Abstract
A new model of tumor metabolism is proposed that describes how the complementary metabolic functions of the local stroma and the tumor cells contribute to cancer progression. Cancer cells alter the metabolism of cancer-associated fibroblasts to obtain lactate and amino acids, which are utilized for energy production, rapid growth, and resistance to chemotherapy drugs. Because the microenvironment provides glutamine, cancer cells are able to replenish TCA cycle intermediates and to act as nitrogen donors for nucleotide synthesis. Moreover, adipocytes in the microenvironment attract cancer cells through the secretion of inflammatory cytokines and proteases. The cancer cells then induce metabolic changes in the adipocytes in order to acquire free fatty acids which are oxidized by cancer cells to generate energy for proliferation. Increasing knowledge about the metabolic symbiosis within the tumor has led to novel therapeutic strategies designed to restrict metabolic adaptation, including inhibiting lactate transporters and repurposing anti-diabetic drugs (thiazolidinediones, metformin).
Background
The identification of cancer as a genetic disease, compellingly established by the detection of genomic derangements within malignant cells, led researchers to focus on alterations in tumor suppressor genes and oncogenes. However, in the last decade our genetic view has been expanded by the observation that tumors are thriving organs with multiple cell types within a distinctive extracellular matrix (ECM), and that all these components can impact tumor progression and response to therapies. This view has added significant complexity to the study of human tumors, since it takes into consideration the effects of fibroblasts, mesothelial, immune cells, adipocytes, and endothelial cells on tumor growth. During transformation and metastasis, cancer cells recruit these cell types to surround themselves with a supportive tumor microenvironment (TME). Over time, the tumor and the adjacent cells co-evolve and even metastasize together (1). Stromal cells are recruited by paracrine growth factors (e.g. PDGF, VEGF) secreted by cancer cells and then, in turn, secrete cytokines (e.g. HGF, TGF-β, CCL5) (2–4) which accelerate the aggressiveness of cancer cells. The progression of cancer is further supported by the TME, in which low levels of inflammation mediated by immune cells create microenvironmental conditions promoting the invasion of epithelial tumor cells (5). Three dimensional organotypic cultures using primary cells have allowed to model these complex interactions in cell culture (6, 7).
These reciprocal interactions between cancer and stromal cells have been worked out in detail, but minimal emphasis has been placed on the metabolic alterations in the TME. While it is now accepted that cancer cells undergo unique metabolic alterations that facilitate growth (8), most studies of cancer cell metabolism have narrowly focused on changes in the cancer cells, generally ignoring the possible contributions of the TME. However, it has long been known that, in normal tissue, different cell types cooperate to adapt to metabolic demands. For example, adipocytes provide energy to exercising muscle fibers by supplying fatty acids (9) and lactate is shuttled between muscle fibers for use as energy (10).
Given that normal cells have codependent metabolic relationships, that tumors of different origins use different metabolic pathways, and that, even within the same tumor, epithelial cancer cells have different metabolic states (11), it is probable that individual cell types within and surrounding a tumor also have different, interdependent, metabolic states. Our goal is to review the substrates (lactate, amino acids, and fatty acids) that cancer cells use to generate energy, with a focus on how adjacent stromal cells serve as a unique and targetable source of these metabolic building blocks (Fig. 1).
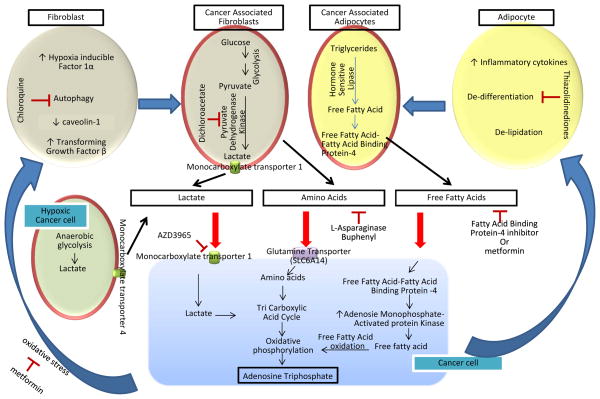
Figure 1.
Metabolic adaptations in the tumor microenvironment and therapeutic strategies. Stromal cells form a complex metabolic hub in their interactions with cancer cells. Cancer-associated fibroblasts (CAFs) are metabolically activated by signals (in the form of cytokines or oxidative stress) from cancer cells, resulting in the release of energy rich metabolic intermediates such as lactate and amino acids. These metabolites are then taken up via specific transporters to generate ATP. Oxygen availability also dictates metabolic heterogeneity since cancer cells in hypoxic areas use anaerobic glycolysis to generate lactate which is subsequently taken up by normoxic cancer cells and used for ATP production. Cancer-associated adipocytes (CAAs) also undergo metabolic alterations induced by cancer cells including heighted activity of hormone sensitive lipases (HSL) which produces free fatty acids (FFA), that once released by CAAs, are taken up by cancer cells. Intracellular FFA are chaperoned by fatty acid binding proteins such as FABP4. FA are oxidized in mitochondria to generate ATP. This complex relationship between cancer cells and stromal cells favors cancer growth/migration/invasion and metastasis. However it also provides multiple therapeutic targets. Some of the promising strategies include targeting pyruvate dehydrogenase kinase in CAFs with dichloroacetate (DCA), inhibiting lactate transporters with AZD3965, promoting breakdown of non-essential amino acid asparagine with L-Asparaginase, preventing induction of the CAA phenotype with thiazolidinediones (TZDs), and targeting the FABP4 protein to block use of FFAs as a source of energy. The diabetes drug metformin may also be used to reduce oxidative stress in CAFs and inhibit uptake of FFA in cancer cells.
HIF-1α: hypoxia inducible factor 1a, TGF-β: transforming growth factor β, PDK: pyruvate dehydrogenase kinase, SLC6A4: glutamine transporter, MCT: monocarboxylate transporter, TCA: tricarboxylic acid cycle, AMPK: AMP-activated protein kinase, OXPHOS: oxidative phosphorylation.
Exchange of lactate between stroma and tumor
Lactate is an end product of glycolysis during anaerobic metabolism, a gluconeogenic precursor, and a regulator of the cellular redox state. Under steady-state conditions, lactate is either used within cells or secreted into the extracellular space where it is available for neighboring cells (“cell-cell lactate shuttle” (12)). In exercising muscles, lactate is produced by rapidly contracting fast-twitch muscle fibers through glycolysis and is exported outside of the cell through monocarboxylate transporter (MCT) 4. Lactate is then either transported to the liver to be utilized as a substrate for gluconeogenesis (“Cori cycle”) or taken up by adjacent slow-twitch oxidative fibers through MCT1 and, as a precursor for glucose, replenishes energy stores.
A similar mechanism has been described in tumor cells (11, 13). Regulated by the O2 gradient, hypoxic tumor cells distant from blood vessels use anaerobic glycolysis, producing lactate, which is released by the cell through MCT4. Lactate then diffuses along a concentration gradient to the well-oxygenated tumor cells lining the tumor vasculature, enters the cells through MCT1, and is oxidized to produce energy through oxidative phosphorylation. The Dewhirst lab in 2008 showed that, when MCT1 is inhibited, the oxidative tumor cells are forced to utilize glucose for energy production and, since glucose is not available, the tumors necrose (11). These findings demonstrated, for the first time, that metabolic cooperation exists between aerobic and hypoxic tumor cells, leading to metabolic heterogeneity within the tumor organ. These results also proved that cancer cells are capable of the opportunistic optimization of available metabolic substrates within their microenvironment in order to increase tumor survival and growth.
Evidence that trafficking of energy resources also occurs between tumor and stromal cells began to emerge as early at 2006, when colorectal cancers were analyzed immunohistochemically and shown to overexpress lactate dehydrogenase (LDH) 5 and MCT1, suggesting that they metabolize glucose through anaerobic glycolysis and export lactate. Interestingly, adjacent fibroblasts expressed proteins involved in lactate uptake and metabolism (MCT1/2, LDH1) and a low level of GLUT1. Based on the immunohistochemical expression patterns of LDH5, MCT1/2, and GLUT1 in the cancer cells and fibroblasts, the authors’ hypothesis was that the fibroblasts use tumor-derived lactate as an energy source (13). This hypothesis was subsequently supported by findings from the Lisanti laboratory which demonstrated that cancer cells utilize lactate produced by cancer-associated fibroblasts (CAFs). In co-culture experiments, MCF7 breast cancer cells induced oxidative stress and activated HIF-1 in adjacent fibroblasts, which resulted in autophagic destruction of the mitochondria and increased anaerobic glycolysis and lactate production by the stromal cells (14, 15). The lactate produced by the CAFs was then utilized by the cancer cells, a phenomenon termed the “reverse Warburg effect” (16). In this bi-directional exchange, the cancer cells actively induce metabolic changes in normal fibroblasts which act to convert them into CAFs; during this process TGF-β ligands activate HIF-1α and NFκB and caveolin-1 is downregulated resulting in impaired mitochondrial function. Because oxidative phosphorylation is reduced, CAFs, in turn, perform aerobic glycolysis and secrete lactate, which is used as an energy source by the cancer cells (17). Caveolin-1 plays a central role in stromal metabolic changes, however, the clinical relevance of caveolin-1 in cancer is unclear. Caveolin-1 is upregulated in breast, ovarian cancer, and hepatic cancer while it is down regulated in pancreatic and renal cancer (18). Similarly, high levels of lactate efflux by stromal cells, as measured by MCT4, have been associated with decreased survival in triple-negative breast cancers (19). Based on these combined data, normalization of stromal metabolism and reprograming of TME metabolic pathways could be a rational way to target tumor growth.
Stromal cell-supplied amino acids are utilized by tumor cells
It has long been recognized that glutamine is the amino acid most highly utilized by cancer cells and that many cancer cells are reliant on the presence of exogenous glutamine, a phenomenon termed “glutamine addiction”. Cancer cells use glutamine to replenish TCA cycle intermediates (anaplerosis), as a nitrogen donor for nucleotide and amino acid synthesis, and for protein translation (20). In fact, glutamine is at the intersection of genetic, epigenetic, and metabolic aberrations in cancer, as exemplified in an elegant study by Terunuma et al. Here, metabolomic profiling of breast tumors and tumor-adjacent normal tissue showed that 2-hydroxyglutarate (2HG) was 4.6-fold higher in tumors than normal tissue, was functionally linked to both glutamine metabolism and MYC activation and resulted in a global increase in DNA methylation and a poor clinical outcome (21). This study adds to numerous studies indicating a relationship between MYC activation and glutamine utilization (22).
In a process that mirrors the transferring of lactate, cancer cells are also able to obtain glutamine from the TME. Metabolomic profiling of CAFs revealed an overall catabolic phenotype in which CAFs produce several potent metabolic substrates for cancer cell use, including glutamine (23). The hypothesis that cancer cells take advantage of glutamine produced by CAFs is supported by the behavior of MCF7 breast cancer cells cultured with CAFs. In co-culture the cancer cells show: i) reduced glutamine synthesis (measured by glutamate amine ammonia ligase (GLUL)), ii) increased glutamine catabolism (measured by expression of glutaminase (GLS) and glutamate dehydroxylase 1 (GLUD1)), and iii) increased expression of a glutamine uptake transporter (SLC6A14) (24).
In another example of altered amino acid metabolism, both cancer and stromal cells (tumor-associated macrophages and CAFs) increase consumption of tryptophan and arginine through modified expression of enzymes, including indoleamine 2,3-dioxygenase, tryptophan 2,3-dioxygenase, arginase, and nitric oxide synthase (25–27). The depletion of tryptophan and arginine not only results in the production of tumor-promoting metabolites (e.g. kynurenine, ornithine, and nitric oxide) that have known roles in migration, invasion, and cell survival, it also suppresses activation of T cells and immunosurveillance (25, 27, 28). In a second example, p62, an adaptor protein for the atypical protein kinase C serine/threonine kinases, has been shown to function as a tumor suppressor in the stroma of prostate cancer, through a mechanism requiring cystine and glutamine uptake as a result of mTORC1/c-myc activation. Loss of p62 in the tumor microenvironment reduced mTORC1/c-myc, impaired expression of key cystine and glutamine transporters and reduced NADPH production through the pentose phosphate pathway, changes that resulted in impaired glutathione (GSH) production. This metabolic reprogramming promoted tumor growth through production of IL-6, TGF-β and maintenance of the CAF phenotype (29).
Stromal cystine may also promote drug resistance in chronic lymphocytic leukemia (CLL) (30). Mesenchymal stromal cells take up cystine and convert it to cysteine, which is released into the microenvironment and taken up by CLL cancer cells for GSH synthesis. High GSH expression in the CLL tumor cells augments survival and reduces drug induced cytotoxicity. This effect of GSH in cancer cells is not consistent with the effects of p62 loss in stromal cells described above, which indicates an association between impaired GSH production and the promotion of tumor growth. Such inconsistencies demonstrate the complex and at times contradictory metabolic effects in different components of the TME, which might also be very tumor type specific. Overall, what has emerged from the studies described above is yet another example of the metabolic coupling of cancer cells and the tumor stroma, in which the TME serves as an essential and renewable source of several types of amino acids that are used as metabolic building blocks for cancer cells.
Fatty acids fuel tumor growth
Adipocytes are a major constituent of the TME in renal cell, breast, and ovarian cancer, but, until recently, they have been considered passive bystanders to cancer progression. Reports in breast (31) and ovarian (32) cancer demonstrated that normal tumor-adjacent adipocytes undergo at least three functional changes that support tumor growth. First, in the presence of cancer cells, adipocytes increase secretion of inflammatory cytokines (i.e. IL-6 and IL-1β), proteases (i.e. MMP-11/stromelysin-3), and plasminogen activator inhibitor-1 (PAI-1). Second, dedifferentiation of the adjacent adipocytes occurs, as evidenced by a loss of terminal differentiation markers. In a positive feedback loop, MMP-11/stromelysin-3 further promotes adipocyte de-differentiation (33). Third, there are fewer lipid droplets in adipocytes, indicating decreased lipid accumulation and/or delipidation. These functional changes characterize the transformation of normal adipocytes into “cancer-associated adipocytes” (CAAs) which are primed to supply energy, in the form of fatty acids, to adjacent cancer cells.
One of the first clues that cancer cells obtain energy substrates from adjacent adipocytes came from the observation that, in both patient samples and in vitro co-culture experiments, ovarian cancer cells adjacent to adipocytes not only accumulate lipids but also have an increased rate of fatty acid β-oxidation (32). Upon further investigation, activation of AMP-activated protein kinase (AMPK) was noted in cancer cells cultured with adipocytes. AMPK is a central metabolic sensor, that when activated (phosphorylated), induces energy producing processes, including fatty acid oxidation. It is evident that a similar phenomenon occurs in prostate cancer since the translocation of lipids from adipocytes to prostate cancer cells has been visualized by FTIR spectroscopy (34). Interestingly, prostate cancer cells use almost no glucose and rely almost entirely on fatty acid oxidation for energy production (35), resulting in the limited utility of F18-2DG PET imaging in patients with well-differentiated prostate cancer (36).
While adipose tissue is predominately comprised of adipocytes, it also contains endothelial cells, macrophages, and fibroblasts. This amalgam of stromal cells acts as an endocrine organ, regulating energy homeostasis in the TME through the secretion of adipokines and inflammatory cytokines, a process that seems to be particularly relevant in breast and ovarian cancer (37). Cancer-associated cachexia is the clinical manifestation of this exploitive relationship between cancer cells and adipose tissue. In patients with advanced cancer, cachexia is a result of adipose atrophy induced by increased lipolysis in adipocytes. Accordingly, mice lacking the ability to increase lipolysis (deleted hormone sensitive lipase (Hsl) or adipose triglyceride (Atgl) genes) do not develop cachexia (38). These findings regarding HSL and ATGL in cachexia suggest that the metabolic exchange between stromal and cancer cells can be uncoupled and raises the possibility of novel metabolically targeted therapeutic approaches for cancer.
Clinical–Translational Advances
Broadly, therapeutic approaches aimed at reversing metabolic reprograming in the TME include drugs that inhibit the cancer cell’s ability to seize the metabolism of stromal cells or drugs that interrupt the cancer cell’s ability to use the energy resources produced by the stromal cells (Table 1).
Table 1.
Clinically tested metabolic therapeutics for cancer
| Agent | Stromal targets | Mechanism(s) of action | Clinical testing in cancer |
|---|---|---|---|
| Chloroquine | CAF | Inhibits autophagy | Phase I ongoing (NCT00224978), |
| Thiazolidinediones (TZD) | CAA | Inhibit dedifferentiation | Ongoing |
| L-asparaginase | CAF | Decrease glutamine | Approved for ALL |
| Buphenyl | CAF | Decrease glutamine | Phase I (NCT00002909, NCT00005639) |
| AZD3965 | CAF | Inhibit lactate transporters (MCTs) | Phase I ongoing (NCT01791595) |
| Dichloroacetate | CAF | Inhibit lactate production | Phase I ongoing (NCT01111097) |
| FABP-4 inhibitor | CAA | Decrease fatty acid uptake | NA |
| Metformin | CAF CAA |
Anti-oxidant Decrease fatty acid uptake |
Phase I/II ongoing (several), |
CAF: cancer-associated fibroblast, CAA: cancer-associated adipocyte, FABP: fatty acid binding protein
Treatments aimed at inhibiting the cancer cell’s ability to co-opt the metabolism of stromal cells are primarily focused on stopping the induction of CAF and CAA phenotypes. An anti-malarial drug, chloroquine, blocks oxidative stress and autophagy in fibroblasts, preventing the production of high energy mitochondrial fuels and disrupting the ability of cancer cells to induce a CAF metabolic phenotype (39). Based on promising pre-clinical results, several clinical trials are underway to determine if chloroquine is an effective adjuvant treatment for cancer (40). The challenge of chloroquine use as a cancer therapeutic is the paradoxical role of autophagy in cancer cells. Specifically, autophagy can promote tumor growth in some contexts and suppress tumor growth in other contexts; therefore, it is possible that the anti-autophagic effect of chloroquine could stimulate cancer growth.
Therapies aimed at interrupting the transformation of adipocytes into CAAs by inhibiting dedifferentiation include a class of anti-diabetic drugs, thiazolidinediones (TZDs). TZDs are ligands for the transcription factor, peroxisome proliferation-activated receptor (PPARγ), which regulates the terminal differentiation of adipocytes (41). In liposarcoma, a tumor characterized by dysfunctional adipocyte differentiation, clinical testing demonstrated that a TZD, troglitazone, inhibited de-differentiation of adipocytes (42). Unfortunately, TZD use has been associated with cardiovascular side effects and bladder cancer, resulting in the withdrawal of two TZDs, rosiglitazone and pioglitazone, from the market. These adverse events may hamper further development of TZDs as cancer therapeutics.
Effort has also been made to develop therapies aimed at interrupting the ability of cancer cells to use the energy resources produced by the stroma. The strategy of blocking the energy substrates of cancer cells has a precedent in pediatric acute lymphoblastic leukemia (ALL), since L-asparaginase (Elspar®) treatment kills ALL cells by depriving the cells of asparagine and glutamine. Unfortunately, significant toxicity prohibits use of the medication in adults (43). Another agent (Buphenyl®), which decreases plasma glutamine, has undergone phase I testing for the treatment of solid tumors (44, 45) but awaits advanced trials to test its clinical efficacy. An inhibitor of MCT 1/2 (AZD3965), takes aim at lactate transfer between tumor cells and tumor/stromal cells and is in phase I trials (46). In addition, the pyruvate dehydrogenase kinase inhibitor, dichloroacetate, reduces lactate production and may be utilized to interfere with the “reverse Warburg effect” (47). Interruption of the use of free fatty acids by cancer cells is being attempted using fatty acid binding protein-4 (FABP4) inhibitors and metformin. The inhibition of FABP4, which binds reversibly long chain fatty acids, was shown to have a protective effect in ovarian cancer in vitro and in mouse models (32).
At least one therapy, metformin, may work by both interrupting the cancer cell-induced metabolic changes in the stroma and blocking cancer cell use of stromal supplied energy. Metformin is a commonly used treatment for diabetes that has been associated with improved cancer outcomes in epidemiologic studies (48) and is currently undergoing phase II/III testing as adjuvant treatment in several cancer types. In the TME, metformin has been shown to block adipocyte-mediated lipid accumulation in ovarian cancer cells (49) and to restore caveolin-1 expression in fibroblasts, reversing the CAF phenotype induced by cancer cells (15). If metformin proves to attenuate pro-tumorgenic metabolic changes in the TME, it might also be repurposed in the primary prevention of cancer, since it is likely that these changes are important in the early steps of carcinogenesis. Moreover, since metformin has an excellent safety profile, its use can be justified in those at high risk, but without evidence of disease.
Recent reports indicating that targeted therapies lead to a metabolic response of the entire tumor organ extend the concept of metabolic interdependence in the TME even further. In a pre-clinical study, treatment of various tumors with receptor tyrosine kinase (RTK) inhibitors (sunitinib, sorafenib) induced anaerobic glycolysis and lactate production in cancer, stromal, and endothelial cells (50). When the RTK inhibitor treatment was stopped, the metabolism of the tumor organ adapted again by reducing glycolysis and shifting to an increased TCA cycle, leading to lipogenesis and rapid tumor regrowth. The mechanistic insight gained from this reversal was exploited by combining RTK inhibitor treatment and treatment with a fatty acid synthase inhibitor (Orlistat), a combination abrogating tumor regrowth and metastasis (50). As outlined above, significant efforts are underway to develop therapeutics that target the recruitment of metabolic substrates from tumor stroma, representing a new frontier in cancer drug discovery.
Summary
The study of stroma-tumor metabolic interactions, while still in its infancy, has added yet another level of complexity to our efforts to understand the tumor organ. While untangling the metabolic pathways regulated in cancer and stromal cells appears to be a formidable task, several common themes have emerged:
Cancer cells impose a self-serving metabolic program on stromal cells, recruiting them to supply energy substrates that support tumor survival and aggression.
The metabolic networks in both cancer and stromal cells have high plasticity, and are capable of fast temporal and spatial metabolic adaptation based on changing environmental cues.
Stromal cells detoxify the tumor microenvironment to reduce cancer cell apoptosis/autophagy and provide nutrients
A feasible next step in delineating the complex metabolism of the TME is to use advanced untargeted mass-spectrometry profiling approaches as well as flux analysis, coupled with sophisticated bioinformatics analysis and standardization (51, 52). These approaches will allow us to obtain an integrated picture of the metabolic changes within the different tumor compartments. It is evident that we must study the tumor organ as a functional metabolic domain if we are to understand the metabolic dependence of cancer cells on the TME and identify novel therapeutics that are able to slow/limit tumor growth.
Acknowledgments
The authors thank Gail Isenberg for editing the manuscript.
Grant Support
I.L. Romero was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH under award number K12HD000849 and the American Board of Obstetrics and Gynecology. E. Lengyel was supported by the NCI of the NIH under award numbers R01CA111882 and R01CA169604, and a grant from Bears Care, the charitable beneficiary of the Chicago Bears Football Club.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orimo A, Gupta P, Sgroi D, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL 12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Karnoub A, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 4.Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL, et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest. 2014:124. doi: 10.1172/JCI74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White EA, Kenny HA, Lengyel E. Three-dimensional modeling of ovarian cancer. Advanced Drug Deliv Rev. 2014 Jul 14; doi: 10.1016/j.addr.2014.07.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–79. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50:S138–S43. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587:5591–600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonveaux P, Schroeder VF, Wergin MC, Verrax J, Rabbani ZN, DeSaedeleer CJ, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985;17:22–34. [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role of tumor-associated stroma. Cancer Res. 2006;66:632–7. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Outschoorn UE, Whitaker-Menezes D, Pavlides S, Chiavarina B, Bonuccelli G, Trimmer C, et al. The autophagic tumor stroma model of cancer or “battery-operated tumor growth” a simple solution to the autophagy paradox. Cell Cycle. 2010;9:4297–306. doi: 10.4161/cc.9.21.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–76. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 17.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, et al. Caveolin-1−/− null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol. 2009;174:746–61. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Che G. Value of caveolin-1 in cancer progression and prognosis: Emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (review) Oncol Lett. 2014;8:1409–21. doi: 10.3892/ol.2014.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–17. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–33. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terunuma A, Putluri N, Mishra P, Mathe EA, Dorsey TH, Yi M, et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest. 2014;124:398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Simon MC. Molecular pathways: targeting MYC-induced metabolic reprogramming and oncogenic stress in cancer. Clin Cancer Res. 2013;19:5835–41. doi: 10.1158/1078-0432.CCR-12-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, et al. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9:3485–505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, et al. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: Implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12:1085–97. doi: 10.4161/cbt.12.12.18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012;22:335–41. doi: 10.1016/j.semcancer.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, et al. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013;8:e55146. doi: 10.1371/journal.pone.0055146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF, Hung WC. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014;16:410. doi: 10.1186/s13058-014-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 29.Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–35. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol. 2012;14:276–86. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirat B, Bochet L, Dabek M, Daviaud D, Dauviller S, Majad B, et al. Cancer- associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 32.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt M, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andarawewa KL, Montrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862–71. doi: 10.1158/0008-5472.CAN-05-1231. [DOI] [PubMed] [Google Scholar]
- 34.Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–4. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology. 2007;72:226–33. doi: 10.1159/000112946. [DOI] [PubMed] [Google Scholar]
- 37.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipocyte tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–41. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das S, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–8. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–33. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 40.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 41.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–56. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 42.Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951–6. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet. 2005;44:367–93. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert J, Baker SD, Bowling MK, Grochow L, Figg WD, Zabelina Y, et al. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clin Cancer Res. 2001;7:2292–300. [PubMed] [Google Scholar]
- 45.Carducci MA, Gilbert J, Bowling MK, Noe D, Eisenberger MA, Sinibaldi V, et al. A Phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin Cancer Res. 2001;7:3047–55. [PubMed] [Google Scholar]
- 46.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–94. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res. 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 49.Tebbe C, Chhina J, Dar SA, Sarigiannis K, Giri S, Munkarah AR, et al. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget. 2014;5:4123–41. doi: 10.18632/oncotarget.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G, et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell metabolism. 2014;20:280–94. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Ser Z, Locasale JW. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem. 2014;86:2175–84. doi: 10.1021/ac403845u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Members MSIB, Sansone SA, Fan T, Goodacre R, Griffin JL, Hardy NW, et al. The metabolomics standards initiative. Nat Biotechnol. 2007;25:846–8. doi: 10.1038/nbt0807-846b. [DOI] [PubMed] [Google Scholar]