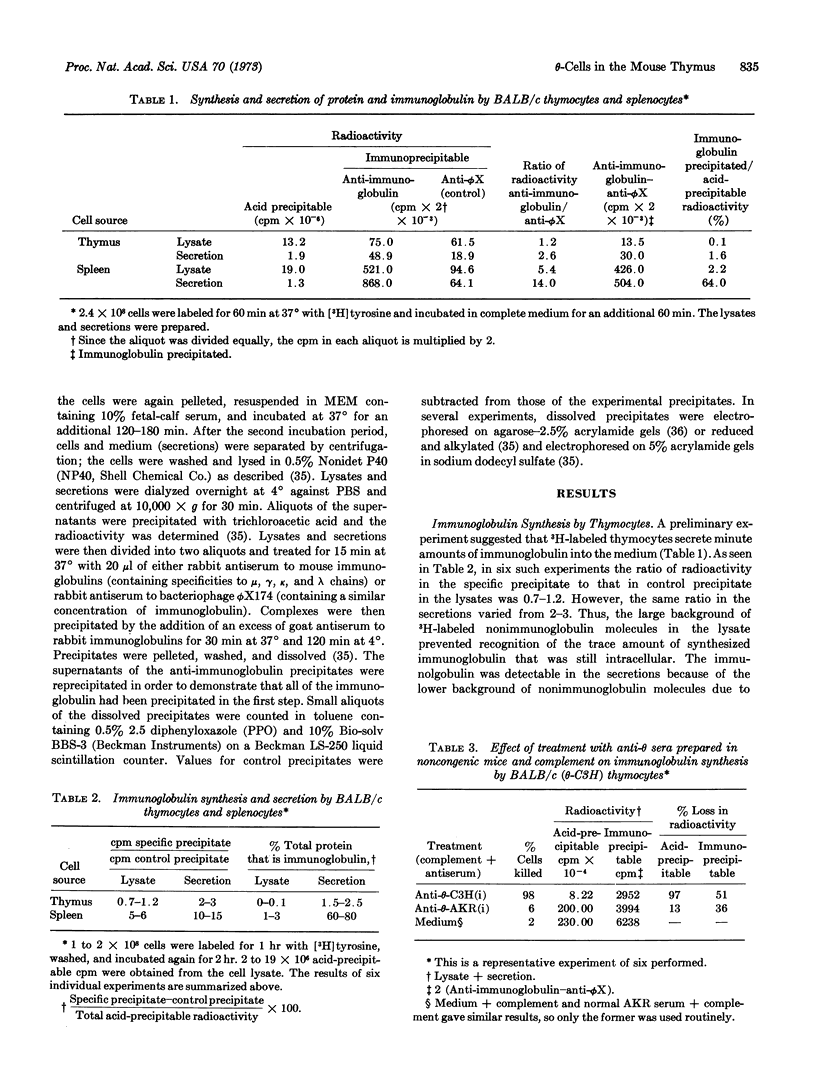
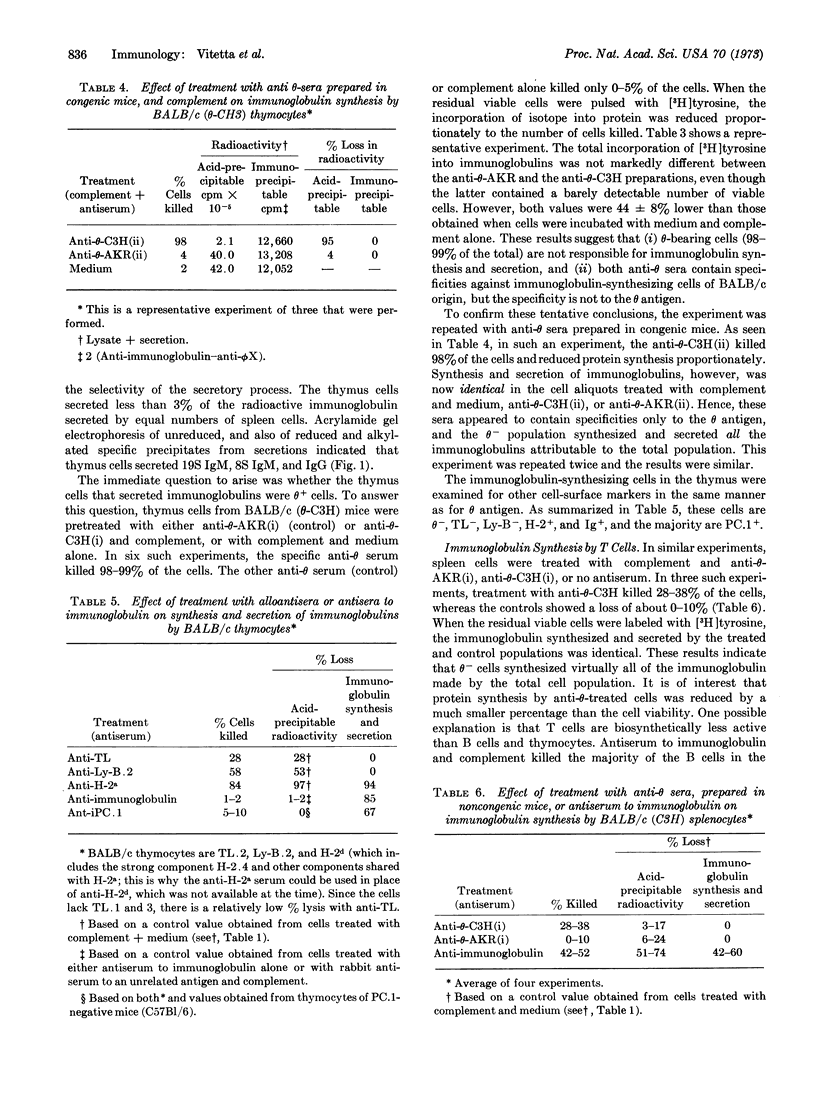
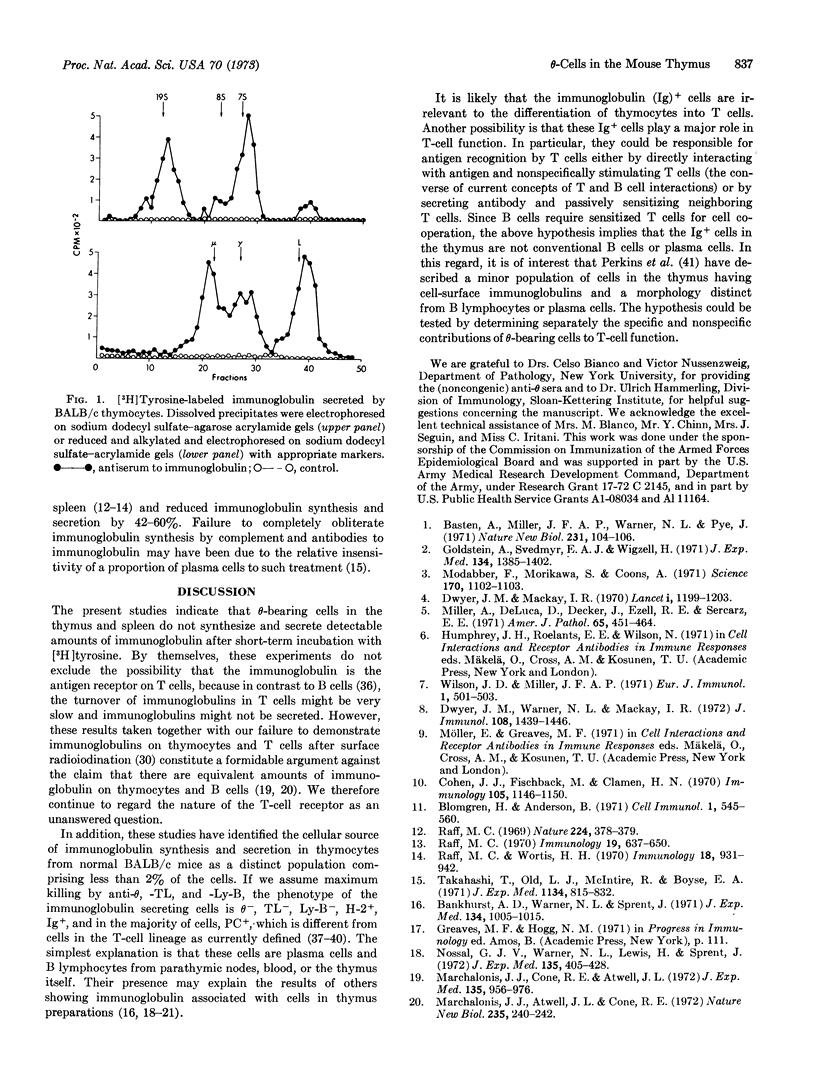
Abstract
The mouse thymus contains cells that synthesize and secrete minute amounts of immunoglobulin (Ig). These cells were studied by a combination of cytotoxic tests (with alloantisera prepared in congenic strains of mice) followed by pulse labeling of the surviving cell population with [3H]tyrosine. The immunoglobulin-synthesizing cells constitute less than 2% of the total cell population and are θ-, TL-, Ly-B-, Ig+, H-2+, and PC+. Since this phenotype is not characteristic of cells of the thymocyte lineage, the Ig+ cells are probably plasma cells and/or bone marrow-derived (B) lymphocytes.
Similar studies of spleen cells indicate that immunoglobulin-synthesizing and secreting cells are θ-.
Keywords: T and B cells, cytotoxic tests
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankhurst A. D., Warner N. L., Sprent J. Surface immunoglobulins on thymus and thymus-derived lymphoid cells. J Exp Med. 1971 Oct 1;134(4):1005–1015. doi: 10.1084/jem.134.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Flaherty L., Stockert E., Old L. J. Histoincompatibility attributable to genes near H-2 that are not revealed by hemagglutination or cytotoxicity tests. Transplantation. 1972 Apr;13(4):431–432. doi: 10.1097/00007890-197204000-00013. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Ceppellini R., Bonnard G. D., Coppo F., Miggiano V. C., Popisil M., Pellegrino M., Curtoni E. S. Mixed leukocyte cultures and HL-A antigens. II. Inhibition by anti-HL-A sera. Transplant Proc. 1971 Mar;3(1):63–70. [PubMed] [Google Scholar]
- Cohen J. J., Fschbach M., Claman H. N. Hydrocortisne resistance of graft vs host activity in mouse thymus, spleen and bone marrow. J Immunol. 1970 Nov;105(5):1146–1150. [PubMed] [Google Scholar]
- Dwyer J. M., Mackay I. R. Antigen-binding lymphocytes in human fetal thymus. Lancet. 1970 Jun 6;1(7658):1199–1202. doi: 10.1016/s0140-6736(70)91787-3. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L., Mackay I. R. Specificity and nature of the antigen-combining sites on fetal and mature thymus lymphocytes. J Immunol. 1972 May;108(5):1439–1446. [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L., Mackay I. R. Specificity and nature of the antigen-combining sites on fetal and mature thymus lymphocytes. J Immunol. 1972 May;108(5):1439–1446. [PubMed] [Google Scholar]
- Golstein P., Erik M. D., Svedmyr A. J., Wigzell H. Cells mediating specific in vitro cytotoxicity. I. Detection of receptor-bearing lymphocytes. J Exp Med. 1971 Dec 1;134(6):1385–1402. doi: 10.1084/jem.134.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Inhibition of human mixed lymphocyte reaction by antibodies to immunoglobulin light chain determinants. Clin Exp Immunol. 1971 Sep;9(3):313–328. [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Rajewsky K. Evidence for surface-associated immunoglobulin on T and B lymphocytes. Eur J Immunol. 1971 Dec;1(6):447–452. doi: 10.1002/eji.1830010608. [DOI] [PubMed] [Google Scholar]
- Lance E. M., Cooper S., Boyse E. A. Antigenic change and cell maturation in murine thymocytes. Cell Immunol. 1970 Nov;1(5):536–544. doi: 10.1016/0008-8749(70)90040-7. [DOI] [PubMed] [Google Scholar]
- Lesley J. F., Kettman J. R., Dutton R. W. Immunoglobulins on the surface of thymus-derived cells engaged in the initiation of a humoral immune response. J Exp Med. 1971 Sep 1;134(3 Pt 1):618–629. doi: 10.1084/jem.134.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Atwell J. L., Cone R. E. Isolation of surface immunoglobulin from lymphocytes from human and murine thymus. Nat New Biol. 1972 Feb 23;235(60):240–242. doi: 10.1038/newbio235240a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason S., Warner N. L. The immunoglobulin nature of the antigen recognition site on cells mediating transplantation immunity and delayed hypersentivity. J Immunol. 1970 Mar;104(3):762–765. [PubMed] [Google Scholar]
- McDevitt H. O., Benacerraf B. Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. doi: 10.1016/s0065-2776(08)60477-0. [DOI] [PubMed] [Google Scholar]
- Miller A., DeLuca D., Decker J., Ezzell R., Sercarz E. E. Specific binding of antigen to lymphocytes. Evidence for lack of unispecificity in antigen-binding cells. Am J Pathol. 1971 Nov;65(2):451–465. [PMC free article] [PubMed] [Google Scholar]
- Modabber F., Morikawa S., Coons A. H. Antigen-binding cells in normal mouse thymus. Science. 1970 Dec 4;170(3962):1102–1104. doi: 10.1126/science.170.3962.1102. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. D., Karnovsky M. J., Unanue E. R. An ultrastructural study of lymphocytes with surface-bound immunoglobulin. J Exp Med. 1972 Feb 1;135(2):267–276. doi: 10.1084/jem.135.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Owen J. J. Thymus-derived lymphocytes: their distribution and role in the development of peripheral lymphoid tissues of the mouse. Eur J Immunol. 1971 Jan;1(1):27–30. doi: 10.1002/eji.1830010105. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Riethmüller G., Rieber E. P., Seeger I. Suppression of graft versus host reaction by univalent anti-immunoglobulin antibody. Nat New Biol. 1971 Apr 21;230(16):248–250. doi: 10.1038/newbio230248a0. [DOI] [PubMed] [Google Scholar]
- Rychlíková M., Démant P., Iványi P. Histocompatibility gene organization and mixed lymphocyte reaction. Nat New Biol. 1971 Apr 28;230(17):271–272. doi: 10.1038/newbio230271a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., McIntire K. R., Boyse E. A. Immunoglobulin and other surface antigens of cells of the immune system. J Exp Med. 1971 Oct 1;134(4):815–832. doi: 10.1084/jem.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Bianco C., Nussenzweig V., Uhr J. W. Cell surface immunoglobulin. IV. Distribution among thymocytes, bone mrrow cells, and their derived populations. J Exp Med. 1972 Jul 1;136(1):81–93. doi: 10.1084/jem.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Cell surface immunoglobulin. V. Release from murine splenic lymphocytes. J Exp Med. 1972 Oct 1;136(4):676–696. doi: 10.1084/jem.136.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Miller J. F. T and B rosette-forming cells. Eur J Immunol. 1971 Dec;1(6):501–503. doi: 10.1002/eji.1830010622. [DOI] [PubMed] [Google Scholar]



