Abstract
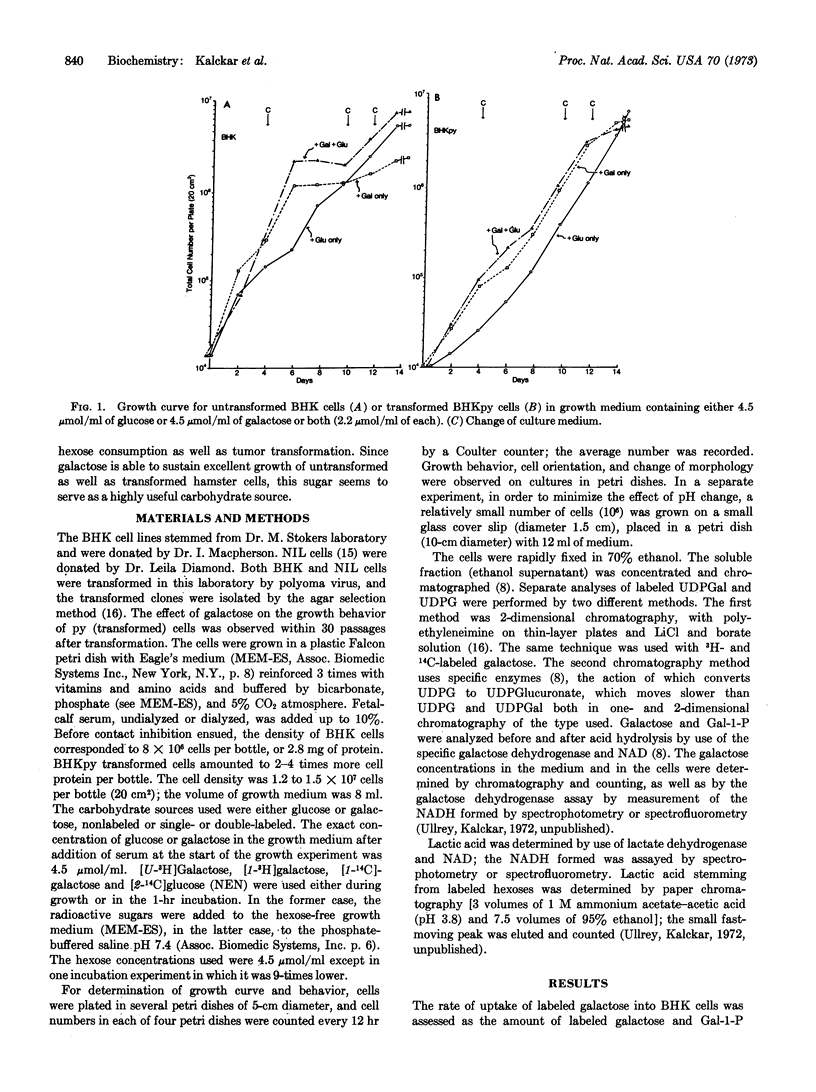
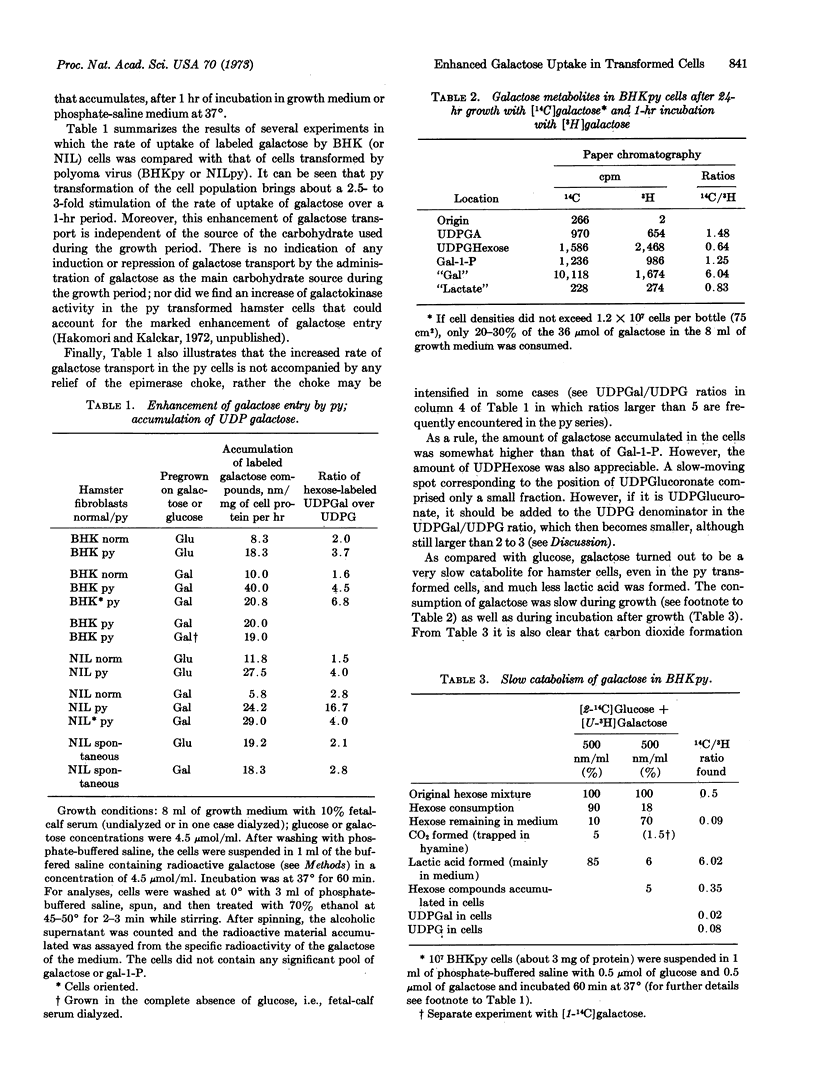
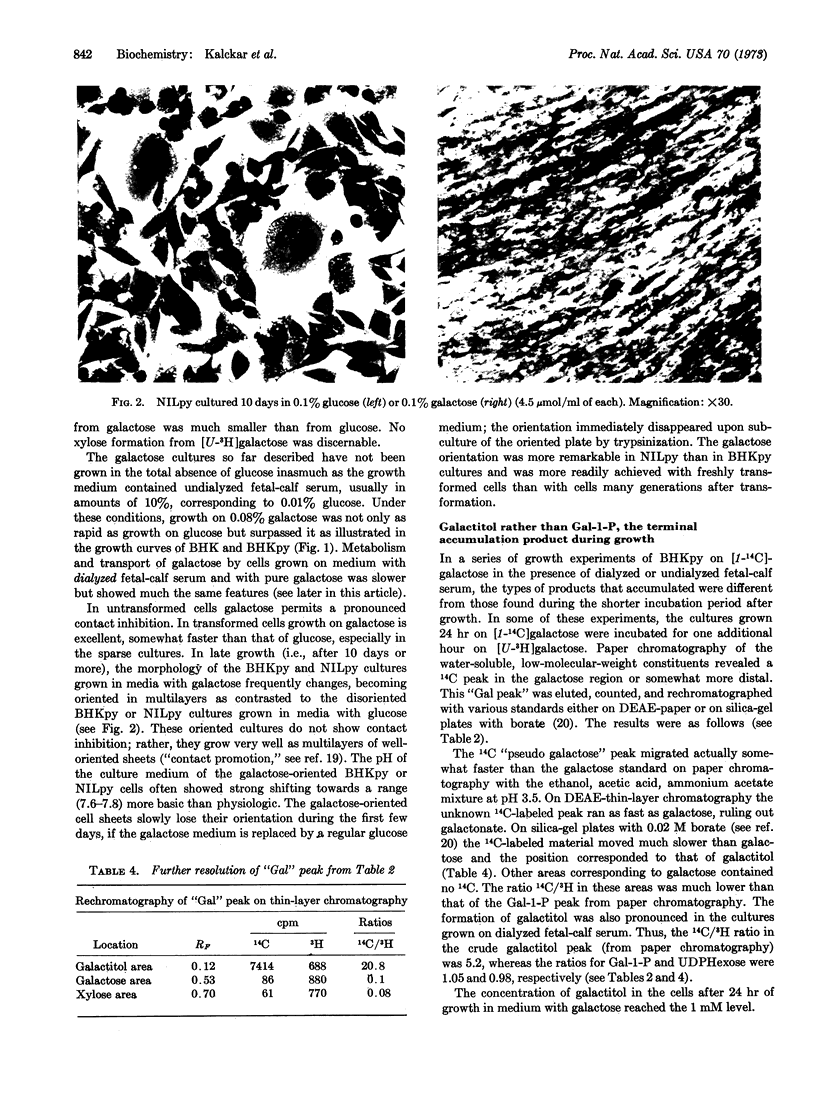
Untransformed as well as polyoma virus-transformed hamster cells can be grown equally well on a slow catabolite like galactose as on a rapid catabolite like glucose. The rate of uptake of galactose is greatly enhanced in the transformed cells as compared with untransformed cells, and this enhancement of entry was as markedly expressed in galactose-grown cultures as in glucose-grown cultures. Since the transformed cultures grown in glucose medium consume practically all of their carbohydrate, contrary to the galactose-grown cultures, problems dealing with regulation of transport by substrate concentrations have to be dealt with also. The galactose captured by the cells accumulates initially as galactose, α-galactose-1-phosphate, and UDP-galactose. However, after a 24-hr growth on a galactose growth medium, the product accumulated was almost exclusively galactitol. In spite of the enhancement of entry of galactose into the transformed cells, the metabolic pathway becomes stalled even before it has reached the stage of glucose-1-phosphate, largely due to a choke of the enzyme UDP-galactose-4-epimerase (EC 5.1.3.2). Among the sparse amounts of catabolic products generated by the transformed cells from galactose, carbon dioxide (allebeit no D-xylose) and lactic acid were found, both of them in much smaller amounts than seen if glucose is the carbohydrate source. Also, growth of transformed cells on a galactose medium gradually tends to become oriented, a phenomenon that could be called “contact promotion.” Subsequent addition of glucose disturbs the oriented growth and interferes with contact promotion.
Keywords: tumor transformation, nonglycolytic hexose, accumulation of galactitol
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh C. L., Tytell A. A. Propagation of the human diploid cell WI-38 in galactose medium. Life Sci. 1967 Feb 15;6(4):371–380. doi: 10.1016/0024-3205(67)90006-9. [DOI] [PubMed] [Google Scholar]
- Diamond L. Two spontaneously transformed cell lines derived from the same hamster embryo culture. Int J Cancer. 1967 Mar 15;2(2):143–152. doi: 10.1002/ijc.2910020209. [DOI] [PubMed] [Google Scholar]
- EAGLE H., BARBAN S., LEVY M., SCHULZE H. O. The utilization of carbohydrates by human cell cultures. J Biol Chem. 1958 Sep;233(3):551–558. [PubMed] [Google Scholar]
- EBNER K. E., HAGEMAN E. C., LARSON B. L. Functional biochemical changes in bovine mammary cell cultures. Exp Cell Res. 1961 Dec;25:555–570. doi: 10.1016/0014-4827(61)90190-2. [DOI] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- GOLDBERG E. B., NITOWSKY H. M., COLOWICK S. P. THE ROLE OF GLYCOLYSIS IN THE GROWTH OF TUMOR CELLS. IV. THE BASIS OF GLUCOSE TOXICITY IN OXAMATE-TREATED, CULTURED CELLS. J Biol Chem. 1965 Jul;240:2791–2796. [PubMed] [Google Scholar]
- Hakomori S. Cell density-dependent changes of glycolipid concentrations in fibroblasts, and loss of this response in virus-transformed cells. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1741–1747. doi: 10.1073/pnas.67.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Hatanaka M., Todaro G. J., Gilden R. V. Altered glucose transport kinetics in murine sarcoma virus-transformed BALB-3T3 clones. Int J Cancer. 1970 Mar 15;5(2):224–228. doi: 10.1002/ijc.2910050209. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSELBACHER K. J., ANDERSON E. P., KURAHASHI K., KALCKAR H. M. Congenital galactosemia, a single enzymatic block in galactose metabolism. Science. 1956 Apr 13;123(3198):635–636. doi: 10.1126/science.123.3198.635. [DOI] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALCKAR H. M., ROBINSON E. A. SOME ABERRATIONS IN METABOLIC PATTERNS OF MALIGNANT CELLS. Biochem Z. 1963;338:763–770. [PubMed] [Google Scholar]
- Kijimoto S., Hakomori S. Enhanced glycolipid: -galactosyltransferase activity in contact-inhibited hamster cells, and loss of this response in polyoma transformants. Biochem Biophys Res Commun. 1971 Aug 6;44(3):557–563. doi: 10.1016/s0006-291x(71)80119-5. [DOI] [PubMed] [Google Scholar]
- Kozak L. P., Wells W. W. Studies on the metabolic determinants of D-galactose-induced neurotoxicity in the chick. J Neurochem. 1971 Nov;18(11):2217–2228. doi: 10.1111/j.1471-4159.1971.tb05080.x. [DOI] [PubMed] [Google Scholar]
- Lie S. O., McKusick V. A., Neufeld E. F. Simulation of genetic mucopolysaccharidoses in normal human fibroblasts by alteration of pH of the medium. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2361–2363. doi: 10.1073/pnas.69.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- MAIO J. J., RICKENBERG H. V. Metabolic block in utilization of galactose by strain L tissue culture cells. Science. 1961 Oct 6;134(3484):1007–1008. doi: 10.1126/science.134.3484.1007. [DOI] [PubMed] [Google Scholar]
- MAXWELL E. S. The enzymic interconversion of uridine diphosphogalactose and uridine diphosphoglucose. J Biol Chem. 1957 Nov;229(1):139–151. [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau R., Kohlbacher M., Shaw S. N., Amos H. Enhancement of hexose entry into chick fibroblasts by starvation: differential effect on galactose and glucose. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3407–3411. doi: 10.1073/pnas.69.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. G., Donnell G. N., Hodgman J. E., Bergren W. R. Differences in uridine diphosphate galactose-4-epimerase between haemolysates of newborns and of adults. Nature. 1967 Apr 15;214(5085):283–284. doi: 10.1038/214283a0. [DOI] [PubMed] [Google Scholar]
- Robinson E. A., Kalckar H. M., Troedsson H., Sanford K. Metabolic inhibition of mammalian uridine diphosphate galactose 4-epimerase in cell cultures and in tumor cells. J Biol Chem. 1966 Jun 25;241(12):2737–2745. [PubMed] [Google Scholar]
- Sakiyama H., Gross S. K., Robbins P. W. Glycolipid synthesis in normal and virus-transformed hamster cell lines. Proc Natl Acad Sci U S A. 1972 Apr;69(4):872–876. doi: 10.1073/pnas.69.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENFELS K., KURZ G. [On the specificity of galactose dehydrogenase from Pseudomonas saccharophila and its use as an analytical aid]. Biochem Z. 1962;335:559–573. [PubMed] [Google Scholar]
- Weinstein A. N., Segal S. Thin-layer chromatographic separation of hexitols and corresponding hexoses. Anal Biochem. 1967 Sep;20(3):558–561. doi: 10.1016/0003-2697(67)90304-1. [DOI] [PubMed] [Google Scholar]