Abstract
In asthma, airflow obstruction is thought to result primarily from inflammation-triggered airway smooth muscle (ASM) contraction. However, anti-inflammatory and smooth muscle-relaxing treatments are often temporary or ineffective. Overproduction of the mucin MUC5AC is an additional disease feature that, while strongly associated pathologically, is poorly understood functionally. Here we show that Muc5ac is a central effector of allergic inflammation that is required for airway hyperreactivity (AHR) to methacholine (MCh). In mice bred on two well-characterized strain backgrounds (C57BL/6 and BALB/c) and exposed to two separate allergic stimuli (ovalbumin and Aspergillus extract), genetic removal of Muc5ac abolishes AHR. Residual MCh responses are identical to unchallenged controls, and although inflammation remains intact, heterogeneous mucus occlusion decreases by 74%. Thus, whereas inflammatory effects on ASM alone are insufficient for AHR, Muc5ac-mediated plugging is an essential mechanism. Inhibiting MUC5AC may be effective for treating asthma and other lung diseases where it is also overproduced.
Introduction
Asthma is a major health problem. Worldwide, it affects more than 300 million people. In the U.S., it affects approximately 10 million and costs $50-60 billion per year to treat. Asthma is characterized by airflow obstruction and structural changes associated with acute and chronic lung inflammation. Accordingly, the most widely used therapies are bronchodilators and anti-inflammatory agents1. However, treatment results are often temporary or incomplete, and alternative strategies are lacking. We propose that this is due to an incomplete understanding of the mechanisms by which airflow obstruction and symptoms of dyspnea, cough, and wheezing are caused by the combined effects of inflammation, bronchoconstriction, and additional non-contractile mechanisms. Non-contractile factors include airway wall thickening due to edema and structural remodeling2, loss of airway liquid surface tension due to plasma extravasation and fibrin accumulation3, and airway occlusion due to mucus hypersecretion4. With even just a small amount of ASM constriction, these non-contractile components can geometrically amplify airway resistance and increase the propensity for airways to close5-8.
In human asthma and in animal models, mucin overproduction and hypersecretion are prominent pathological findings9-14. In its most severe setting--fatal asthma--autopsy studies show that patients die from severe airflow obstruction caused by bronchoconstriction coupled with widespread mucus plugging15-17. Even in mild to moderate asthma, where there is minimal baseline airflow limitation, patients respond to the inhaled cholinergic agonist methacholine (MCh) with exaggerated obstruction. Existing dogma labels this airway hyperreactivity (AHR) to MCh as resulting from excessive ASM contraction1. Thus, unlike fatal asthma, where mucus hypersecretion is well recognized, in non-fatal exacerbations the potential obstructive role of mucus has been largely overlooked1. In fact, in mild to moderate disease, mucin overproduction is highly prevalent12, and the genomic region on chromosome 11p15 that encodes airway polymeric mucins is associated with AHR18. Furthermore, mucin overproduction and changes in its biochemical/biophysical properties correlate with asthma exacerbations4,19,20. These associations in humans are supported in animal models where mucin overproduction and AHR to MCh are both induced, and where in addition to causing ASM contraction, MCh is also known to cause mucin secretion21,22. Thus, the obstructive effects of ASM contraction may be amplified by acute thickening of mucus on airway surfaces. While these associations are strong, establishing the causative significance of mucus secretion in asthma has been hampered by a lack of experimental evidence and the absence of effective clinical interventions that target the chief glycoprotein components of airway mucus, the secreted polymeric mucins MUC5AC and MUC5B.
MUC5B predominates in healthy human lungs, and its levels in asthma remain stable or in some cases decrease10,12,23. On the other hand, MUC5AC levels in asthma increase significantly and consistently4,10,12,13. Mice display similar tightly controlled expression patterns: High baseline Muc5b gene expression remains stable following antigen challenge while low baseline Muc5ac gene expression increases dramatically24. Furthermore, whereas Muc5b is required for airway homeostasis and anti-bacterial defense, Muc5ac is not essential for these functions25, and in lung injury Muc5ac is actually detrimental26. These findings implicate Muc5ac as a deleterious and dispensable glycoprotein component of airway mucus. Thus, it is suitable to investigate as a potential target for reducing obstruction in asthma.
In allergic asthma, a biphasic response occurs after allergen inhalation. Acutely, IgE cross-linking induces mast cell and basophil histamine release, causing a brief episode of airflow obstruction (what is most commonly thought of as an “asthma attack”). The acute early-phase response lasts a few minutes to a few hours, and it is followed by a late-phase response that lasts for several hours to several days. Clinically, the late-phase is considered to be the main driver of disease progression27. Accordingly even after baseline lung function is restored following an acute asthma episode, the ensuing late-phase displays sustained pathophysiology hallmarked by AHR to inhaled agonists such as MCh, inflammation characterized by recruitment of eosinophils and IL-13-producing type 2 T helper cells, and airway remodeling linked to induced MUC5AC production28.
MUC5AC/Muc5ac induction is a well-characterized epithelial response to allergic (type 2) inflammation. IL-13 has been implicated in the pathogenesis of human asthma29 and in animal models30,31. However, compared to its reported effects on leukocytes32 or ASM33, the relative importance of IL-13 signaling in epithelia34 and the mechanisms by which it elicits asthma-like pathophysiology are unclear. MUC5AC/Muc5ac gene expression is induced by IL-13 through direct stimulation of airway epithelial cells24,30,31,34-36. In mice, Muc5ac expression is central to the allergic mucous phenotype. It is induced maximally 48-72 h after antigen challenge24, times that are concordant with late-phase human asthma. We thus hypothesized that eliminating this type 2 inflammation effector would protect against asthma-like disease in mice.
Here we show the functional significance of Muc5ac in allergic AHR, inflammation, and mucus obstruction. In Muc5ac−/− mice37, we demonstrate that even in the presence of ongoing inflammation, significantly reduced heterogeneous mucus plugging abolishes AHR.
Results
Muc5ac is a major polymeric mucin in allergic mouse lungs
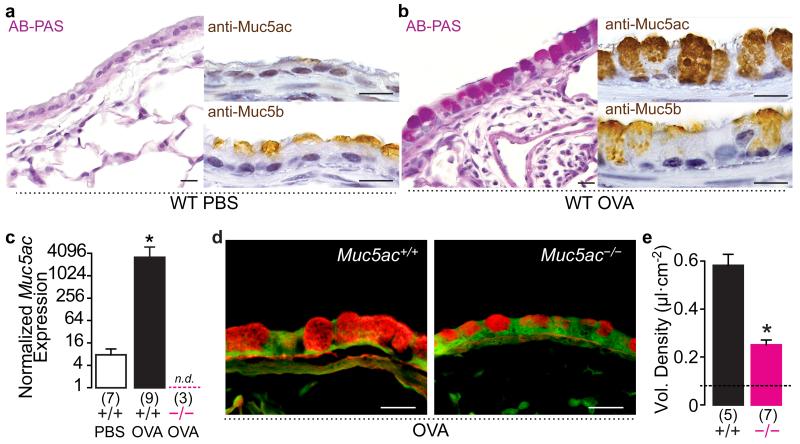
In wild type (WT) mice, immunohistochemical analyses demonstrated constitutive Muc5b production that was similar in healthy and ovalbumin (OVA) sensitized and challenged airways (Figs. 1a,b). OVA challenge induced Muc5ac protein and alcian blue-periodic acid Schiff’s positive glycoconjugate production (Figs. 1a,b), which were marked by a ~400-fold increase in Muc5ac transcripts (Fig. 1c). In OVA challenged Muc5ac−/− mice, Muc5ac transcripts were absent (Fig. 1c), and bronchial epithelial mucin content was significantly reduced (Fig. 1d,e). We thus examined the development of allergic asthma-like phenotypes in Muc5ac−/− mice.
Fig. 1. Mucous metaplasia is significantly reduced in Muc5ac−/− airways.
(a and b) Mucin production in saline (a) and OVA (b) challenged WT mice. AB-PAS, anti-Muc5ac, and anti-MUC5B staining shows up-regulated Muc5ac and sustained MUC5B in allergic airways. (c-e) Muc5ac mRNA increased 383-fold in OVA challenged WT mice (+/+), but it was undetectable in Muc5ac knockout (−/−) mice (c), resulting in a 67% reduction in mucous metaplasia detected by periodic acid fluorescent Schiff’s staining (d and e). Scale bars, 20 μm (a, b, and d). Mean, s.e.m. (c and e). ‘*’, p<0.05 (one-tailed) by unpaired t-test (c and e), with Welch’s correction for unequal variances in c. ‘n.d.’, not detected. Numbers in parentheses, ‘N’ mice. ‘Vol.’, volume. Dashed line in e, baseline in saline challenged WT mice.
Muc5ac is required for AHR to MCh in allergic mouse lungs
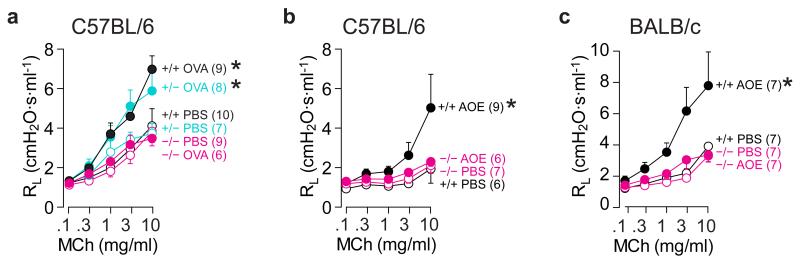
To determine the consequences of disrupting Muc5ac expression on airflow obstruction, we assessed lung mechanical function and AHR to MCh. OVA challenge did not alter baseline lung resistance (Supplementary Fig. 1a), demonstrating that inflammatory and mucous metaplastic changes did not disrupt steady state airflow measurably in this model of late-phase asthma. However, when exposed to MCh, OVA challenged WT and heterozygous mice both demonstrated significant AHR. By contrast, Muc5ac−/− mice were protected (Fig. 2a and Supplementary Fig. 1b). To confirm these findings, we also assessed AHR in C57BL/6 and BALB/c WT and congenic Muc5ac−/− mice exposed to aerosolized Aspergillus oryzae extract (AOE). AOE is a fungal allergen pertinent to human asthma that upon mucosal sensitization elicits strong asthma-like responses in mice38. In WT animals, AOE challenge caused AHR to MCh, but in both Muc5ac−/− lines, AHR was abolished (Figs. 2b,c and Supplementary Fig. 1b). Importantly, protection occurred irrespective of C57BL/6 and BALB/c strain-specific airway and tissue localized pathophysiologies. To partition AHR on airway and tissue scales, we assessed lung mechanics by measuring input impedance. As reported previously39, AHR was strongly influenced by tissue resistance and elastance in C57BL/6 mice (Figs. 3a and Supplementary Fig. 2) and by tissue and central airway resistance in BALB/c mice (Figs. 3b and Supplementary Fig. 2); Muc5ac deficiency was protective irrespective of background strain. Thus, in diverse models of allergic asthma, Muc5ac was required for AHR to MCh.
Fig. 2. Muc5ac is required for AHR to MCh.
Lung resistance (RL) responses to MCh in WT (+/+, black), Muc5ac+/- (turquoise), and Muc5ac−/− (magenta) mice after saline (open circles), OVA (closed circles in a), or Aspergillus oryzae extract (AOE; closed circles in b and c). Mean, s.e.m. (a-c). ‘*’, p<0.05 between slopes of best-fit regression lines by one-way ANOVA. Numbers in parentheses, ‘N’ mice.
Fig. 3. Muc5ac mediates mouse strain-specific allergic airway pathophysiology.
Conducting airway resistance (ΔRAW), tissue resistance (ΔGTI) and tissue elastance (ΔHTI) were measured using oscillatory mechanics to assess input impedance. (a) In WT C57BL/6 mice, Aspergillus oryzae extract (AOE)-induced AHR (closed black circles) was dominated by changes in peripheral tissue mechanics (ΔGTI and ΔHTI) compared to changes in RAW. (b) In WT BALB/c mice, AOE-induced AHR (closed black circles) had central airway (ΔRAW) and tissue dominance (ΔGTI), whereas ΔHTI was not significantly different following AOE (n.s.) in WT mice. In both congenic strains, responses were significantly blunted in Muc5ac−/− mice (closed magenta circles). Mean, s.e.m. ‘*’, p<0.05 between slopes of best-fit regression lines by one-way ANOVA. ‘n.s.’, not significant. Numbers in parentheses, ‘N’ mice.
Mucin secretion alone is not sufficient to cause AHR
Because acute mucin secretion was required for AHR to MCh with no residual effect attributable to ASM hypercontractility (Figs. 2 and 3), we next determined whether mucin secretion was sufficient to acutely induce AHR independently of bronchoconstriction. In airway epithelial cells, P2Y2 purinergic receptor stimulation is a major pathway for mucin secretion40. P2Y2 receptors are activated by the endogenous agonists ATP and UTP40,41. ATP is rapidly dephosphorylated to adenosine, a signaling molecule that itself induces bronchoconstriction; ATP was thus inappropriate for these studies. Since UTP and its metabolites do not cause acute ASM contraction42, we challenged WT C57BL/6 mice with OVA or saline, and 72 h later we mechanically ventilated them and measured immediate changes in secretion and lung resistance in response to UTP. In OVA challenged WT mice, although UTP rapidly stimulated secretion (Figs. 4a,b), there was no disruption of airflow (Fig. 4c). Thus, although Muc5ac was critical for allergic AHR in the presence of ASM-mediated bronchoconstriction following MCh, UTP-induced mucin secretion alone did not evoke an AHR response.
Fig. 4. Acute mucin secretion does not cause AHR.
WT mice were OVA or PBS challenged, and then anesthetized, ventilated, and exposed to UTP (0.1-100 mg/ml) or vehicle (saline). (a) In OVA challenged lungs fixed immediately after dose response tests, there was an acute decrease in periodic acid fluorescent Schiff’s (PAFS) staining in UTP (right panel) compared to vehicle (‘-UTP’, left panel) treated mice, demonstrating acute UTP-induced secretion. Scale bars, 20 μm. (b) Quantitation of UTP-induced changes in PAFS staining in OVA challenged mice. (c) No effect of UTP on RL in either PBS or OVA challenged WT mice. Mean, s.e.m. in b and c. Numbers in parentheses, ‘N’ mice. ‘Vol.’, volume. Dashed line in b, baseline in saline challenged WT mice. ‘*’, p < 0.05 by t Test.
Allergic inflammation fails to drive AHR in Muc5ac null mice
Type 2 inflammation is critical for AHR in allergic mouse models30,31. Given the essential role for Muc5ac in AHR identified here (Figs. 2 and 3), and the requirement for Muc5b in inflammatory cell clearance25, we determined whether Muc5ac expression mediated AHR by modulating allergic inflammation. Compared to saline challenged mice, there were significant increases in leukocyte numbers that varied with environmental exposure (OVA vs AOE) and genetic diversity (C57BL/6 vs BALB/c) (Supplementary Table 1). In WT C57BL/6 mice, there was an 8.5-fold difference in eosinophilic responses to OVA and AOE; between WT BALB/c and C57BL/6 strains, there was a 9.7-fold difference in eosinophil numbers in response to AOE (Fig. 5). However, between WT and Muc5ac−/− mice in each of these groups, eosinophilic inflammation was not significantly different (Fig. 5, Supplementary Fig. 3, and Supplementary Table 1). Thus, full protection from AHR in the absence of Muc5ac occurred even though asthma-like allergic inflammation was sustained.
Fig. 5. Muc5ac deficiency does not reduce allergic inflammation.
WT (black) and Muc5ac−/− (red) mice had similar numbers of eosinophils in lung lavage fluid irrespective of OVA or Aspergillus oryzae extract (AOE) antigen challenge or strain background variables. For all groups, saline challenged mice had <104 eosinophils in lung lavage fluid (see Supplementary Table 1). Mean, s.e.m. N = 6-11 mice/group.
Reduced heterogeneous airway closure in Muc5ac null mice
Since AHR was abolished in Muc5ac−/− mice even though inflammation remained intact, we hypothesized that AHR was mediated by physical obstruction of the airway lumen by Muc5ac. Obstruction could result from uniformly thickened mucus causing an increase in resistance to airflow via homogeneous changes in mean airway lumen diameter. Alternatively, obstruction could result from non-uniform mucus aggregates causing an increase in resistance to airflow via heterogeneous occlusion and airway closure (Fig. 6a). To compare these, AOE challenged WT and Muc5ac−/− mice were mechanically ventilated and treated with increasing doses of MCh as shown in Fig. 2. During peak changes in resistance following 10 mg/ml MCh, lungs were fixed transmurally with methacarn to preserve airway geometry and enable quantification of secreted mucus in situ43.
Fig. 6. Muc5ac deficiency protects against heterogeneous airflow obstruction.
(a) Models of the effects of uniform vs. non-uniform obstruction on airflow. (b and c) Changes in peak total lung and airway resistances (ΔR) following 10 mg/ml MCh in WT and Muc5ac−/− mice (n = 7 per genotype) (b) were fit to Poiseuille’s Law (equation in c) to predict changes in airway lumen diameter (red point and dashed arrow). Viscosity (η) and length (l) were held constant. (d-f) Mean (d) and distributions (e,f) of airway lumen diameters measured in methacarn fixed lungs; changes in mean lumen diameter were also fit to the equation in c (black points and dashed arrow). (g-i) Contributions of mucus, airway epithelium, and smooth muscle to changes in composite airway wall/mucus (AW/M) thickness. Mean, s.e.m. in b, d, g-i. x-axis labels bin maximum values in e, f. N = 4 WT and 5 Muc5ac−/− mice. ‘*’, p < 0.05 by t Test.
We generated a model of homogenous obstruction by fitting changes in resistance and airway lumen diameter to Poiseuille’s Law. In AOE challenged Muc5ac−/− mice, resistance (R) to airflow decreased 2.3-fold (Fig. 6b). Assuming laminar flow, Poiseuille’s Law predicts a 23% increase in mean lumen diameter uniformly (Fig. 6c,magenta dashed line). However, our histological measurements showed that mean lumen diameters improved by 39% and 32% in bronchi and bronchioles, respectively (Fig. 6d). If applied to the model above, airflow resistances would be 3 to 4-fold improved (Fig. 6c, black lines), but they were not. Thus, a model in which the prevention of AHR in Muc5ac−/− mice was the result of protection from uniform mucus thickening and homogenous airflow obstruction is incongruent with our observations. Indeed, the poor fit of the findings above, the anatomically diverse responses observed in our AHR studies (Figs. 3a and 3b), and previous data from murine and computational models2,8 more strongly support heterogeneous obstruction as the major determinant of AHR.
To determine whether Muc5ac-mediated heterogeneous obstruction contributes to AHR, we analyzed the distributions of individual airway lumen sizes relative to their anatomical locations. We observed non-uniform distributions of lumen sizes. In Muc5ac−/− lungs, there was a right-ward shift in the distribution of airway lumen sizes compared to WT (Fig. 6e,f), which was consistent with protection from AHR. Furthermore, in WT mice, we also observed markedly greater proportions of bronchiolar airways with diameters of ≤100 μm (Fig. 6f), a measurement consistent with a previously calculated threshold for airway closure8. Because components such as the epithelial and ASM layers and secreted mucus can all influence airway closure, we next determined how these were affected in Mu5ac−/− mice.
In our functional studies, epithelial mucin production/secretion and ASM contraction were both required for AHR by (Figs 2-4). We thus compared the specific contributions of secreted mucin, epithelial thickening, and ASM to anatomically localized changes in airway sizes. In bronchi, overall airway diameter (including ASM, epithelium, mucus, and lumen) was 539±78 μm in WT mice and 683±31 μm in Muc5ac−/− mice (p = 0.051, one-tailed t-Test); in the bronchioles these values were 274±15 μm and 310±22 μm, respectively (p = 0.096, one-tailed t-Test). Though not statistically significant as a combined group, these data suggested that secreted Muc5ac in the airway lumen could have some effect(s) on the behavior of underlying tissues. We therefore derived a measure of composite airway wall/mucus thickness by subtracting airway lumen diameters (Fig. 6d) from overall airway diameters. In the bronchi, this was not significantly different between WT and Muc5ac−/− mice (103±21 μm vs. 76±9 μm, respectively; p = 0.124, one-tailed t-Test). By contrast, in the bronchioles, differences in composite airway wall/mucus thickness were significant (71±14 μm in WT vs. 44±10 μm in Muc5ac−/−; p = 0.006, one-tailed t-Test). Mucus contribution to composite airway wall/mucus thickness was reduced in Muc5ac−/− mice compared to WT as expected since a major mucin component was missing (Fig. 6g). However, there were also significant differences in epithelial tissue and ASM components (Fig. 6h,i). Epithelial hypertrophy is directly related to mucin production9, so its contribution was also predictable (Fig. 6h). By contrast, the effect of Muc5ac deficiency on the contribution of ASM, which was significantly lower in Muc5ac−/− bronchi and bronchioles, was surprising (Fig. 6i).
Collectively, these findings reinforce the concept AHR is caused by contractile and non-contractile factors that contribute interdependently. Since AHR in allergic mouse lungs was dependent on a combination of ASM contraction and Muc5ac secretion (Figs. 2-4), and since changes in airway structure and function did not fit models of homogenous changes in airflow, these findings further support the hypothesis that epithelial Muc5ac secretion during MCh-induced ASM constriction causes the formation of mucus plugs that potentiate heterogeneous airflow disruption.
Muc5ac mediates heterogeneous mucus occlusion in mice
In WT airways, mucus plugs were present, and Muc5ac was abundant (Fig. 7a); in Muc5ac−/− airways, plugs were infrequent, and mucus was sparse (Fig. 7b). Throughout the lungs, mean lumen mucin volume density, a homogeneous measure of mucus content across airway surfaces, was reduced by 32% (Table 1). Thus, as with lumen diameter, assuming uniform mucus accumulation across all airway surfaces and fitting these to Poiseuille’s Law results in changes in resistance that exceed those observed experimentally.
Fig. 7. Muc5ac deficiency reduces mucus plugging.
(a and b) Inhaled MCh caused mucus plugging in WT allergic mouse airways (aw). Muc5ac protein (a) and PAFS staining (b) in AOE challenged WT (Muc5ac+/+) and Muc5ac−/− mouse airways. Arrow in A, region shown at higher magnification in right panel. (c-f) Extent and distribution of mucus plugging. Mean occlusion per mouse across all measured airways (c). Distribution of the extent (d) and localization (e and f) of occlusions. Arrows in e and f, starts of upper quartiles. Scale bars, 100 and 20 μm (a); 50 μm (b). Mean, s.e.m. (c). Median-quartiles (d). ‘*’, p<0.05 (one-tailed) by unpaired t-test (c) and Mann-Whitney test (d). Numbers in parentheses, ‘N’ mice (c), ‘N’ airways (d-f).
Table 1. Measurements of secreted mucus in mice during bronchoconstriction.
| Histological measurement | WT | Muc5ac −/− | p-value |
|---|---|---|---|
| Vmucus per surface area (mm3 cm−2) | |||
| All airways | 0.71±0.08 | 0.48±0.43 | 0.313 |
| Axial bronchi | 1.16±0.09 | 0.84±0.11* | 0.032* |
| Non-terminal bronchioles | 0.58±0.13 | 0.30±0.06* | 0.041* |
| Vmucus per anatomic region (mm3 mm−3) | |||
| All airways | 3.9±1.1 | 2.3±0.6 | 0.185 |
| Axial bronchi | 0.8±0.1 | 0.6±0.2 | 0.122 |
| Non-terminal bronchioles | 3.1±1.0 | 1.7±0.5* | 0.099 |
| % occlusion per anatomic region | |||
| All airways | 18±4.3 | 7.2±1.4 | 0.020* |
| Axial bronchi | 20.1±5.7 | 11.7±2.6 | 0.097 |
| Non-terminal bronchioles | 16.8±1.3 | 6.3±1.2 | 0.022* |
Values are means ± s.e.m.
p < 0.05 by unpaired t-test. N = 4 WT and 5 Muc5ac−/− mice.
We thus determined the extent and distribution of mucus occlusion percentage, a sensitive measure of the fractional volume of airspaces occupied by mucus. This was used previously to quantify the prevalent but heterogeneous airway plugging observed in fatal asthma, which showed show that >98% of airways in these patients were occluded with mucus by 20% or more15. In Muc5ac−/− airways, overall mean lumen occlusion percentage was reduced by ~60% (Table 1 and Fig. 7c). Similar to human asthma15, plugging was also heterogeneous in mice (Fig. 7d). In WT airways, median occlusion was 6.7% compared to 0% in Muc5ac−/− airways. Furthermore, in WT mice only 12% of airways were occlusion free, and 32% were occluded by ≥20%. In Muc5ac−/− mice, 51% of airways were occlusion free, and only 8.2% were occluded by ≥20%.
To further dissect the sources of heterogeneous obstruction, we determined the distribution of occlusions by airway generation. In WT mice, occlusions of ≥20% were common in axial bronchi (50% of airways) and bronchioles (31% of non-terminal airways) (Fig. 7e,f). In Muc5ac−/− mice, occlusions of ≥20% were present in only 17.4% of axial bronchi (2.7-fold reduction) and 7.2% of bronchioles (4.6-fold reduction). Thus, disruption of the Muc5ac gene in mice resulted in protection from a heterogeneous airway plugging phenotype that was similar to the pathology observed in fatal human asthma15.
Discussion
AHR in asthma is currently thought to result primarily from inflammation-triggered ASM hypercontractility1. However, there is growing evidence that in addition to ASM-driven narrowing, obstruction is also mediated by significant non-contractile factors2,4,8,44. Here we report that in allergically inflamed mouse lungs, Muc5ac is an essential non-contractile mediator of AHR. These findings have important implications with respect to mechanisms of asthma pathophysiology. They could also inform the development of more effective treatments that target MUC5AC. These could especially benefit patients in whom bronchodilators or corticosteroids are ineffective1,45.
In our studies, mucin secretion and ASM contraction were both required for AHR. Although there were C57BL/6 and BALB/c strain-specific differences between central airway and peripheral tissue responses, there was no residual AHR to MCh in Muc5ac−/− mice at whole lung or partitioned airway and tissue scales. Thus, our data challenge the notion that AHR in allergic asthma is dominated by exaggerated ASM contraction. That dogma is grounded on studies showing ASM hypertrophy in pathological tissues, hypercontractility of excised ASM from asthma patients, and on protection provided by bronchodilating β-agonist treatments1. Such findings show association and suggest necessity for ASM hypercontractility in AHR, but they do not prove its sufficiency. Our studies also showed that inducing mucin secretion without bronchoconstriction did not evoke AHR. Thus, in mice, while ASM contraction and mucin secretion are both necessary for acute AHR, neither alone is sufficient. Rather, ASM contraction and mucin secretion mediate AHR cooperatively. These findings highlight the need to better understand coordinated contractile and non-contractile mechanisms of airflow obstruction in asthma2.
In addition to finding that ASM contraction and Muc5ac secretion were both required for AHR, we also found an apparent interdependence between mucus secretion and ASM-mediated airway constriction. Airway diameters and ASM thickness were both significantly reduced in allergic Muc5ac−/− lungs after MCh (Fig. 6). This may reflect effects of mucus secretion on airway surface tension and the propensity for airway closure, or it may demonstrate the ability of airways with less mucus to remain open following deep lung inflation maneuvers that are administered between MCh doses in our experimental set up. In asthma patients, these could affect the ability of deep breaths to maintain open airways. Alternatively, during dose-response tests in our studies, enhanced passage of aerosolized MCh through open airways in Muc5ac−/− mice could have increased its deposition in the terminal bronchioles, which have little to no smooth muscle or mucin expression and thus contribute minimally to AHR. Lastly, changes in ASM constriction could have been related to changes in ASM hypertrophy in allergic Muc5ac−/− mice. However, this is unlikely since the total amount of ASM was not different between allergic WT and Muc5ac−/− mice (Supplementary Table 2). Nonetheless, further investigation is needed to determine the potential significance and mechanisms of these intriguing interactions between ASM and non-contractile components.
To this end, other non-contractile factors of AHR have been studied2,3,8. Plasma extravasation and fibrin accumulation can reduce airway liquid surface tension and cause acute airway closure3. Studies in animal and computational models present this alternative to ASM dominance as heterogeneous closure occurring via “sudden liquid bridge formation”2,8. Our findings are consistent with this conceptual framework, which we advance by defining Muc5ac as a key molecular mediator. Indeed, it is plausible that aqueous-soluble factors become enriched within hydrated and heterogeneously distributed Muc5ac-containing mucus aggregates. Alternatively, each could occur independently yet synergize to mediate airway closure during bronchoconstriction.
Our studies also demonstrated that Muc5ac mediates AHR independently of the degrees of allergic inflammation. Differences in genetic and stimulus dependent allergic inflammation were striking (Fig. 5, Supplementary Fig. 3, and Supplementary Table 1). However, even though inflammation is critical for allergic AHR30,31, and secreted mucins can modulate immune function and resolving inflammation25,26,46, eosinophils in OVA and AOE challenged Muc5ac−/− lungs did not differ from WT (Fig. 5). By contrast, mucus plugs throughout Muc5ac−/− airways were significantly reduced in abundance and severity (Fig. 7). Thus, protection from AHR was mediated not by altered inflammation but through reduced plug formation: In constricting airways, acute physical obstruction caused by Muc5ac was essential for AHR. This segregation between Muc5ac function and preserved type 2 inflammation is consistent with in vivo findings in nematode infected mice. “Allergic” type 2 inflammation stems from off-target anti-helminth responses. In nematode infected Muc5ac−/− mice, even though intestinal type 2 inflammation is sustained, animals do not effectively control infections in the absence of Muc5ac37. Rather than affecting inflammation, the type 2 inflammation effector Muc5ac (but not the homeostatic intestinal mucin Muc2) directly impairs worm viability37. It remains to be determined whether the mechanisms by which Muc5ac selectively inhibits worm growth also promote mucus aggregation and plugging in the airways.
Indeed, biochemical/biophysical mechanisms by which secreted mucus occludes airways in asthma are poorly understood. Our current understanding of mucins in asthma is largely limited to anecdotal evidence of altered physical properties19, to associations drawn from pathological studies showing increased goblet cell numbers and mucin contents in biopsies from a wide range of disease severities4,47, and to pervasive airway plugging in autopsy specimens obtained from fatal cases15-17. Some studies do show that patients have persistently abnormal mucus and mucociliary clearance4,11,19,20,23,48,49. However, the obstructive consequences of mucin secretion in AHR and non-fatal exacerbations have remained unclear. This may be due in part to a lack of appreciation of interrelated ASM contractile and epithelial secretory processes. MCh induces both ASM contraction and epithelial mucin secretion21,22. In the airways, mucins become hydrated50, swelling to produce a thick gel that amplifies the obstructive effects of narrowing initiated by ASM2,4. Applying strategies that combine relaxing ASM with reducing mucin production and secretion and/or enhancing mucociliary clearance could improve disease treatment.
While potentially beneficial, clearance-enhancing mucolytics and expectorants have been disregarded as asthma treatments. At achievable doses, existing therapies such as hypertonic saline and N-acetylcysteine show poor efficacy and tolerability because the high concentrations that are needed to clear mucus plugs are irritating and cause bronchoconstriction4,51-53. For example, although N-acetylcysteine is the only FDA-approved reducing agent available as an inhaled adjunct mucolytic, clinical data do not support its use54, reflecting its low mucolytic activity at high mucin concentrations55,56 and at neutral airway pH (7.14 in mice57). We speculate that improved inhaled mucolytic agents can reduce plugging and AHR in asthma. However, it will be critical for any strategy that reverses or prevents obstruction, to do so safely while also protecting mucus-mediated defense.
Muc5ac is one of two polymeric mucins that are the predominant glycoproteins in airway mucus. The other, Muc5b, is expressed abundantly at baseline and remains present following allergic inflammation in mice (Figs. 1a,b). Muc5b is critical for homeostatic defense. Muc5b−/−, but not Muc5ac−/−, mice have severely impaired mucociliary clearance functions, resulting in accumulated particles and bacteria in the airways, and ultimately in death due to uncontrolled infection25. In humans with asthma, as MUC5AC expression increases, MUC5B expression either remains stable or decreases dramatically10,12, with divergent MUC5AC:MUC5B expression ratios associated with worse AHR10. Thus, targeting MUC5AC, while preserving or enhancing MUC5B, should be considered for any improved mucolytic therapies.
Two possible means for controlling the effects of MUC5AC on AHR are through targeting gene expression mechanisms or the glycoprotein itself. Critical expression signals include IL-13 and epidermal growth factor receptor (EGFR) ligands34-36. A monoclonal anti-IL-13 antibody has shown promise in early human trials29, and a small molecule inhibitor of MAPK13 was effective in allergic mice58. However, a recent trial of EGFR blockade in COPD failed because at doses that achieved minimal changes in mucin production, adverse effects occurred59. Transcriptional control of MUC5AC/Muc5ac gene expression through regulators including SPDEF (SAM pointed domain-containing Ets transcription factor), Notch, and Hypoxia Inducible Factor-124,60-62 have also been identified, but they are not currently targetable.
As an alternative to controlling gene expression, more selective results could potentially be yielded by disrupting the secreted MUC5AC polymer or by blocking its assembly. Recent studies have revealed mechanisms for controlling mucin disulfide bond-mediated polymerization63 and patterns of selective mucin glycosylation23,49. These may provide better targets for preventing mucus aggregation and airway plugging acutely. Specifically, by determining which glycan or oligomerization mechanisms are specific for MUC5AC versus MUC5B, novel strategies could be designed to reduce AHR by selectively inhibiting MUC5AC-mediated plugging while maintaining MUC5B-mediated airway defense. Future studies are directed at investigating these possible mechanisms. Since excessive MUC5AC is also a common problem in airway diseases such as chronic obstructive pulmonary disease and cystic fibrosis, whose inflammation and MUC5AC gene induction pathways differ significantly from allergic asthma, targeting the physical glycoprotein and polymeric properties of MUC5AC could have broader therapeutic applications.
Methods
Experimental animals
Studies were performed with approval of the animal care and use committees of the University of Colorado (approval number 97412(01)E) and the MD Anderson Cancer Center (approval numbers 05-04-046, 05-05-04334, and 00001214-RN00). Muc5ac−/− mice were generated previously37. Mice were backcrossed onto congenic C57BL/6 and BALB/c lines using marker assisted analysis at the MD Anderson Research Animal Support Facility-Smithville, Animal Genetics Services lab. After achieving 100% carriage of 77 C57BL/6 and 82 BALB/c strain specific markers, lines were backcrossed 3-5 additional generations. Mice were either bred as homozygous nulls or heterozygotes. C57BL/6 and BALB/c mice from the Jackson Labs (Bar Harbor, ME) were used for congenic backcrossing and as WT controls. Male and female mice were used starting at 7-8 wks age. Before and after exposures, mice were housed under specific pathogen-free conditions in ventilated cages (≤5 mice per cage) with a 12 h light/dark cycle and with food and water available ad libitum. Mice were placed into experimental and control groups chronologically.
Ovalbumin and Aspergillus oryzae induced lung inflammation
For studies in ovalbumin challenged mice, animals were sensitized i.p. with 4 weekly injections of 20 μg ovalbumin, 2.25 mg alum in saline (Sigma, St. Louis, MO). Seven days after the last injections, sensitized mice were then exposed to 5 ml of a 2.5% (w/v) ovalbumin aerosol delivered until the full volume was administered9. For A. oryzae extract (AOE) challenge, mice were exposed by nose-only inhalational challenge. AOE is a commercially available fungal protease/peptidase complex produced by submerged fermentation of Aspergillus oryzae (catalog no. P6110 Sigma). Although marketed as a “protease,” our own microscopic analysis shows that it is a crude extract rich in fungal particles, although no live organisms were detected when samples were cultured. In addition, endotoxin levels were <50 units/ml (Endosafe-PTS, Charles River Laboratories, Wilmington, MA). AOE was used previously to elicit allergic inflammation in mice following bolus intranasal application38. For aerosol exposures here, a 5 ml volume of a 10% v/v solution of AOE was made in PBS containing 2.5% w/v ovalbumin (Sigma) and 1% v/v antifoam A (Sigma). Aerosols were generated using an Ultravent jet nebulizer, which when driven at 30 psi, generates particles with a mean mass aerodynamic diameter of <1 μm64. In mice, >80% of the particles generated are respirable and deposit in the lower airways efficiently64. Exposures were made in custom built nose-only chambers and lasted until a 5 ml volume was delivered (40-45 min). Mice were AOE challenged once weekly for 4 wks. Allergic inflammation was confirmed by lung lavage and Giemsa staining. Muc5ac mRNA levels were determined by qPCR as described previously24.
Airway hyperreactivity
Lung function was measured 72 h post OVA challenge or 48 h post AOE challenge using a flexiVent (Scireq, Montreal, Quebec, Canada). Mice were anesthetized with 2.0 g/kg urethane (i.p.). This was sufficient to provide up to 4 h of sedation, though experiments lasted only 20-40 min. Mice were tracheostomized with a blunt beveled 18 G Luer stub adapter. Once ventilated (150 breaths/min, 10 ml/kg, against 3 cmH2O positive end expiratory pressure - PEEP), mice were paralyzed by continuous infusion of succinylcholine chloride (10 μg/g/min, i.p.). Baseline total lung resistance (RL) using a single compartment model and the input impedance parameters, Newtonian (airway) resistance (RAW), tissue resistance (GTI), and tissue elastance (HTI) were determined. Changes in RL, RAW, GTI, and HTI in response to successive, increasing doses of methacholine chloride (MCh; Sigma, St. Louis, MO, Cat no. A2251; 0.1-10 mg/ml) or uridine triphosphate trisodium salt (UTP; Sigma, Cat no. 94370; 0.1-100 mg/ml) administered by an in-line ultrasonic nebulizer were assessed. Heart rates were monitored by EKG. At 10 mg/ml MCh, heart rates often fell below 200 beats/min and did not recover. At this point, no further dose response measurements were made.
Mucin production and secretion
To assess mucin production, mouse lungs were fixed by intratracheal formalin administration and stained using periodic acid fluorescent Schiff’s (PAFS) to assess and quantify intracellular glycoconjugates9,65. To assess secretion, WT C57BL/6 mice were OVA challenged, ventilated on a flexiVent, and exposed to inhaled UTP to induce secretion (0.1-100 mg/ml) or inhaled saline to control for ventilator or vehicle induced secretion (7 doses repeated over the same intervals used for UTP). After UTP or saline exposures, lungs were immediately fixed intratracheally with formalin. Acute mucin secretion in airway tissues was assessed by measuring changes retained PAFS positive contents. Secretion was defined as decreased staining due to acute depletion of intracellular stores in UTP vs. saline treated animals.
Mucus plugging and airway measurements
Tissues were fixed by adapting a method previously used to preserve colonic mucus using methanol based Carnoy’s fixative (methacarn)43. Methacarn is a highly permeant non-crosslinking preservative that rapidly penetrates tissues transmurally while minimizing mucus disruption and tissue shrinkage43. Animals were carried through full dose response curves. During peak increases in RL following 10 mg/ml MCh, mice were rapidly euthanized by anesthetic overdose and cessation of mechanical ventilation. Mice remained connected to the ventilator, which by default stopped at end-expiration (3 cm H2O PEEP). Skin covering the lower sternum was removed, and 400 μl of methacarn was injected into the pleural space. The trachea was ligated immediately below the tracheal cannula. These steps took less than 15 seconds, thus minimizing changes in airway constriction and mucus transport. Lungs were fixed in situ for 30-60 min, then removed and immersion fixed with the trachea still ligated for 24-48 h in methacarn at 4°C. Tissues were transferred to 100% methanol (changed twice over a 48 h period). While remaining fully immersed in methanol, left lungs were sectioned into uniform 1.92 mm thick filets and imaged to calculate volume by Cavalieri estimation (319 ± 31 mm3 and 322 ± 24 mm3 for WT and Muc5ac−/− mice, respectively). Tissues were then paraffin processed and sectioned (5 μm thickness).
Histological assessments were made using AB-PAS and PAFS to assess total mucin, Muc5ac (clone 45M1, 1:100 dilution), and MUC5B (custom polyclonal antisera, 1:2,000) production37,65,66. Intracellular mucin content levels and the extent of mucus plugging were examined by systematic uniform random sampling67. Stained slides were scanned by two blinded investigators using an Olympus BX63 microscope and cellSens software (Olympus USA, Center Valley, PA). At least 5 axial bronchi per mouse were analyzed. For non-axial “bronchiolar” airways, samples were analyzed by selection of every fifth 20× image in a continuous x−/y− map of all lung samples per slide. Mucin and airway area were calculated using point-grid counting. Basement membrane length and airway circumferences were measured in cellSens. Data were calculated as volume densities (volume per unit surface area of basement membrane)9,65 and as extracellular mucus volume fractions relative to lung and airway volumes (multiplied by 100 to express as % occlusion)67.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 5.04 with a p-value cut-off of 0.05 considered significant. Inflammation, mucin production, and mucin secretion data were compared between groups using unpaired one-way student’s t-tests with corrections made for unequal variances (Welch’s), or non-Gaussian distribution (Mann-Whitney) where appropriate. For multiple comparisons, ANOVA with a Dunnett post-hoc correction or Kruskal-Wallis test for non-Gaussian distribution was used. For dose response tests, curves were compared using ANOVA for repeated measures, and using ANOVA with a Dunnett post-hoc correction to compare slopes after linear regression against log-transformed MCh doses. Both analyses of dose response tests provided congruent results. Sample sizes were chosen based on the numbers of WT saline vs. allergic mice required to achieve statistically significant difference.
Supplementary Material
Acknowledgements
We thank G. Francis, F. Benavides, and J. Parker-Thornburg for their assistance in generating mutant and congenic mice. Discussions with Dr. W.M. Foster of the Duke University Medical Center were helpful in the design of our aerosol system. This work was supported by the National Institutes of Health Grant R01HL080396, R21ES023384 (C.M.E.); R21HL120770 (D.A.S.); UH2HL123442 (C.M.E. and D.A.S.); R01HL097000 (B.F.D.); R01HL070952 (M.R.B.); R01DK097075, R01HL092188, R01HL098294, and R01HL119837 (H.K.E.); P01HL114457 (M.R.B. and H.K.E.); R01HL109517 (W.J.J.); and grants by the American Heart Association 14GRNT19990040 (C.M.E.) and the Crohn’s and Colitis Foundation of America (H.K.E.). T.A.W. was supported by the intramural research program at the National Institute of Allergy and Infectious Diseases/National Institutes of Health. Additional support was provided by National Institutes of Health Cancer Center Support Grants P30CA016672 (M.D. Anderson Cancer Center, including the Research Animal Support Facility-Smithville, Animal Genetics Services) and P30CA046934 (University of Colorado).
D.S. R, F.T., D.R.L., A.A.F., D.N.H., M.A.McG., M.M.McE., O.W.W., E.S., and M.G.R. bred animals, performed exposures, performed studies of lung function, inflammation, and mucin production/secretion. K.N.K., T.A.W., H.K.E., M.R.B., M.J.T., D.A.S., and B.F.D. assisted in study design and data analysis. C.M.E. performed conceptualized the study, performed, experiments, and wrote the manuscript.
Footnotes
None of the authors had any Conflicts of Interest to declare.
References
- 1.Fanta CH. Asthma. N Engl J Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. doi:10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 2.Bosse Y, Riesenfeld EP, Pare PD, Irvin CG. Annu Rev Physiol. 2010;72:437–462. doi: 10.1146/annurev-physiol-021909-135851. doi:10.1146/annurev-physiol-021909-135851. [DOI] [PubMed] [Google Scholar]
- 3.Wagers SS, et al. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin.Invest. 2004;114:104–111. doi: 10.1172/JCI19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy JV, Dickey BF. Airway mucus function and dysfunction. New England Journal of Medicine. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundblad LK, et al. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–774. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy D, Milic-Emili J. Closing volume in asymptomatic asthma. The American review of respiratory disease. 1973;107:559–570. doi: 10.1164/arrd.1973.107.4.559. [DOI] [PubMed] [Google Scholar]
- 7.Wagers SS, et al. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol (1985) 2007;102:221–230. doi: 10.1152/japplphysiol.01385.2005. doi:10.1152/japplphysiol.01385.2005. [DOI] [PubMed] [Google Scholar]
- 8.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl.Physiol. 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 9.Evans CM, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J.Respir.Cell Mol.Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. doi:10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest(J) 2002;122:320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 12.Ordonez CL, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. doi:10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 13.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001;163:511–516. doi: 10.1164/ajrccm.163.2.2001038. [DOI] [PubMed] [Google Scholar]
- 14.Zudhi Alimam M, et al. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. American Journal of Respiratory Cell and Molecular Biology. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 15.Kuyper LM, et al. Characterization of airway plugging in fatal asthma. Am.J.Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 17.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J.Clin.Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Collaborative Study on the Genetics of Asthma A genome-wide search for asthma susceptibility loci in ethnically diverse populations. The Collaborative Study on the Genetics of Asthma (CSGA) Nat Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. doi:10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan JK, Richardson PS, Fung DC, Howard M, Thornton DJ. Analysis of respiratory mucus glycoproteins in asthma: a detailed study from a patient who died in status asthmaticus. Am.J Respir.Cell Mol.Biol. 1995;13:748–756. doi: 10.1165/ajrcmb.13.6.7576713. [DOI] [PubMed] [Google Scholar]
- 20.Innes AL, et al. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180:203–210. doi: 10.1164/rccm.200807-1056OC. doi:10.1164/rccm.200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat.Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 22.Foster WM, et al. MARCKS-related peptide modulates in vivo the secretion of airway Muc5ac. Am J Physiol Lung Cell Mol Physiol. 2010;299:L345–352. doi: 10.1152/ajplung.00067.2010. doi:10.1152/ajplung.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem.J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young HW, et al. Central Role of Muc5ac Expression in Mucous Metaplasia and Its Regulation by Conserved 5′; Elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy MG, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. doi:10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koeppen M, et al. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal immunology. 2013;6:762–775. doi: 10.1038/mi.2012.114. doi:10.1038/mi.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busse WW, Lemanske RF., Jr Asthma. New England Journal of Medicine. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 28.Holt PG, Sly PD. Th2 cytokines in the asthma late-phase response. Lancet. 2007;370:1396–1398. doi: 10.1016/S0140-6736(07)61587-6. doi:10.1016/S0140-6736(07)61587-6. [DOI] [PubMed] [Google Scholar]
- 29.Corren J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. doi:10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 31.Grunig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. The Journal of allergy and clinical immunology. 2003;111:677–690. doi: 10.1067/mai.2003.1333. quiz 691. [DOI] [PubMed] [Google Scholar]
- 33.Perkins C, et al. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. The Journal of experimental medicine. 2011;208:853–867. doi: 10.1084/jem.20100023. doi:10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuperman DA, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat.Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 35.Zhen G, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol.Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeyama K, et al. Epidermal growth factor system regulates mucin production in airways. Proc.Natl.Acad.Sci.U.S.A. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasnain SZ, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. The Journal of experimental medicine. 2011;208:893–900. doi: 10.1084/jem.20102057. doi:10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kheradmand F, et al. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;281:L394–402. doi: 10.1152/ajplung.2001.281.2.L394. [DOI] [PubMed] [Google Scholar]
- 40.Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. British journal of pharmacology. 1991;103:1053–1056. doi: 10.1111/j.1476-5381.1991.tb12299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lethem MI, et al. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol.Biol. 1993;9:315–322. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- 42.Govindaraju V, Martin JG, Maghni K, Ferraro P, Michoud MC. The effects of extracellular purines and pyrimidines on human airway smooth muscle cells. The Journal of pharmacology and experimental therapeutics. 2005;315:941–948. doi: 10.1124/jpet.105.089698. doi:10.1124/jpet.105.089698. [DOI] [PubMed] [Google Scholar]
- 43.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc.Natl.Acad.Sci.U.S.A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundgren JD, Vestbo J. The pathophysiological role of mucus production in inflammatory airway diseases. Respir Med. 1995;89:315–316. doi: 10.1016/0954-6111(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 45.Israel E, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. doi:10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 46.Shan M, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. doi:10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hogg JC. The pathology of asthma. Clinics in chest medicine. 1984;5:567–571. [PubMed] [Google Scholar]
- 48.O’Riordan TG, Zwang J, Smaldone GC. Mucociliary clearance in adult asthma. The American review of respiratory disease. 1992;146:598–603. doi: 10.1164/ajrccm/146.3.598. doi:10.1164/ajrccm/146.3.598. [DOI] [PubMed] [Google Scholar]
- 49.Innes AL, et al. The H antigen at epithelial surfaces is associated with susceptibility to asthma exacerbation. Am J Respir Crit Care Med. 2011;183:189–194. doi: 10.1164/rccm.201003-0488OC. doi:10.1164/rccm.201003-0488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a009597. doi:10.1101/cshperspect.a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daviskas E, et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur Respir J. 1996;9:725–732. doi: 10.1183/09031936.96.09040725. [DOI] [PubMed] [Google Scholar]
- 52.Falliers CJ, Cato A. Controlled trial of bronchodilator-mucolytic aerosols, combined and separate. Annals of allergy. 1978;40:77–83. [PubMed] [Google Scholar]
- 53.Rao S, Wilson DB, Brooks RC, Sproule BJ. Acute effects of nebulization of N-acetylcysteine on pulmonary mechanics and gas exchange. The American review of respiratory disease. 1970;102:17–22. doi: 10.1164/arrd.1970.102.1.17. [DOI] [PubMed] [Google Scholar]
- 54.Tam J, Nash EF, Ratjen F, Tullis E, Stephenson A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. The Cochrane database of systematic reviews. 2013;7 doi: 10.1002/14651858.CD007168.pub3. CD007168, doi:10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillissen A, et al. Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitro. Respir Med. 1997;91:159–168. doi: 10.1016/s0954-6111(97)90052-4. [DOI] [PubMed] [Google Scholar]
- 56.Suk JS, et al. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19:1981–1989. doi: 10.1038/mt.2011.160. doi:10.1038/mt.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jayaraman S, Song Y, Verkman AS. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol Cell Physiol. 2001;281:C1504–1511. doi: 10.1152/ajpcell.2001.281.5.C1504. [DOI] [PubMed] [Google Scholar]
- 58.Alevy YG, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122:4555–4568. doi: 10.1172/JCI64896. doi:10.1172/JCI64896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodruff PG, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:438–445. doi: 10.1164/rccm.200909-1415OC. doi:10.1164/rccm.200909-1415OC. [DOI] [PubMed] [Google Scholar]
- 60.Tsao PN, et al. Notch signaling prevents mucous metaplasia in mouse conducting airways during postnatal development. Development. 2011;138:3533–3543. doi: 10.1242/dev.063727. doi:10.1242/dev.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen G, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. doi:10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park KS, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. doi:10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schroeder BW, et al. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol. 2012;47:178–185. doi: 10.1165/rcmb.2011-0421OC. doi:10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster MW, Yang Z, Potts EN, Michael Foster W, Que LG. S-nitrosoglutathione supplementation to ovalbumin-sensitized and -challenged mice ameliorates methacholine-induced bronchoconstriction. Am J Physiol Lung Cell Mol Physiol. 2011;301:L739–744. doi: 10.1152/ajplung.00134.2011. doi:10.1152/ajplung.00134.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Piccotti L, Dickey BF, Evans CM. Assessment of intracellular mucin content in vivo. Methods Mol Biol. 2012;842:279–295. doi: 10.1007/978-1-61779-513-8_17. doi:10.1007/978-1-61779-513-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy MG, et al. Mucin production during prenatal and postnatal murine lung development. Am J Respir Cell Mol Biol. 2011;44:755–760. doi: 10.1165/rcmb.2010-0020OC. doi:10.1165/rcmb.2010-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. doi:10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.