Abstract
In muscle and other mechanically active tissue, cell membranes are constantly injured and their repair depends on the injury induced increase in cytosolic calcium. Here we show that injury-triggered Ca2+ increase results in assembly of ESCRTIII and accessory proteins at the site of repair. This process is initiated by the calcium binding protein - Apoptosis Linked Gene (ALG)-2. ALG-2 facilitates accumulation of ALG-2 interacting protein X (ALIX), ESCRT III, and Vps4 complex at the injured cell membrane, which in turn results in cleavage and shedding of the damaged part of the cell membrane. Lack of ALG-2, ALIX, or Vps4B each prevents shedding, and repair of the injured cell membrane. These results demonstrate Ca2+-dependent accumulation of ESCRTIII-Vps4 complex following large focal injury to the cell membrane and identify the role of ALG-2 as the initiator of sequential ESCRTIII-Vps4 complex assembly that facilitates scission and repair of the injured cell membrane.
Introduction
Due to mechanical activity the myofiber’s cell membrane (sarcolemma) is exposed to constant injury1, 2. In the face of frequent injury, muscle cells depend on efficiently repairing tears in their sarcolemma – this is a Ca2+-dependent process2, 3. Cell membrane repair involves use of intracellular compartments, which in case of muscle cells include mitochondria4, lysosomes5, 6, and caveolae7. These compartments perform different functions during repair of the injured cell membrane. Mitochondria accumulate at the site of injury, lysosomes fuse with the injured cell membrane to secrete acid sphingomyelinase, and caveolae internalize pores in the injured cell membrane7-10. Thus intracellular compartments facilitate repair of injured cell membrane by various processes including clotting, patching or removing the damaged portions of the injured cell membrane10-12. The process of endocytic removal of plasma membrane wound by pore forming toxins (pores of <100nm) has been shown to involve exocytosis of lysosomes and endocytosis through caveolae as well as ESCRT-mediated budding of intraluminal vesicles in the late endosome8. Shedding (ectocytosis) of small (<100nm) plasma membrane wounds, facilitated by ESCRTs, has also been shown to aid in repair of such cell membrane wounds13,14.
Unlike pore forming toxins, exercise and other muscle injuries lead to large (micron scale) wounds in the cell membrane, but the processes leading to their repair is not well elucidated1, 15. Repair of large mechanical injury to muscle involves increased caveolar endocytosis7, which is initiated by acid sphingomyelinase (ASMase) released due to injury-triggered lysosomal exocytosis16. We found that muscle cell injury also triggers lysosome exocytosis resulting in ASMase secretion, a defect in which compromises repair of the injured myofibers6. In addition to triggering caveolar endocytosis, ASMase also causes shedding of plasma membrane vesicle17. However, role of plasma membrane shedding (ectocytosis) in repair of large cell membrane wound has not been established.
Irrespective of the size of the cell membrane wound and the cellular mechanism involved in its repair, a common feature of all of these processes is their dependence on the increase in cytosolic Ca2+. Thus, to understand the molecular process involved in repair of muscle cell membrane we analyzed Ca2+-induced changes in muscle cell membrane proteome. This identified that Ca2+ triggers accumulation of ESCRT complex at the cell membrane. Live imaging of muscle and other cells undergoing repair from large (>1μm) focal injury confirmed Ca2+-triggers translocation of ESCRTs and accessory proteins at the site of repair. Ca2+- and injury-triggered ESCRT accumulation is initiated by a calcium-binding protein ALG-2 (Apoptosis Linked Gene-2) and its interacting protein ALIX (ALG-2 interacting protein X)18. Injury triggered ESCRT assembly culminates in accumulation of Vps4, which aids in cleavage and shedding of the damaged cell membrane. Shedding and repair of injured cell membrane was prevented in cells lacking ALG-2, ALIX, or Vps4B. Our findings demonstrate repair of large cell membrane injury as a new role of ESCRT-mediated membrane scission. This adds to the known role of ESCRTs in the formation of intraluminal vesicles19, retrovirus budding20, 21, cytokinesis22-24, and repair of nanometer scale cell membrane wounds due to pore forming toxins8. Similar to the latter21, 24, 25, we find ESCRT-mediated cell membrane repair makes use of a non-ESCRT protein (ALG-2) to initiate assembly of ESCRTIII and Vps4 complexes. These results point to a role of ESCRT complex in repair of large injuries, and identify the mechanism for ESCRTIII assembly triggered by large cell membrane injury.
Results
Acute increase in Ca2+ changes cell membrane proteome
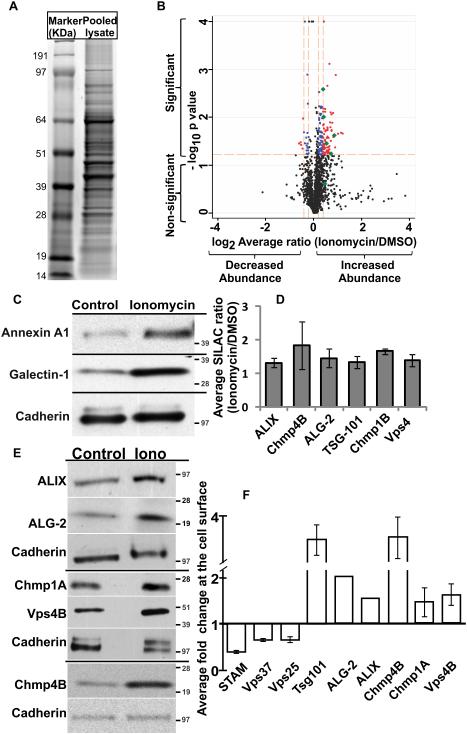
To quantify Ca2+-induced changes in the muscle cell membrane proteome we carried out mass spectrometry for quantitative proteomics with stable isotope labeling by amino acids in cell culture (SILAC). Using this approach we previously identified mitochondria as a cellular compartment needed for the repair of injured myofibers4. Here we extended this approach to mononucleated muscle cells and analyzed the change in cell membrane proteome of C2C12 myoblasts treated for 5 minutes with either 0.1% DMSO (control) or with 10μM Ca2+ ionophore - ionomycin. To obtain proteins reproducibly altered in response to ionomycin we carried out 6 replicate pairs such that in 5 replicates SILAC labeled cells were treated with ionomycin and in 1 replicate they were treated with DMSO. Each pair was then mixed and proteins were resolved by SDS-PAGE (Fig. 1A). This identified 1556 protein pairs that were detected in two or more replicates (Supplementary Data1). Averaging the ratio of extracted spectral intensity of each SILAC labeled and unlabeled peptide detected for a protein provided ionomycin-induced fold-change for each protein. The log2 fold change SD across the 1556 proteins was +/− 0.4. Next, we calculated each protein’s p value independently using one sample t-test to determine the statistical significance of difference in the values obtained for a given sample with respect to the null hypothesis of a change in ratio of 1. Proteins that differed significantly (p<0.06; one sample t-test vs ratio of 1.0) from an unaltered ratio of 1 (log2=0) were identified. Applying filters of p-value <0.06 and log2 SD +/− 0.2 (ratio cut-off of ≥1.15 or ≤0.87), narrowed the list to top 10% of altered proteins (130 increased and 24 decreased) (Supplementary Data 2, Fig. 1B). Due to the small fold changes, we attempted to validate Ca2+-triggered changes in the cell surface level of proteins reported by MS analysis independently by Western Blot (WB) analysis. We found that while the magnitude of change varies between MS and WB analyses, each of the dozen proteins assayed by WB analysis exhibited the same trend of Ca2+-triggered change at the cell surface level as predicted by MS analysis. Representative proteins that showed ionomycin induced increase in cell membrane level by MS analysis - Galectin-1 (fold change 1.76±0.01; p-value 0.008) and Annexin A1 (fold change 1.27±0.03; p-value 0.026) also showed increased level by WB analysis (Fig. 1C). Similarly proteins whose level did not change eg: Cadherins (fold change 0.94-1.1; p-value >0.72) (Supplementary Data1) were found to be unaltered by WB analysis using pan-Cadherin antibody (Fig. 1C).
Figure 1. Ca2+ triggers recruitment of ESCRTIII complex at the cell membrane.
SILAC labeled and unlabeled C2C12 myoblasts treated for 5 minutes with Ionomycin (10μM) or with DMSO (0.1%) were pooled, and the cell membrane proteins extracted. (A) A representative coomassie stained 1D SDS-PAGE gel (n=6) used for differential cell membrane proteomic analysis. The gel shows molecular weight marker and the cell membrane protein pool (Pooled lysate). The gel lane for pooled lysate was cut into slices and processed for proteomic analysis by mass spectrometry (MS). (B) Scatter (volcano) plot shows the fold change (log2 average ratio) and −log10 p value (one sample t-test vs ratio of 1.0) for the proteins quantified in at least two of the six independently replicated experiments. Black spots represent protein whose abundance was not altered in response to ionomycin-triggered Ca2+ increase or the alteration did not reach the designated statistical significance across replicates. Red symbols represent proteins with p<0.06 (one sample t-test vs ratio of 1.0) and fold change +/− 0.4 SD, while blue symbols mark proteins with p<0.06 and fold change +/− 0.2 SD. Green symbols show the ESCRT and related proteins that show fold change greater than +/− 0.2 SD. (C) Western blot analysis of change in the cell membrane level of representative non-ESCRT proteins that were identified by MS analysis to be increased (Annexin A1- and Galectin-1) or not altered (Cadherin) at the cell surface by ionomycin-induced Ca2+ increase. The molecular weight (KDa) markers are marked for each blot. (D) Plot showing average change in cell surface level of ESCRT proteins as assessed by MS analysis. Specific protein level were quantified by ion chromatogram of peptides (generated by IP2 Census quantitation software) such that the values for all peptides corresponding to a protein were averaged to obtain ratio shown for the abundance of this protein in Ionomycin versus DMSO treated cells – error bars represent SD. (E) Representative Western blots (n=3) with corresponding molecular weight (KDa) markers, and (F) averaged ratio for ionomycin triggered change in cell surface level of proteins in ESCRTIII and accessory proteins – error bars represent SE.
The list of up-regulated proteins identified by MS analysis included various endosomal proteins - notably ESCRT III members Chmp4B and Chmp1a were amongst the top 15 altered proteins (Supplementary Data2). Chmp6 level also increased by 1.2-fold, but did not reach the applied statistical threshold of p-value <0.06 (Supplementary Data1). We also detected ESCRT binding proteins – ALIX, Vps4 and ALG-2 (Fig. 1B (green symbol), Fig. 1D). However, we did not observe any proteins from traditional ESCRT 0, II and only member of the ESCRT I complex (Tsg-101). Therefore, we examined Ca2+-triggered changes in the cell surface level of all ESCRT complexes ESCRT0 (STAM) ESCRT I (Vps37) and ESCRT II (Vps25) by WB. We found that Ca2+ triggered increase in cell surface level of ESCRT III and interacting proteins (including Tsg-101), but did not increase the cell membrane level of all proteins tested for ESCRT 0-II complexes (Fig. 1E, F, Supplementary Fig. 1A). To monitor the effect of ionomycin induced global increase in cellular Ca2+ level we immunostained ESCRT proteins in untreated or ionomycin treated cells. Ionomycin caused punctate distribution of both the ESCRT III associated proteins tested (Vps4B and ALG-2), but it did not affect the distribution of ESCRT 0 member STAM, which remained diffusely distributed in the cytosol (Supplementary Fig. 1B). Next, we used live imaging to monitor the effect of Ca2+ increase on localization of ESCRT III and associated proteins in myoblasts. Live TIRF imaging of cells showed that Chmp4B and Chmp1A puncate start forming at the cell surface within 90 seconds of being treated with ionomycin (Supplementary Fig.2 A-D). Similar response was observed for fluorescently tagged Vps4, Tsg101 and ALG-2 (Supplementary Fig. 2E-L). Together, the above observations show that Ca2+-triggers rapid assembly of ESCRTIII-Vps4 complex into punctae at the cell surface.
Ca2+-triggers ESCRT recruitment at the injured cell membrane
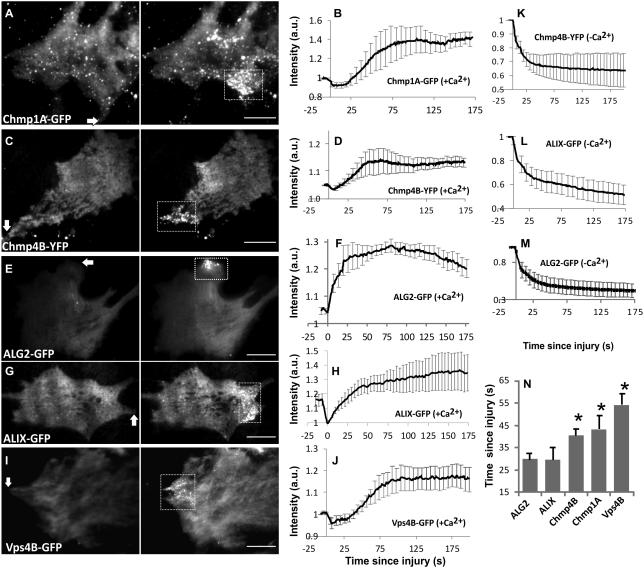
Having established Ca2+-triggered cell surface accumulation of ESCRTIII complex, we examined if injury triggered increase in Ca2+ also leads to ESCRT accumulation at the cell membrane. Confocal or TIRF imaging of cell membrane following focal injury by a pulsed laser resulted in accumulation of the punctae containing ESCRTIII (Chmp1A and Chmp4B – Fig. 2A-D) and accessory proteins ALG-2, ALIX and Vps4B (Fig. 2E-J) at the site of repair. This accumulation was dependent on the presence of extracellular calcium at the time of injury. Cells injured in the absence of extracellular Ca2+ lacked any ESCRT accumulation or formation of any puncta. Moreover, due to lack of cell membrane repair, following injury these proteins also leaked from the cell resulting in injury-triggered decrease in their cellular level (Fig. 2K-M). In the presence of calcium, formation of the puncta initiated within the first minute and peaked over the next minute (Fig. 2B,D,F,H,J). Examination of the injury-triggered kinetics of puncta formation identified that punctae for ALG-2 and its binding partner ALG-2 interacting protein X (ALIX) began assembling within 30 s of injury, followed by Chmp (~45 s post injury) and Vps4B (~60s) punctae (Fig. 2N). These differences in time of accumulation of ESCRTIII and Vps4B were statistically significant as compared to ALG-2 and ALIX, but initiation of ALG-2 accumulation was indistinguishable from that of ALIX.
Figure 2. Injury triggers recruitment of ESCRTIII and accessory proteins at the site of repair.
C2C12 myoblasts expressing fluorescently tagged (A,B) Chmp1A, (C,D) Chmp4B, (E,F) ALG-2, (G,H) ALIX, and (I,J) Vps4B proteins were injured using a pulsed laser. Representative TIRF images (A,C,E,G,I) show change in the level of ESCRT and accessory proteins at the injured cell membrane. The kinetics of accumulation of these proteins at the injured cell membrane when cells were injured in the (B, D, F, H, J) presence or the (K, L, M) absence of extracellular calcium. Images show the cells 5s prior to injury (Pre-injury) and at the time point following injury when the intensity of the respective protein at the site of injury has reached its peak value (Post-injury) and the plots show average of n>4 cells each. (N) Kinetics of protein accumulation at the injured cell membrane was imaged and the chart shows average time each protein takes to initiate accumulation following injury (n>10 cells each). Arrows on the image show the site of injury and boxes mark the site of repair where proteins accumulate. Scale bars represent 10μm and (*) represents P-value <0.03 (paired 2-tailed t-test with ALG-2) and error bars represent SE.
ESCRT recruitment is independent of cell type and MVB
To assess if ESCRT accumulation was unique to muscle cells we assessed this in HeLa cells. For this we made use of HeLa cells stably expressing near endogenous levels of Chmp4B-GFP and Vps4A-GFP26. Both these and other proteins we assessed were found to accumulate at the site of repair in manner similar to the myoblasts indicating cell type independence of this response (Fig. 3A-B).
Figure 3. ESCRT accumulation at injured cell membrane correlates with ALG-2 accumulation but not MVB fusion.
HeLa cells stably expressing low levels of (A) Chmp4B-GFP and (B) Vps4A-GFP, were injured by the pulsed laser at sites marked by the arrow. Images taken 5s before injury (Pre-injury) and at the peak of the respective protein accumulation (Post-injury) show injury triggered accumulation of these proteins similar to C2C12 cells. (C-F) HeLa cells co-expressing CD63-mCherry with (C, D) ALG2-GFP, or (E, F) Chmp4B-YFP were injured by laser (marked by arrow) and accumulation of ALG2 and Chmp4B punctae (green) was monitored together with CD63 (red) by dual-color TIRF imaging. Plots show intensity of CD63 at the site of injury where individual ALG-2 or Chmp4B punctae (marked by white circles) form. Note lack of co-localization between CD63-mCherry vesicle and the nascent ALG-2 or Chmp4B punctae. (G, H) Images of injury-triggered fusion of CD-mCherry (red) labeled vesicles (V1 and V2) together with accumulation of Vps4B-GFP (green) at the site of injury. Note that injury-triggered fusion of CD63-mCherry vesicles does not correlate spatially (V1) or temporally (V2) with formation of Vps4B puncate. (H) Images showing exocytosis of vesicles V1 and V2 as CD63-mCherry released by them diffuses into the cell membrane. (I) Images and (J) averaged plot (n=5) showing accumulation of ALG-2 mCherry and ALIX-GFP following focal laser injury. (K) Images showing accumulation of ALG-2 and Chmp4B in HeLa cell stably expressing Chmp4B-mCherry and transiently expressing ALG-2 following focal injury (white arrow). Zoom of the site of repair as ALG-2 (green) accumulates followed by Chmp4B (red) is shown and the individual channels (red and green) corresponding to the color combined zoomed images are shown below each image. Scale bar represents 10μm for A, B, I, and K; 2μm for C, E, and G.
We have previously shown that Ca2+ triggers exocytosis of CD63 positive late endosomes27, 28, a process associated with cell membrane repair29. Thus, while fusion of MVB leading to release of ILVs containing ESCRTs could provide a mechanism for the appearance of ESCRTs at the cell surface, the sequential nature of the assembly of ALIX, Chmp4B/Chpm1A and Vps4B (Fig. 2N) argues against such a possibility. To directly evaluate this possibility we simultaneously imaged ESCRT appearance and fusion of CD63-mCherry labeled MVBs. None of >30 nascent punctae we monitored for each protein (ALG-2, Chmp4B, or Vps4B) appeared coincident with CD63-labeled vesicles (Fig. 3C-F). Even when we monitored CD63 labeled vesicles that underwent injury-triggered exocytosis at the site of repair, the fusing vesicle did not deliver ESCRT puncta (Fig. 3G,H). These results show Ca2+-triggered MVB fusion is not the mechanism for ESCRT accumulation at the injured cell membrane. To directly examine if the sequential buildup of ESCRTIII and accessory proteins involved their accumulation on the same location as ALG-2, we simultaneously expressed ALG-2 with low levels of ALIX or Chmp4B in the same cell. By monitoring injury-triggered accumulation of these proteins we observed ALG-2 accumulated simultaneously (in space and time) with ALIX (Fig. 3I,J). However, this was different for the ESCRTIII protein Chmp4B, which accumulated after ALG-2, but even here the nascent Chmp4B punctae co-localized with the ALG-2 punctae (Fig. 3K). This observation is reminiscent of sequential accumulation of ALIX and ESCRT III during other cellular processes regulated by ESCRTs21, 22 (Supplementary Fig. 4).
ALG-2 initiates ESCRT recruitment at injured cell membrane
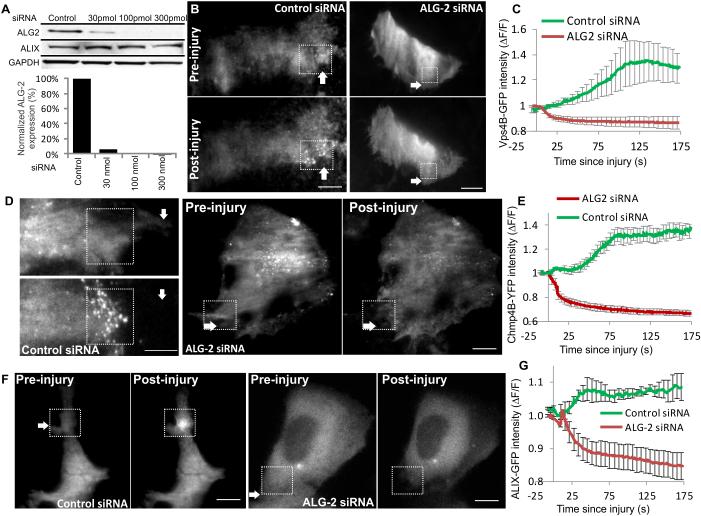
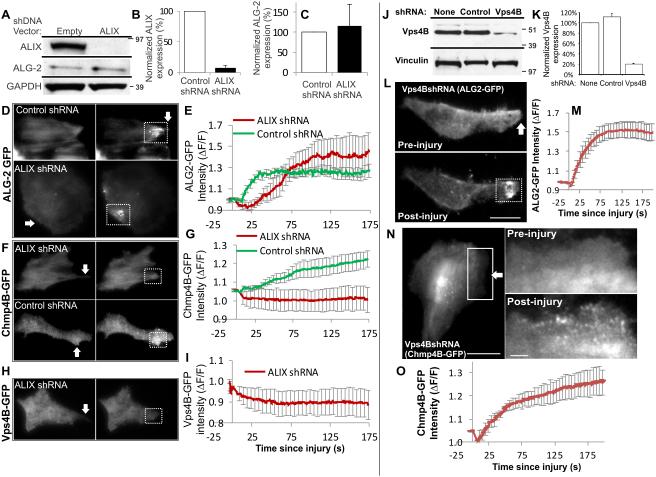
An important feature of ESCRT III accumulation at the injured cell membrane is its Ca2+-dependence. This could be on account of the EF-hand containing Ca2+-binding protein ALG-2 that has been shown to associate with ALIX, TSG-101, and Chmp4B in a Ca2+-dependent manner30. To examine if ALG-2 regulates Ca2+-triggered accumulation of ESCRTs we tested if lack of ALG-2 affects injury-triggered accumulation of ESCRT III and accessory proteins. By use of ALG-2 targeted siRNA in HeLa cells we reduced ALG-2 expression to well below 10% of the endogenous level (Fig.4A). Unlike the control siRNA transfected cells, the cells lacking ALG-2 expression failed to accumulate Vps4B (Figure 4B, C) and Chmp4B (Fig 4D, E) following injury. In view of the co-accumulation of ALG-2 with ALIX we tested if ALG-2 regulates ALIX accumulation or if these two proteins accumulate independently. Again, while the control siRNA had no effect on ALIX accumulation, lack of ALG-2 prevented injury-triggered accumulation of ALIX (Fig. 4F, G). While this indicates requirement of ALG-2 for ALIX accumulation, it is plausible that ALG-2 and ALIX may have complimentary role in their accumulation. In such an event ALG-2 will have a regulatory function rather than a recruitment function and each of these proteins will be needed for the co-accumulation. To test this possibility next we generated a pool of HeLa cells stably expressing ALIX shRNA vector or the control empty shRNA vector. Cells with ALIX shRNA showed <10% of ALIX protein level as compared to the empty vector control cells (Fig. 5A,B). In view of the direct interaction between ALG-2 and ALIX we tested the level of ALG-2 in the ALIX knocked down cells and found ALIX knockdown does not effect expression level of ALG-2 protein (Fig. 5A,C). We then assessed the effect of ALIX knockdown on injury-triggered ALG-2 accumulation and found that lack of ALIX did not prevent, but caused a slight delay in the accumulation of ALG-2 (Fig. 5D,E). Further, similar to the lack of ALG-2, lack of ALIX also prevented injury-triggered accumulation of Chmp4B (Fig. 5F-G) and Vps4B (Fig. 5H-I). Above observations that ALG-2 and ALIX are required for injury-triggered recruitment of ESCRT III and Vps4 complex and following injury they begin accumulating prior to ESCRT III and Vps4 (Fig. 2N) point to sequential recruitment of injury-triggered ESCRT accumulation, which is initiated by ALG-2 and mediated by ALIX.
Figure 4. ALG-2 is required for injury-triggered ALIX and ESCRT III assembly.
Control and ALG-2 specific siRNAs were transfected in HeLa cells and its effect was assessed on (A) expression of ALG-2 and ALIX (see Supplementary Fig. 5A for uncropped blots), (B-G) injury-triggered accumulation of (B, C) Vps4B-GFP, (D, E) Chmp4B-YFP, and (F, G) ALIX-GFP. TIRF images show cells 1s prior to injury (Pre-injury) and 125s following injury (post-injury). Plots show change in intensity of indicated protein at the site of repair in 4 cells each. The site of injury is marked by white arrow while the site of repair is marked by the dotted box. Scale bar represents 10μm and the error bars indicate SE.
Figure 5. ALIX regulates injury-triggered accumulation of ESCRT III but not of ALG-2.
(A) Western Blot analysis of ALIX and ALG-2 expression in HeLa cells stably expressing ALIX or empty shRNA vector (Supplementary Fig. 5B shows uncropped blots). Quantification of averaged (B) ALIX, and (C) ALG-2 expression normalized to GAPDH from 3 independent experiments. (D-I) TIRF images and quantification of control and ALIX knockdown cells during the course of repair from laser injury (white arrow) to assess the effect on injury-triggered accumulation of (D, E) ALG2-GFP, (F, G) Chmp4B-GFP, and (H, I) Vps4B-GFP at the site of repair. The plot shows the averaged values for change in the level of respective proteins within the site of repair contained by the dotted box. (J) Western Blot analysis of Vps4B expression in HeLa cells stably expressing Vps4B shRNA or empty shRNA vector (Supplementary Fig. 5B shows uncropped blots). (K) Quantification of averaged Vps4B expression normalized to GAPDH in 3 independent experiments. (L-O) TIRF images and quantification of injury-triggered accumulation of (L, M) ALG2-GFP, and (N, O) Chmp4B-GFP at the site of repair in Vps4B knockdown cells prior to and following repair from laser injury. Injury site is marked by white arrow and the plot shows the averaged values (n=5 cells) for the accumulation of respective proteins. Scale bar represents 10μm and the error bars indicate SE.
A key test of this model would be that preventing later events in the sequential recruitment pathway (ESCRT III/Vps4 recruitment) should not affect the earlier steps (ALG-2/ALIX recruitment). We thus examined the effect of the lack of Vps4B on Chmp4B and ALG-2 accumulation. For this we generated a HeLa cell line stably knocked down for Vps4B to <10% of endogenous level (Fig. 5J,K). We observed that Vps4B depletion had no effect on injury-triggered accumulation of ALG-2 (Fig. 5L,M) or Chmp4B (Fig. 5N,O), indicating that Vps4B is dispensable for Chmp4B and ALG-2 accumulation. This finding thus establishes the sequential recruitment model for injury-triggered ESCRT III recruitment.
ESCRTs facilitate repair of large cell membrane wound
While the ALG-2, ALIX and Vps4B knockdown each had different effects on injury-triggered recruitment of proteins, in all cases we observed increased number of cells failed to survive injury. This suggested that proper assembly of the entire ESCRTIII-Vps4 complex is needed for proper repair of cell membrane to proceed. To examine this possibility we assessed cell membrane repair using the commonly used assay of FM dye entry following laser injury31, 32, which recapitulates the Ca2+-dependent repair of cell membrane injury6, 9 (Supplementary Figure 3A). As a first step towards exploring the link between cell membrane repair and injury-triggered ESCRT assembly we examined if cell membrane repair kinetics occurs at the same timescale as injury-triggered ESCRTIII accumulation. Monitoring ESCRT III assembly using Chmp4B-GFP and repair by following entry of the FM4-64 dye we observed Chmp4B accumulation at the site of repair coincides with that of cessation of FM dye entry, indicating efficient cell membrane repair (Fig. 6A, B). To examine if this correlation is causally linked with repair we next assessed cell membrane repair in cells knocked down for the ESCRT III assembly and accessory proteins.
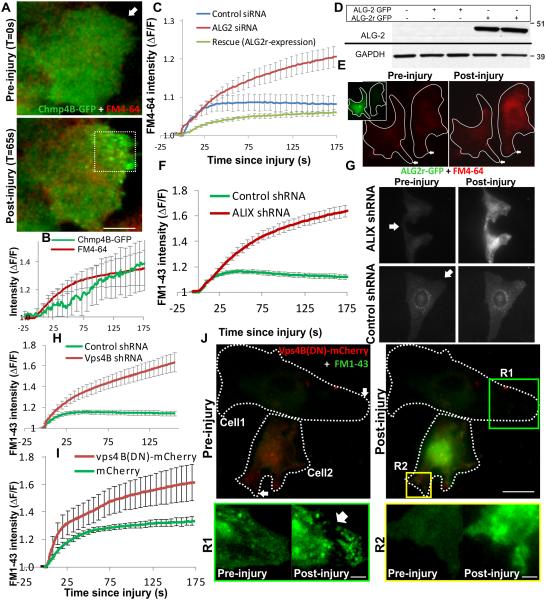
Figure 6. Ca2+-triggered ESCRT/Vps4B assembly allows cell membrane repair.
Following laser injury of myoblasts in presence of FM dye, repair of cell membrane was monitored by following dye entry, which results in increase in FM-dye fluorescence. Cellular dye fluorescence was normalized to the time point prior to injury (ΔF/F). (A) Live cell TIRF (green) and widefield (red) images of a myoblast expressing Chmp4B-GFP (green) and injured in the presence of FM4-64 dye (red) by laser (arrow). (B) The plot shows kinetics of cell membrane repair (FM 4-64 intensity) and Chm4B-GFP accumulation at the injured cell membrane. (C) Plot showing FM 4-64 entry following laser injury of 10-12 cells transfected for 72 hours with control siRNA (blue line) or ALG-2 siRNA with no subsequent treatment (red line) subsequent transfection with siRNA resistant ALG2-GFP plasmid (ALG2r-GFP). (D) Western blot analysis of expression of ALG2-GFP and ALG2r-GFP in cell lysates from HeLa cells transfected with these plasmids with or without ALG-2 siRNA and probed with ALG2 antibody. Bands corresponding to the GFP-tagged protein are shown here and the uncropped blots are shown in Supplementary Fig. 5C (E) Images showing laser injury-induced uptake of FM4-64 dye (red) in HeLa cells expressing or not expressing ALG2r-GFP (inset, green cell). (F) Kinetics of injury-triggered FM 1-43 entry in stable clones of HeLa cells expressing ALIX shRNA (red symbol) or empty vector (control; green symbol) averaged for 10-15 cells in each condition. (G) Representative images of a pair of ALIX shRNA or empty vector control cells injured (at the site marked by the arrow) in presence of FM 1-43 dye. (H) Kinetics of injury-triggered FM 1-43 entry in stable clones of HeLa cells expressing Vps4B shRNA (red symbol) or empty vector (control; green symbol) averaged for 10-15 cells in each condition. (I) Kinetics of injury-triggered FM 1-43 entry in up to 15 C2C12 myoblasts each transiently expressing mCherry (green) or Vps4B(DN)-mCherry. (J) Representative images of FM1-43 dye entry following injury of a pair of C2C12 myoblasts expressing (Cell2) or not expressing (Cell1) Vps4B(DN)-mCherry for 24 hours. Zoomed inset R1 and R2 show the region near the site of injury for cell 1 (lacking) and cell 2 (expressing) Vps4B(DN)-mCherry. Arrow in R1 shows the site where the damaged membrane was excised from the repaired cell and the diffraction limited dots represent the vesicles released during excision of the membrane. Error bars represent SE, and scale bars represent 10μm for the whole cell images and 2μm for the zoomed regions R1 and R2.
In view of the role of ALG-2 in initiating injury-triggered ESCRT assembly, we first examined the effect of ALG-2 knockdown. While control siRNA expressing cells repaired within a minute of injury, cells depleted for ALG-2 failed to prevent FM-dye entry (Fig. 6C, red plot; Supplementary Fig. 3B). To assess the specificity of the response we generated ALG-2 cDNA (ALG-2r) that is unaffected by the ALG-2 siRNA used in this study (Fig. 6D). Expression of GFP-tagged ALG-2r (ALG2r-GFP) rescued the repair defect caused by siRNA-mediated knockdown of ALG-2 (Fig. 6E, C-green plot). This establishes a need of ALG-2 in repair of injured cell membrane. We next assessed the role of ALIX in this process. Similar to ALG-2, cells lacking ALIX failed to repair from a focal laser injury (Fig. 6F,G). These results indicate that independent of the approach used, blocking injury-triggered ESCRT assembly prevents repair of the injured cells. We next tested if ESCRT III assembly alone is sufficient for repair or repair also requires assembly of the Vps4 complex. To address this we used two complimentary approaches. In the first approach we used HeLa cells knocked down for Vps4B and found that cells lacking Vps4B failed to repair from injury (Fig. 6H). In the second approach we monitored repair of HeLa cells by blocking Vps4 function by the expression of dominant negative Vps4B cDNA - Vps4B(DN) mutant33. Compared to cells expressing mCherry protein (Fig. 6I) or no exogenous proteins (Fig. 6J) cells that expressed Vps4B(DN)-mCherry for as little as 16 hours fail to repair and prevent FM dye entry (Fig. 6I,J). The need for Vps4B activity is suggested by a closer comparison of the fate of the cell membrane at the site of injury (R1, R2). While R1 (not expressing Vps4B(DN)) shows the injured part of the cell membrane was excised from the rest of the repaired cell and fragmented into smaller vesicles (Fig. 6J zoom of post-repair R1 image), in the case of R2 (the cell expressing Vps4B(DN)) the injured part of the membrane remained contiguous with the rest of the cell body and no vesicles were shed by the injured cell membrane. This latter behavior is similar to what we observed for cells injured in the absence of calcium (Supplementary Fig. 3A, R1 v/s R2) as well as cells lacking ALG2 or Vps4B expression (Supplementary Fig. 3B, C - region R3 v/s R4 and R5 v/s R6). In view of the known role of ESCRT III-Vps4B complex in membrane scission above observation indicate that membrane scission and vesiculation is required for repair and is mediated by ESCRTIII-Vps4B complex.
We next examined if shedding of the vesicles also occurs following injury of muscle fibers in an intact muscle tissue. For this we used intact biceps muscle and carried out focal ex vivo injury of individual myofibers in the intact muscle using pulsed laser in presence of FM1-43 dye to label the cell membrane and all of the released extracellular vesicles. Following injury we observed as the injured myofibers repaired they shed FM dye-labeled vesicles into the endomysial space and these vesicles originated from the site of injury (Supplementary Movie1, Supplementary Fig. 3D, E). As with the ESCRT III assembly (Fig. 6A,B), we found that the time course of extracellular vesicle shedding by the injured myofiber (indicated by red line in Supplementary Fig. 3E) occurred co-incident with the repair of the damaged cell membrane – as indicated by plateauing of FM dye entry in the cell (Supplementary Fig. 3E). This indicates that the Ca2+ triggered vesiculation to remove damaged membrane is a phenomenon that extends beyond the mononucleate muscle cells not only to other types of mononucleated cells but also multinucleate muscle fibers that have fully differentiated in vivo.
Discussion
ESCRT complex is involved in cell membrane repair in a manner that closely parallels involvement of this complex in retroviral budding and cytokinesis (Supplementary Fig. 4). Similar to these latter processes, injury triggers sequential assembly of ESCRT III without triggering assembly of most of the known ESCRTs 0-II member. ESCRT assembly at the injured cell membrane is dependent on ALIX (Fig. 5A-C). It is suggested that following injury ALIX may associate with cell membrane through its endosomal lipid (LBPA - lysobisphosphatidic acid) binding domain13. However, we find that injury-triggered ALIX accumulation depends on ALG-2, absence of which prevents ALIX accumulation (Fig. 4F,G). As accumulation of ALG-2 itself is not prevented by the lack of ALIX (Fig. 5D,E), ALG-2 appears to be the regulator of ALIX accumulation and not vice versa. However, both ALG-2 and ALIX are needed for the accumulation of ESCRTIII and Vps4 complexes at the injured cell membrane (Fig. 4, 5). Based on these features we conclude that ALG-2 is the initiator and its binding partner ALIX the facilitator of ESCRT III recruitment. Binding between ALIX and ALG-2 occurs through a motif in ALIX that partially overlaps with that of Cep55 (centrosome protein of 55 kDa) – the protein responsible for ESCRTIII assembly during cytokinesis34. By the virtue of its ability to form a homodimer, ALG-2 can function as an adaptor bridging two interacting proteins such as ALIX and Tsg10135. This could be the basis for the presence of Tsg101 - the sole ESCRTI/ESCRTII protein to accumulate at the site of cell membrane repair. In view of above, the role of ALG-2 during cell membrane repair appears to parallel that of Cep55 during cytokinesis and Gag during HIV budding. As these are ESCRT0 proteins (for their respective processes), we propose that ALG-2 is the ESCRT0 protein for cell membrane repair (Supplementary Fig. 4).
ALG-2 is amongst the best conserved penta-EF (PEF) hand protein. PEF domain contains an evolutionarily primitive Ca2+-binding motif found in a number of protein families including calpains and S100 proteins. Calpain has been implicated in the repair of injured cell membrane36 and we have recently identified a similar role for S100 protein – S100A1137. Identification of a role of ALG-2 in cell membrane repair adds further to the importance of EF hand proteins in facilitating cell membrane repair. As cell membrane injury is arguably amongst the earliest and ubiquitous calcium-dependent cellular process, the ancient origin and widespread presence of EF hand in proteins may have facilitated their use to sense injury-triggered calcium entry and initiate a repair response. So far, the only function attributed to the calcium binding ability of ALG-2 has been the Ca2+sensor at ER exit sites, where it stabilizes membrane association of Sec31 and facilitates ER exit38. Our finding that ALG-2 can assemble at injured cell membrane in response to Ca2+- increase identifies a new feature of this protein, which we find is needed for repair of injured cell membrane. Further, ALG-2 involvement in repair is noteworthy due to the initial identification of ALG-2 as a Ca2+-dependent regulator of cell death39. While cells that fail to repair will undergo necrotic cell death, we believe that ALG-2 may regulate activation of cell death in those cell that close the cell membrane breach, but fail to fully recover from the injury. Such a role of ALG-2 will be of great relevance to disease models where cell membrane repair is inefficient6, 40.
ALG-2 and ALIX mediated ESCRTIII and Vps4 accumulation facilitates cleavage of injured cell membrane. Cell membrane cleavage proceeds with the involvement of ESCRTIII and Vps4 complex. Accumulation of Vps4 is required for the cleavage of damaged region of the injured cell membrane, as lack of Vps4 and blocking its function by use of dominant negative Vps4 prevents cleavage of the injured cell membrane (Fig. 6H-J and S3B,C). Cleavage of injured cell membrane results in formation of small extracellular vesicles that are shed from the site of injury in cultured cells and in muscle tissue (Fig. 6J, Supplementary Fig. 3). Shedding of injured cell membrane as small extracellular vesicles by ESCRTIII-Vps4 complex is distinct from blebbing of injured cell membrane and from our recent demonstration of actin-mediated large-scale retraction of damaged cell membrane37, 41. Formation and shedding of extracellular vesicles is a key step in the process of regulating tissue inflammation and repair following injury42. Thus, in addition to facilitating repair of the injured cell membrane, involvement of ESCRT-mediated shedding of injured cell membrane may also aid tissue repair by activating inflammatory signaling through the release of extracellular vesicles.
In summary, results presented here point to the role of ESCRTs in repair of large focal injuries and identify the sequential process by which ESCRT III and Vps4 complexes are assembled at the site of injury. It remains to be seen if this mechanism is conserved even in case of small cell membrane injuries such as by the pore-forming toxins. Additionally, work using knockout mouse models would help address the in vivo role of ESCRTs in the repair and regeneration of muscles injured by exercise or due to genetic deficits. With increasing recognition of extracellular vesicles in the process of tissue inflammation and repair, ESCRT-mediated vesiculation could also offer insights into extracellular signaling required to initiate inflammation and stem cell activation for efficient regeneration of muscle and other tissues.
Methods
Cell culture
Mouse C2C12 myoblasts (ATCC, Manassas, VA) were routinely cultured in Dulbecco’s Modified Eagle Medium (DMEM) with Glutamax, high glucose, 110 mg/L sodium pyruvate (Invitrogen, USA) supplemented with 10% fetal bovine serum (Hyclone, Thermo Fisher Scientific, USA) and with 100 μg/mL Penicillin and 100 μg/mL Streptomycin (Invitrogen, USA) at 37°C and 5% CO2. HeLa cells (ATCC, Manassas, VA) and clones stably expressing low levels of specific ESCRT proteins26 were grown in DMEM (without pyruvate, Gibco, catalog number 11965-092) with 10% FBS and 0.4μg/ml Geneticin at 37°C and 5% CO2 using standard sterile techniques. For SILAC labeling cells were cultured for 6 generations in media containing regular Lysine and Arginine isotopes (unlabeled) or with media that has 13C6 arginine and 15N2, 13C6 Lysine as the sole source of these amino acids (labeled). To test complete replacement of lysines and arginine by their heavy counterparts C2C12 cells were cultured in the presence of 13C6 arginine and 15N2, 13C6 Lysine as described above and all proteins from five random gel slices from five different locations of the gel for a crude protein extract were subjected to tryptic digestion. The resulting peptide mixture was analyzed by MS, which showed a >99% incorporation of the SILAC label. These labeled cells were then used for subsequent treatment and preparation of plasma membrane proteome along with unlabeled cells cultured simultaneously in unlabeled media.
Plasmids and stable cell lines
C2C12 or Hela were transfected with the indicated plasmid using Lipofectamine LTX (Life technologies) or Fugene 6 (Roche Biochemicals) respectively as per manufacterers’ instructions. Expression was allowed for 24-48 hours. The plasmids used to make stably expressing/knocked down HeLa cell lines are based on the pLNCX2 plasmid (Clontech) with GFP-Chmp4B, GFP-Vps4A (A gift by Sanford Simon26) or shRNA-Vps4B (A gift from Yasuo Ariumi). The ORF was sequenced completely. The following retroviral constructs were used for this study: pLNCX2-mEGFP-Chmp4B (NM_176812), pLNCX2-mEGFP-Vps4A21, pLV-Vps4Bi, pLV-ALIXi and non-targeting control shRNA sequence pLV-shCon as described elsewhere43. Sense and antisense sequences of the oligonucleotides used for the Vps4B shRNA and the production and use of the lentiviruses as described previously43 and in Table 1. Stable HeLa cell lines expressing tagged ESCRTs or vps4A shRNA were created and tested for any detectable adverse effects on cell growth. Retroviral vectors expressing the desired shRNA were transfected into HEK293T cells with plasmids expressing VSVG and MLV-Gag-Pol using Fugene 6 according to manufacturers’ instructions. Viruses were harvested 24 h later, filtered through a 0.45 μm Millex HP PES filter (Millipore) and added to HeLa cells. 24 h later, selection was started using 400 μg/ml Geneticin (Invitrogen) and continued for at least one week. Clones expressing low levels of the protein or knocked down for vps4B were selected by limiting dilution. The clones selected also showed no morphological aberration and obvious difference in growth. ALG-2 siRNA used has is described in Table 1.
Table 1.
shRNAs and siRNAs used in the study. Names and sequence of the siRNAs used in the study to silence expression of ALG-2, ALIX, and Vps4B genes are listed.
| siRNA/shRNA | Sequence |
|---|---|
|
ALIX
shRNA |
5’-GATCCCCGGAGGTGTTCCCTGTCTTGTTCAAGAGACAAGACAGGGAACACCTCCTTTTTGGAAA-3’ (sense)
5’ - AGCTTTTCCAAAAAGGAGGTGTTCCCTGTCTTGTCTCTTGAACAAGACAGGGAACACCTCCGGG-3’ (antisense) |
|
Vps4B
shRNA |
5’-GATCCCCGGAGAATCTGATGATCCTGTTCAAGAGACAGGATCATCAGATTCTCCTTTTTGGAAA-3’ (sense) 5’-AGCTTTTCCAAAAAGGAGAATCTGATGATCCTGTCTCTTGAACAGGATCATCAGATTCTCCGGG-3’ (antisense) |
|
ALG-2
siRNA |
5’-GGUCGAUCAUAUCCAUGUUdTdT-3’ (Sense) 5’-AACAUGGAUAUGAUCGACCdTdT-3’ (Antisense) |
To generate siRNA resistant ALG2, the cDNA was mutated to create 4 conservative point mutations (indicated in lowercase): GaUCcAUaAUcUCCAUGUU. Once the ability of the mutated ALG-2 cDNA to avoid siRNA silencing was established at the protein level this plasmid was used for functional rescue experiments for endogenous ALG-2. The siRNA was custom synthesized by Dharmacon (Thermo Scientific, US). For control siRNA siGLO RISC-Free Control siRNA (Thermo Scientific, US) were used. Hela cells were transfected with 300pmol of siRNAs using oligofectamine according to manufacturers’ instructions.
Ionomycin treatment and SILAC labeling
To label cells using the SILAC strategy, DMEM media without lysine and arginine was custom prepared (Atlanta Biologicals, Lawrenceville, GA) and complete unlabeled and labeled media was then constituted by the addition of either unlabeled lysine and arginine or 13C6 arginine (147.5 mg/mL) and 15N2, 13C6 Lysine (91.25 mg/mL) (Cambridge Isotope Laboratories Inc., Andover, MA) respectively, along with 10% fetal bovine serum (Hyclone, Thermo Fisher Scientific, USA) and 100 mg/mL each of penicillin and streptomycin (Invitrogen, USA), to the above lysine, arginine-depleted medium. Equal number of C2C12 cells grown in parallel in both labeled and unlabeled media for > 6 passages to ensure labeling of all cellular proteins. Subsequently, the cell surface proteins present in both labeled and unlabeled cells were biotinylated using the protocol previously described4, following which labeled/unlabeled cells were treated at room temperature for 5 minutes with 15 mL Hank’s Balanced Salt solution containing 10 μM ionomycin, while corresponding unlabeled/labeled cells were treated at room temperature for 5 minutes with 15 mL of Hank’s Balanced Salt solution containing 0.1% DMSO. Of the 6 independent experiments in 5 experiments labeled cells were treated with ionomycin and the unlabeled cells were treated with DMSO and this was reversed for 1 experimental replicate.
Plasma membrane protein enrichment
Plasma membrane proteins were purified from C2C12 myoblasts as previously described4. Briefly, the cells were washed once with Hank’s Balanced Salt solution, scraped and unlabeled and labeled cells were mixed in a ratio of 1:1. Cells were then collected by ultracentrifugation (>200,000 × g, 20 minutes, 4°C) and the pellet was re-suspended in 2 mL of Hank’s Balanced Salt solution. The cell suspension was homogenized in a tight-fitting 7 mL Dounce homogenizer with 20 strokes of pestle A and 50 strokes of pestle B. The homogenate was then centrifuged at 800g, for 10 minutes at 4°C to obtain the post-nuclear supernatant. The protein concentration was estimated in this supernatant using the Bio-Rad DC™ protein assay kit (Bio-Rad, Hercules, CA). Subsequently, supernatant containing 1.3-2 mg protein was bound to streptavidin beads. The beads were then successively washed with a high salt and a high pH buffer, and finally rinsed twice with water. The beads were resuspended in 2X SDS sample buffer containing 2.5% β-mercaptoethanol.
Sample preparation, in-gel digestion and peptide extraction
The streptavidin beads containing bound biotinylated cell surface proteins were heated at 50°C for 30 minutes to elute the proteins bound to the beads and 1D protein separation was performed using a precast 4-12% SDS-PAGE gel (Bis-tris Novex mini gel, Invitrogen, Carlsbad, CA). The gel was fixed with methanol:water:acetic acid (45:50:5 v/v/v) and subsequently bands were visualized by staining with coomassie blue (Bio-Rad, Hercules, CA). The fractionated gel lane was excised into 35 bands. Each slice was destained with 50% acetonitrile (ACN) (v/v) and 100 mM NH4HCO3, dehydrated with 100% ACN. Gel pieces were rehydrated in 10-20 μl of cold digestion buffer (12.5 ng/μl of gold-grade trypsin (Promega, Madison, WI) in 50 mM NH4HCO3) and incubated at 4°C for 45 minutes. The excess buffer was removed and replaced with 50 mM NH4HCO3. Digestion with trypsin was carried out overnight at 37°C. Tryptic peptides were extracted with 50% acetonitrile with 5% formic acid, repeated twice. Pooled peptides were dried by vacuum centrifugation and resuspended in 10uL of 0.1% trifluoroacetic acid (TFA).
LC-MS/MS
All analyses were carried out on a hybrid Thermo LTQ-OrbitrapXL (San Jose, CA) mass spectrometer coupled online to an Eksigent nano-LC system (AB Sciex, Framingham, MA). Each sample was injected via an autosampler and loaded onto a Symmetry C18 trap column (5μm, 300 μm i.d. × 23 mm, Waters, Milford, MA) for 10 min at a flow rate of 10 μL/min in HPLC grade water with 0.1% formic acid. The sample was subsequently separated by a C18 reverse-phase column (3 μm, 200A, 100 μm × 15 cm, Magic C18, Michrom Bioresources) at a flow rate of 300 nL/min. The mobile phases consisted of water with 0.1% formic acid (A) and 90% acetonitrile (B). A 65 minute linear gradient from 5 to 40% B was employed. Eluted peptides were introduced into the mass spectrometer via Michrom Bioresources CaptiveSpray (Bruker, Freemont, CA). The spray voltage was set at 1.4 kV and the heated capillary at 200 °C. The LTQ-Orbitrap-XL was operated in data-dependent mode with dynamic exclusion in which one cycle of experiments consisted of a full-MS in the Orbitrap (300-2000 m/z) survey scan in profile mode, resolution 30,000, and five subsequent MS/MS scans in the LTQ of the most intense peaks in centroid mode using collision-induced dissociation with the collision gas (helium) and normalized collision energy value set at 35%.
Database Search and IP2 Analysis
For protein identification and quantification we used Integrated Proteomics Pipeline (IP2) version 1.01 software developed by Integrated Proteomics Applications, Inc. (http://www.integratedproteomics.com/). Mass spectral data were uploaded into IP2 software. Files from each lane were searched against the forward and reverse Uniprot mouse database (UniProt release 2010_12 with 16,333 entries) for partially tryptic peptides allowing one missed cleavage, and possible modification of oxidized methionine (15.99492 Da), heavy arginine (6.0204 Da) and heavy lysine (8.0142 Da). IP2 uses the Sequest 2010 (06_10_13_1836) search engine. Mass tolerances were set at +/− 30 ppm for MS and +/− 1.5 Da for MS/MS. Data were filtered based on a 1% protein false discovery rate. All the bands from each lane were summed in the analysis. Census software version 1.77, built into the IP2 platform, was used to determine the ratios of unlabeled and labeled peptide pairs using an extracted chromatogram approach. The distribution of ratios was plotted and correction factors applied to adjust for error in sample mixing. Data were checked for validity by using regression correlation better than 0.5 for each peptide pair.
Bioinformatics Analysis
Biological replicates were combined in IP2 software using Quantitation Compare which calculates the average ratio along with relative standard deviation and t-test p-values. Proteins and corresponding quantitative ratios were filtered based on identification and quantitation in at least two replicates, ratio ≥1.15 or ≤0.87 and t-test p-value <0.059. Protein annotations were acquired using the Keyword and GO terms in the Uniprot Protein Knowledgebase (http://www.uniprot.org/). Proteins of interest were further validated using alternative approaches.
Immunoblot and immunostaining
Cells were lysed with RIPA (Sigma Aldrich, USA) containing protease inhibitor cocktail (Fisher Scientific, USA) for WB analysis or fixed with 4% paraformaldehyde (PFA) or chilled methanol for immunofluorescence analysis. For WB proteins were transferred to nitrocellulose membranes and probed with indicated primary antibody: Chmp4B (ab105768-1:1000 dilution, Abcam, Cambridge, MA); STAM (ab76061, 1:300, Abcam, Cambridge, MA); Vps4B (SAB4200023, 1:500 dilution, Sigma Aldrich, St Louis, MO); Chmp1A (15761-1-AP, 1:500 dilution, Proteintech, Chicago, IL) and Vps25 (15669-1-AP, 1:500 dilution, Proteintech, Chicago, IL), ALG-2 (H00010016-M01, 1:500 dilution, Novus Biologicals, Littleton, CO) and Vps37C (NBP1-56409, 1:500 dilution, Novus Biologicals, Littleton, CO), Galectin-1 (AF1245, 1:500 dilution, R&D systems, Minneapolis, MN), Cadherin (4068, 1/1000 dilution, Cell Signaling Technology, Danvers, MA), ALIX (sc-49268, 1:500 dilution, Santa Cruz Biotech, Dallas, TX), Tsg-101 (sc-7964, 1:500 dilution, Santa Cruz Biotech, Dallas, TX). This was followed by probing with HRP-conjugated secondary antibodies (Sigma Aldrich, St Louis, MO), chemiluminescent Western Blotting Substrate (Fisher, GE Healthcare, USA) and processed on Bio-Lite X-ray film and scanned and quantified by densitometry using BioRad GS-800 densitometer and normalized to the loading control to obtain ionomycin/DMSO fold change values. Uncropped blots corresponding to figure 4-6 are shown in Supplementary Figure 5. For immunostaining, permeabilized cells were bound with appropriate antibodies followed by fluorophore-conjugated secondary antibodies: Alexa Fluor 488 anti-rabbit, Alexa Fluor 594 anti-rat and Alexa Fluor 594 anti-mouse (Life technologies, USA). Nuclei were counterstained with Hoechst 33342. After mounting in mounting medium (Dako, USA), cells were imaged with appropriate filters and 60X-1.45NA objective on Olympus IX81 widefield microscope equipped with Orca-R2 camera (Hamamatsu Photonics, Japan).
Live Imaging
For laser injury assay cells cultured on coverslips were transferred in cell imaging media (CIM; HBSS with 10mM HEPES pH 7.4) or PBS (Sigma Aldrich, USA) into Tokai Hit microscopy stage top ZILCS incubator (Tokai Hit Co., Japan) maintained at 37°C. For laser injury, a 1-2μm-2 area was irradiated for <100 milliseconds with a pulsed laser (Ablate!, 3i Intelligent Imaging Innovations, Inc. Denver, CO, USA). To monitor ESCRT kinetics cells expressing appropriate tagged ESCRT protein was injured and imaged in spinning disc confocal or TIRF mode using IX81 Olympus microscope (Olympus America, PA) equipped with Yokogawa spinning disc confocal or Cell-TIRF (Olympus USA) system respectively. For all imaging a 60X-1.45NA oil objective was used. To quantify cell membrane repair, 1 ug/uL FM1-43 dye (Life technologies, USA) was added to the cell imaging buffer used and cells were imaged at 10 second intervals using IX81 Olympus microscope (Olympus America, PA). FM dye intensity was used to quantify the kinetics of cell membrane repair.
Mouse muscle isolation and membrane injury
Methods involving animals were approved by the local institutional animal research committee, and animals were maintained in a facility accredited by the American Association for Accreditation of Laboratory Animal Care. Biceps muscle were surgically isolated from euthanized 3-month-old C57BL/6 mice in Tyrode's solution containing 128 mM NaCl, 4.7 mM KCl, 1.36 mM CaCl2, 20 mM NaHCO3, 0.36 mM NaH2PO4, 1 mM MgCl2, and 10 mM glucose (pH 7.4). Laser injury was carried out as described above in the Tyrode's buffer containing 1.33 μg/μl FM1-43 dye.
Supplementary Material
Acknowledgements
We acknowledge the generous gifts by Martin Serrano (University College London), Sanford Simon, and Marina Bleck (The Rockefeller University) for ESCRT plasmids and cell lines expressing fluorescently tagged ESCRTs. Hideki Shibata (Nagoya University) for fluorescently tagged ALG-2 plasmid and custom ALG-2 antibody. Yasuo Ariumi (Kumamoto University) for ALIX, Vps4B and control shRNA lentiviral vectors and Aurelia Defour and Anamaris Colberg Poley for their comments. This work was funded by grants from NIH/NIAMS (AR055686, HD040677), and MDA (MDA277389) to JKJ. The imaging and proteomics core received partial support by grants NICHD/NINDS 2R24HD050846-06, NICHD 5P30HD040677, and NIH NCATS UL1RR031988.
Footnotes
Author information: Authors have no competing financial interest.
References
- 1.McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 3.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J. Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma N, et al. Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles. J. Biol. Chem. 2012;287:30455–30467. doi: 10.1074/jbc.M112.354415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennon NJ, et al. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J. Biol. Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 6.Defour A, et al. Dysferlin regulates cell membrane repair by facilitating injury-triggered acid sphingomyelinase secretion. Cell Death. Dis. 2014;5:e1306. doi: 10.1038/cddis.2014.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrotte M, et al. Caveolae internalization repairs wounded cells and muscle fibers. Elife. (Cambridge) 2013;2:e00926. doi: 10.7554/eLife.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrotte M, Fernandes MC, Tam C, Andrews NW. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic. 2012;13:483–494. doi: 10.1111/j.1600-0854.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defour A, Senchandra S, Jaiswal JK. Imaging cell membrane injury and sub-cellular processes involved in repair. JoVE. 2014 doi: 10.3791/51106. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma N, et al. Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles. J. Biol. Chem. 2012;287:30455–30467. doi: 10.1074/jbc.M112.354415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J. Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. ( Pt 11) [DOI] [PubMed] [Google Scholar]
- 12.Idone V, et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez AJ, et al. ESCRT machinery is required for plasma membrane repair. Science. 2014;343:1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 14.Keyel PA, et al. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 2011;124:2414–2423. doi: 10.1242/jcs.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadimitriou JM, Robertson TA, Mitchell CA, Grounds MD. The process of new plasmalemma formation in focally injured skeletal muscle fibers. J. Struct. Biol. 1990;103:124–134. doi: 10.1016/1047-8477(90)90016-6. [DOI] [PubMed] [Google Scholar]
- 16.Tam C, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 2010;189:1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianco F, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vito P, Lacana E, D'Adamio L. Interfering with apoptosis: Ca(2+)-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- 19.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita E, et al. ESCRT-III protein requirements for HIV-1 budding. Cell Host. Microbe. 2011;9:235–242. doi: 10.1016/j.chom.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouvenet N, Zhadina M, Bieniasz PD, Simon SM. Dynamics of ESCRT protein recruitment during retroviral assembly. Nat. Cell Biol. 2011;13:394–401. doi: 10.1038/ncb2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc. Natl. Acad. Sci. U. S. A. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita E, et al. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12889–12894. doi: 10.1073/pnas.1005938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita E, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10541–10546. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleck M, et al. Temporal and spatial organization of ESCRT protein recruitment during HIV-1 budding. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1321655111. doi:10.1073/pnas.1321655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. Plos Biology. 2004;2:1224–1232. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 30.Katoh K, et al. The penta-EF-hand protein ALG-2 interacts directly with the ESCRT-I component TSG101, and Ca2+-dependently co-localizes to aberrant endosomes with dominant-negative AAA ATPase SKD1/Vps4B. Biochem. J. 2005;391:677–685. doi: 10.1042/BJ20050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeil PL, Miyake K, Vogel SS. The endomembrane requirement for cell surface repair. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4592–4597. doi: 10.1073/pnas.0736739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaiswal JK, et al. Patients with a Non-dysferlin Miyoshi Myopathy have a Novel Membrane Repair Defect. Traffic. 2007;8:77–88. doi: 10.1111/j.1600-0854.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 33.Fujita H, et al. A dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane trafficking out of endosomal/lysosomal compartments: class E vps phenotype in mammalian cells. J. Cell Sci. 2003;116:401–414. doi: 10.1242/jcs.00213. [DOI] [PubMed] [Google Scholar]
- 34.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, et al. The mechanism of Ca2+-dependent recognition of Alix by ALG-2: insights from X-ray crystal structures. Biochem. Soc. Trans. 2009;37:190–194. doi: 10.1042/BST0370190. [DOI] [PubMed] [Google Scholar]
- 36.Mellgren RL, et al. Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome, or caspases. Biochim. Biophys. Acta. 2009;1793:1886–1893. doi: 10.1016/j.bbamcr.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaiswal JK, et al. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat. Commun. 2014;5:3795. doi: 10.1038/ncomms4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata H, Suzuki H, Yoshida H, Maki M. ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem. Biophys. Res. Commun. 2007;353:756–763. doi: 10.1016/j.bbrc.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 39.Vito P, Lacana E, D'Adamio L. Interfering with apoptosis: Ca(2+)-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- 40.Bansal D, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 41.Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Blebbing confers resistance against cell lysis. Cell Death. Differ. 2011;18:80–89. doi: 10.1038/cdd.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am. J. Physiol Lung Cell Mol. Physiol. 2012;303:L364–L381. doi: 10.1152/ajplung.00354.2011. [DOI] [PubMed] [Google Scholar]
- 43.Ariumi Y, et al. The ESCRT system is required for hepatitis C virus production. PLoS. ONE. 2011;6:e14517. doi: 10.1371/journal.pone.0014517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.