498, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Collins P.C., Nielsen L.K., Patel S.D., Papoutsakis E.T., and Miller W.M.Characterization of hematopoietic cell expansion, oxygen uptake, and glycolysis in a controlled, stirred-tank bioreactor system. Biotechnol Prog 14,466, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Boehm D., Murphy W.G., and Al-Rubeai M.The effect of mild agitation on in vitro erythroid development. J Immunol Methods 360,20, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Timmins N.E., and Nielsen L.K.Manufactured RBC—rivers of blood, or an oasis in the desert? Biotechnol Adv 29,661, 2011 [DOI] [PubMed] [Google Scholar]