Abstract
OBJECTIVES:
To determine the use and potential interactions of natural health products (NHPs) with conventional medications in children with life-limiting illnesses.
METHODS:
The present study was a retrospective medical record review of palliative care patients <18 years of age who were admitted for respite care to a Canadian paediatric hospice between January 1, 2008 and December 31, 2013. The NHPs were identified according to Health Canada’s inclusion criteria.
RESULTS:
A total of 106 children were included in the present study. Eighty-two (77.4%) had used one or more NHPs: 60 (56%) used vitamins and minerals; 45 (42.5%) used other products including probiotics, omega-3, organic acids and essential fatty acids; 34 (32.1%) used everyday consumer products; 12 (11.3%) used herb or plant-based remedies; and one (0.9%) used homeopathic remedies. Thirty-nine potential NHP-medication and 10 potential NHP-NHP interactions were identified. A considerable number of patients (n=54) used at least one medication and NHP, or two NHPs with potential interactions. The most common type of interaction was pharmacokinetic: decreasing blood concentrations of the medication, NHP or both (43.9% of NHP users); and enhancing the blood concentration of an NHP for NHP-NHP interactions (22% of NHP users).
CONCLUSION:
A high proportion of patients in respite care use NHPs. Most used NHPs and medications that have potential interactions, although there were no adverse clinical manifestations in the present study. It is important to educate health care professionals about NHPs, the evidence available and lack thereof. This could reduce the most serious interactions and improve the alliance between parents and health care providers to balance the potential risks and benefits of NHPs.
Keywords: Complementary medicine, Hospice, Interactions, Natural health products, Paediatric
Abstract
OBJECTIFS :
Déterminer l’utilisation et les interactions potentielles des produits de santé naturels (PSN) avec la médication habituelle chez des enfants ayant une maladie limitant l’espérance de vie.
MÉTHODOLOGIE :
La présente analyse rétrospective de patients en soins palliatifs de moins de 18 ans admis en soins de répit dans un centre canadien de soins palliatifs en pédiatrie s’étalait du 1er janvier 2008 au 31 décembre 2013. Les PSN ont été établis d’après les critères d’inclusion de Santé Canada.
RÉSULTATS :
Au total, 106 enfants ont fait partie de la présente étude. Quatre-vingt-deux (77,4 %) ont utilisé au moins un PSN : 60 (56 %), des vitamines et des minéraux, 45 (42,5 %), d’autres produits y compris des probiotiques, des acides gras oméga 3, des acides organiques et des acides gras essentiels, 34 (32,1 %), des produits de consommation courante, 12 (11,3 %), des remèdes à base d’herbes ou de plantes et un (0,9 %), des remèdes homéopathiques. Trente-neuf interactions potentielles entre des PSN et des médicaments et dix interactions potentielles entre des PSN et des PSN ont été recensées. Un nombre considérable de patients (n=54) a utilisé au moins un médicament et un PSN ou deux PSN ayant des interactions potentielles. Le principal type d’interaction était d’ordre pharmacocinétique : réduire les concentrations du médicament, du PSN ou des deux dans le sang (43,9 % d’utilisateurs de PSN) et accroître la concentration sanguine d’un PSN en cas d’interactions entre deux PSN (22 % des utilisateurs de PSN).
CONCLUSION :
Une forte proportion de patients en soins de répit utilisait des PSN. La plupart des enfants en soins de répit utilisait des PSN et des médicaments susceptibles d’interagir les uns avec les autres, même si la présente étude ne révélait pas de manifestations cliniques indésirables. Il est important d’informer les professionnels de la santé en matière de PSN, des données probantes disponibles ou de l’absence de telles données. Ces mesures pourraient réduire les interactions les plus graves et améliorer l’alliance entre les parents et les dispensateurs de soins pour équilibrer les risques et avantages potentiels des PSN.
Natural health products (NHPs) are used and marketed for the prevention or treatment of an illness or condition, the reduction of health risks or the maintenance of good health. A 2010 survey by Health Canada indicates that three of four Canadians have taken NHPs, and one-third use them daily (1). Moreover, in a 2004 study completed at the emergency department of The Hospital for Sick Children (Toronto, Ontario), approximately 44.9% of children used NHPs or visited a complementary alternative medicine provider (2). Their use is common among Canadian paediatric patients and has increased significantly in the past 15 years (3–5).
A 2010 survey found the incidence of NHP users experiencing side effects or unwanted reactions had increased significantly since 2005 (1). Despite the frequent use and popularity of alternative remedies, the potential benefits and risks of their use are not always clear. This uncertainty is due to several factors: use of alternative medicine is unconventional; preparations rarely meet the required standards of consistency in composition and biological activity; there is a lack of reporting of adverse events and drug interactions (6) due to a lack of professional surveillance; and specific data on organ toxicity are not readily available (7). Furthermore, many complementary health products and practices are not tested for safety or effectiveness in children, and many patients assume NHPs are safe because they are ‘natural’ and are unaware of any risks associated with their use. Thus, patients often do not link causality between NHP use and side effects or adverse reactions they have experienced (8).
The objective of the present study was to describe the reported use of NHPs in children admitted for respite care in a Canadian paediatric hospice (Roger’s House, Ottawa, Ontario). The rate of concurrent use, and the types of potential interactions among NHPs and conventional medications or other NHPs was determined. The results of the present study provide information that may help to improve patients’ safety through better awareness of NHP use in children with reduced life expectancy who are often taking very complex regimens of prescribed medications.
METHODS
Study design
The present study was a retrospective medical record review. Information was collected from 106 consecutive paediatric patients admitted to respite care at Roger’s House, a paediatric hospice, between January 1, 2008 and December 31, 2013. Initially, only data for consecutive active patients were collected. To increase the number of patients included in the study, inactive patients were then included, ie, patients who had not been cared for at Roger’s House during 2013, but who had been cared for between January 1, 2008 and December 31, 2012.
Patients >18 years of age at admission and/or discharge; patients admitted only for end-of-life care, pain and symptom management, or transition to home; and patients for whom data were not available were excluded from the present study.
Data collection
Data collection was based on documentation by health care providers in each patient’s hospice medical record, and was entered into a Research Electronic Data Capture (REDCap®) database.
Patient demographic characteristics (birth date, sex), admission diagnoses, dates of admission and discharge, medication use (dosage, frequency, route of administration) and NHP use (dosage, frequency, route of administration) were collected for each admission to respite care, for up to five admissions. If a patient had >5 admissions during the period examined in the present study, the total number of admissions to respite care was calculated and data were collected only from the first admission, first quartile, middle admission, third quartile and the final admission. For four patients who had several volumes of charts, data were obtained only from the active chart because the other charts were not available.
Ethics approval was granted by the Research Ethics Board of the Research Institute of the Children’s Hospital of Eastern Ontario (Ottawa, Ontario).
NHP classification
Each NHP was identified according to Health Canada’s inclusion criteria: NHPs are defined as “vitamins and minerals, herbal remedies, homeopathic medicines, traditional medicines such as Chinese traditional medicines, probiotics, and other products such as amino-acids and essential fatty acids” (9). Health Canada also indicates that NHPs are products made from natural sources, sold in dosage forms and are designed to maintain or promote health; to restore or correct human health function; or to diagnose, treat or prevent disease.
Anatomical Therapeutic Chemical classification
Anatomical Therapeutic Chemical (ATC) classification system controlled by the WHO was used for the classification of conventional medicines (10).
Statistical analysis
Data were analyzed using SPSS version 21.0 (IBM Corporation, USA).
Sources of data on interaction
Potential interactions between NHP and conventional medications were identified using two databases: Natural Standard (11) and Medline Plus (12). In addition, PubMed Medline (13) and the library databases of the University of Toronto (Toronto, Ontario) (14) were used to search medical literature, articles and reviews on this subject.
RESULTS
Population characteristics
A total of 106 patients were included; 73 were active and 33 were inactive. Sixty-three percent were male and 36.8% female. The mean (± SD) age at admission was 7.8±5.5 years. The diagnosis of each patient was identified and classified into one of seven categories (Figure 1).
Figure 1).
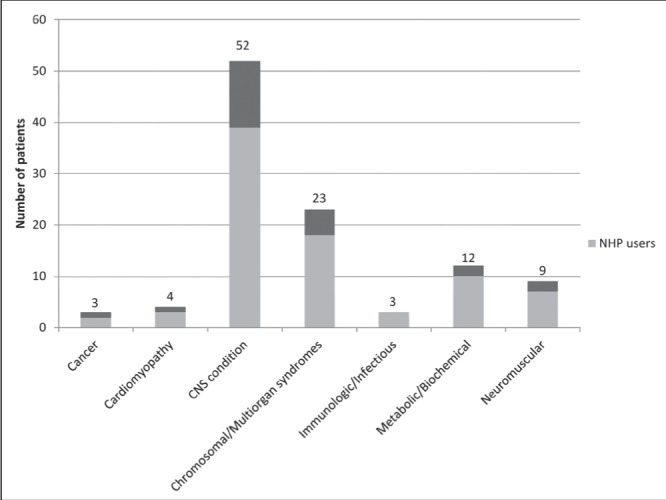
Number of patients in each diagnostic category (n=106). CNS Central nervous system; NHP Natural health product
Medication use
During the five admissions considered for the present study, 84.9% of the patients took at least one drug from the Alimentary Tract and Metabolism group (class A, ATC classification) and 89.6% took at least one drug from the Nervous System class group (class N). In class N, 70.8% used at least one analgesic (class N02), 56.6% an antiepileptic (class N03) and 43.4% a psycholeptic (class N05).
At each of the five admissions considered, each patient was taking a median of five (range zero to 17) prescription medications, not including NHPs.
NHP use
Eighty-two (77.4%) of the patients included in the present study had used ≥1 NHPs: 60 (56.6%) used vitamins or mineral supplements; 12 (11.3%) used herb or plant-based remedies; one (0.9%) used homeopathic remedies; 45 (42.5%) used other products including probiotics, omega-3 fatty acids, essential fatty acids and organic acids; and 34 (32.1%) used over-the-counter products such as enemas or antacids (Table 1).
TABLE 1.
Frequency of use of natural health products (n=106)
| Product | n (%) |
|---|---|
| Vitamins and minerals | 60 (56.6) |
| Vitamin D | 41 (38.7) |
| Multivitamin | 23 (21.7) |
| Vitamins B (B1, B2, B6, B9) | 8 (7.5) |
| Vitamin C | 5 (4.7) |
| Vitamin E | 4 (3.8) |
| Vitamin K1 | 1 (0.9) |
| Calcium | 14 (13.2) |
| Iron | 15 (14.2) |
| Magnesium | 7 (6.6) |
| Zinc | 2 (1.9) |
| Potassium | 1 (0.9) |
| Phosphate | 1 (0.9) |
| Miscellaneous | 45 (42.5) |
| Melatonin | 29 (27.4) |
| Probiotics | 9 (8.5) |
| Docosahexaenoic acid/fish oil/omega 3 | 7 (6.6) |
| L-carnitine | 7 (6.6) |
| L-arginine | 1 (0.9) |
| Creatine | 1 (0.9) |
| Ubiquinone coenzyme Q10 | 1 (0.9) |
| Alpha lipoic acid | 1 (0.9) |
| ‘Everyday consumer products’ | 34 (32.1) |
| Glycerin suppository | 22 (20.7) |
| Fleet® enema (Fleet, USA) | 6 (5.7) |
| Maalox® chewable antacid (Novartis, USA) | 6 (5.7) |
| Gaviscon® antacid (Prestige Brands, Canada) | 2 (1.9) |
| Herbal | 12 (11.3) |
| Sennosides | 4 (3.8) |
| Inulin | 5 (4.7) |
| Corn starch | 1 (0.9) |
| Others | 2 (1.9) |
| Homeopathic remedies | 1 (0.9) |
At each of the five admissions considered in the present study, each NHP user was prescribed a median of two (range zero to nine) NHPs with a median of one (range zero to four) vitamin and one (range zero to 11) NHP not including vitamins.
Potential NHP-medication and NHP-NHP interactions
Despite the unknown composition of some multivitamin products used by 15 patients, 39 potential NHP-medication interactions and 10 NHP-NHP potential interactions (Table 2) (11,12,15–59) were identified. A considerable number of patients (54 [65.8% of children taking NHPs]) had taken at least one pair of medication-NHP or NHP-NHP that could theoretically result in a pharmacodynamic or pharmacokinetic interaction (Figure 2).
TABLE 2.
Potential natural health product (NHP)-medication and NHP-NHP interactions found in our study
| NHP + medication pair | Class | Description of interaction | n (%) |
|
| |||
| Melatonin + anticonvulsants | C | The inhibitory role of melatonin on glutamate receptors (including its free radical scavenging activity), its potentiation of the GABAA-benzodiazepine receptor complex activity and its apparent lack of side effects suggest efficacy in anticonvulsant therapy when used in combination with other classical anticonvulsants. Melatonin may have a useful role in mechanisms of neuroprotection, which indicates possible use in other cases of untreatable epilepsy (11,15,16) | 17 (20.7) |
| Iron + PPIs | B | PPI therapy results in clinically significant iron malabsorption due to gastric acid hyposecretion and the risk of achlorhydria. One study shows that individuals that use of PPI for ≥1 year exhibit significantly decreased mean hemoglobin (≥1.0 g/dL) and hematocrit levels (≥3%) compared with matched controls (11,17) | 13 (15.8) |
| Vitamin D + anticonvulsants | B | Some anticonvulsant agents (phenobarbital, phenytoin, primidone, carbamazepine and oxcarbazepine) may decrease levels of vitamin D by inducing hepatic conversion of vitamin D to inactive metabolites (9,18–21) | 12 (14.6) |
| Calcium + PPIs | B | The stomach acid and the slightly acidic environment are necessary to dissociate ingested calcium (17,22). One study showed a 5.5% decrease in fractional calcium absorption with omeprazole versus placebo (23) | 11 (13.6) |
| Vitamin C + PPIs | B | At nonacidic pH, Vitamin C is unstable and is reversibly metabolized to dehydroascorbic acid. At pH >4, dehydroascorbic acid is susceptible to being hydrolysed to 2,3-diketoglulonic acid; this reaction is irreversible. This intragastric degradation reduces the mean plasma vitamin C level by 12.3% after 28 days of 40 mg/day of omeprazole (11,17,24,25) | 11 (13.4) |
| Vitamin C + acetaminophen | A | Vitamin C may decrease excretion of acetaminophen in the urine, which may increase blood levels of this medication (11,12,26) | 10 (12.2) |
| Magnesium + PPIs | B | The use of PPIs has been associated with decreased magnesium levels, and severe hypomagnesemia has been reported in some cases. This appears to be a class effect, although the mechanisms are poorly understood. The hypomagnesemia has been shown to be refractory to magnesium replacement until PPIs are stopped (1,27–29) | 5 (6.1) |
| Omega 3 + NSAID, acetaminophen | C | Treatment with fish oil capsules reduced the need for NSAID use in rheumatoid arthritis patients. Fish oil treatment increases the inhibitory activity of acetaminophen by cyclooxygenase (11,30) | 5 (6.1) |
| Folate + antiacids/H2 blockers | B | Chronic use of large doses of antacids or the use of H2 blockers may reduce folic acid absorption, but this is likely only significant if dietary folate intake is very low (11) | 4 (4.9) |
| Niacinamide + NSAIDs | C | The use of NSAIDs may reduce the tingling, itching, flushing and warmth associated with oral niacin administration (9) | 4 (4.9) |
| Riboflavin + anticholinergics | A | Anticholinergic agents decrease gastric emptying and intestinal transit rates, which in turn can increase the amount of riboflavin that is absorbed (11,12) | 3 (4.9) |
| Melatonin + azathioprine, corticosteroids | D | Some publications show an in vivo immunostimulatory action of melatonin. Taking melatonin along with immunosuppressants may decrease the effectiveness of these medications (11,12,31,32) | 3 (3.7) |
| Vitamin B12 + PPIs | B | The inhibition of gastric acid secretion by PPI therapy increases intragastric pH, which reduces the bioavailability of dietary vitamin B12 (11,17,25) | 3 (3.7) |
| Niacinamide + Anticonvulsants | A | Niacinamide may increase plasma levels of anticonvulsants, including carbamazepine, diazepam and sodium valproate (11) | 3 (3.7) |
| Vitamin D + thiazides | A | Thiazide diuretics decrease urinary calcium excretion, while vitamin D enhances intestinal calcium absorption. Concomitant use of both agents may cause or exacerbate hypercalcemia (21) | 2 (2.4) |
| Calcium + levothyroxine | B | The administration of calcium and levothyroxine at the same time was found to reduce levothyroxine absorption (33) and was associated with a significant reduction (17 pmol/L at baseline to 15 pmol/L during the calcium period) in mean serum free thyroxine (11,12,34) | 2 (2.4) |
| Iron + ACEIs | F | Iron may suppress dry cough initiated by ACEI use (11,35,36) | 2 (2.4) |
| Vitamin E + NSAID | E | Vitamin E may slow blood clotting. Taking vitamin E along with medications that also slow clotting may increase the chances of bruising and bleeding (11,12) | 2 (2.4) |
| Melatonin + clonidine | D | One study showed a significant decline in melatonin after clonidine administration (17.2 ng/L at 60 min after clonidine intake compared with 32.9 ng/L basal value) (37) | 2 (2.4) |
| Carnitine + anticonvulsants | B | Decreased serum carnitine has been noted in children using phenobarbital, carbamazepine or valproic acid. The new generation of antiepileptics have not shown this interaction (11,38,39) | 2 (2.4) |
| Beta-carotene + PPIs | B | Omeprazole decreased the absorption of a single dose of beta-carotene (11) | 2 (2.4) |
| Creatine + ibuprofen | E | NSAIDs reduce renal blood flow via prostaglandin inhibition, and creatine may be nephrotoxic. Renal function should be monitored (11,12,40) | 1 (1.2) |
| Omega3 + fosinopril | C | Fish oil can increase the hypotensive effects of ACEIs, which may significantly lower blood pressure (11,12,40,42,43) | 1 (1.2) |
| Ubiquinone (coezyme Q10) + ranitidine | A | Transportation of ranitidine may be altered. Ranitidine is a substrate of P-glycoprotein. Thus, coenzyme Q10 affects P-glycoprotein transporter. (11,44) | 1 (1.2) |
| Vitamin D + tacrolimus | B | Tacrolimus is metabolized by CYP3A4 and CYP3A5. Vitamin D requires these enzymes to be activated. Thus, concentration of the active form of Vitamin D may be altered (21) | 1 (1.2) |
| Zinc + PPIs | B | One study showed that suppression of gastric acid secretion by omeprazole reduces intestinal absorption of zinc (245 mg/dL/h to 141 mg/dL/h after omeprazole administration) (11,45) | 1 (1.2) |
| Magnesium + ibuprofen | A/E | Magnesium hydroxide significantly accelerates the absorption of ibuprofen (area under curve of ibuprofen was increased by 66% between 0 h and 1 h with magnesium hydroxide) (46). Another study showed that a tablet containing magnesium hydroxide increased the number of stomach erosions (corpus and antrum) compared with ibuprofen alone (11,47) | 1 (1.2) |
| Iron + minocycline | B | Ferrous sulfate may lead to a significant decrease in intestinal absorption of minocycline absorption. This may be due to chelation of minocycline by iron (11,12,48) | 1 (1.2) |
| Folate + ibuprofen | D | In vitro laboratory evidence suggests folate-dependent enzymes are inhibited by certain NSAIDs (11) | 1 (1.2) |
| Magnesium + cephalexin | B | Magnesium may chelate cephalexin, resulting in decreased absorption of this antibiotic (11) | 1 (1.2) |
| Magnesium + levothyroxine | B | Oral magnesium salts may chelate oral levothyroxine which, in turn, decreases thyroid hormone absorption (11) | 1 (1.2) |
| Zinc + phenytoin | B | Phenytoin may act as a chelator of zinc and may reduce zinc levels (11,49) | 1 (1.2) |
| Magnesium+ tacrolimus | B | In vivo human studies showed tacrolimus reduced renal tubular reabsorption of magnesium, resulting in increased excretion. A significant proportion of people treated with tacrolimus reported hypomagnesemia (11) | 1 (1.2) |
| Thiamine + diuretics | B | Diuretics may increase the excretion of thiamin (11,50,51) | 1 (1.2) |
| Riboflavin + diuretics | B | Diuretic therapy has been shown to decrease plasma concentrations of riboflavin (11) | 1 (1.2) |
| Magnesium + hydrochlorothiazides | B | Long-term use of thiazide diuretics may impair the kidney’s magnesium-conserving ability and lead to hypomagnesemia and hypokalemia (11) | 1 (1.2) |
| Niacinamide +clonidine | E | Taking niacin with clonidine may lower blood pressure, leading to hypotension (12) | 1 (1.2) |
| Selenium + azathioprine | D | Selenium has been shown to stimulate the immune system via cellular and humoral immunity in in vitro animal and human research (11) | 1 (1.2) |
| NHP + NHP pair | Class | Description of interaction | n (%) |
|
| |||
| Calcium+ vitamin D | A | Vitamin D increases calcium absorption from the intestine (11,12,52) | 16 (19.5) |
| Magnesium + vitamin D | A | Vitamin D may enhance the intestinal absorption of magnesium through separate active transport mechanisms in individuals with low magnesium and low vitamin D levels (12,53) | 5 (6.1) |
| Magnesium + calcium | B | Calcium supplements may decrease the absorption of dietary magnesium, but only at very high doses (11,12,54) | 5 (6.1) |
| Zinc + vitamin D | A | Research suggests that vitamin D is involved in zinc absorption (12) | 4 (4.9) |
| Zinc + calcium | B | A study showed that high-calcium diets or supplement could reduce net zinc absorption and zinc balance, and may increase the zinc requirements in adult humans (11,12,55) | 4 (4.9) |
| Iron + riboflavin | A | Riboflavin supplements may improve the way iron supplements work in some individuals with iron deficiency (11) | 4 (4.9) |
| Iron + vitamin C | A | Vitamin C increases iron absorption when taken simultaneously. This effect depends on the patient’s conditions and may not be clinically significant (11,12,56) | 3 (3.7) |
| Magnesium + zinc | B | High doses of zinc appear to decrease magnesium absorption and magnesium balance in healthy adult men (57). Moderately high dietary zinc intake appears to increase the excretion of magnesium in the feces and urine in postmenopausal women (12,58) | 2 (2.4) |
| Iron + calcium | B | Calcium inhibits iron absorption. Long-term effects are unclear because reports measure only immediate iron levels (11,12,59) | 2 (2.4) |
| Chromium + antiacids (calcium carbonate) | B | The concomitant use of antacids and chromium may reduce chromium absorption (11) | 2 (2.4) |
Percentage calculated with respect to total number of patients who were NHP users. ACEI Angiotensin-converting-enzyme inhibitor; NSAID Nonsteroidal anti-inflammatory drug; PPI Proton pump inhibitor. Types of interaction classification: class A: pharmacokinetic interaction, enhance blood concentration of NHP or medication; class B: pharmacokinetic interaction, decrease blood concentration of NHP or medication; class C: pharmacodynamic interaction, enhance therapeutic or physiological effect of NHP or medication; class D: pharmacodynamic interaction, decrease therapeutic or physiological effect of NHP or medication; class E: pharmacodynamic interaction, enhance the side effect of NHP or medication; class F: pharmacodynamic interaction, decrease the side effect of NHP or medication
Figure 2).
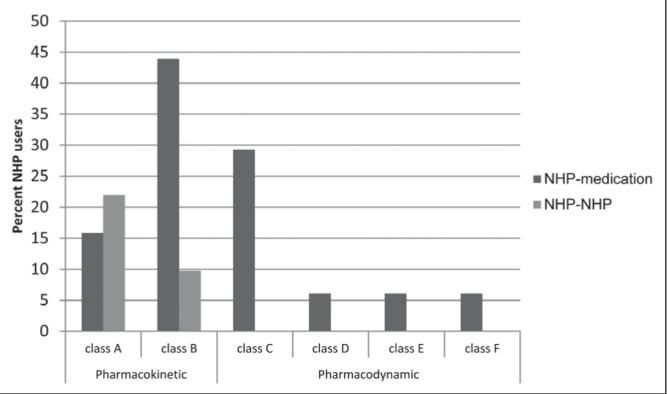
Percentage of patients with each type of potential natural health product (NHP)-medication or NHP-NHP interaction. Only NHP users are included. Some patients may have >1 interaction in each class type. Information regarding interaction class types is presented in Table 2.
A total of 49 (59.8%) children had potential NHP-medication interactions and 18 (21.9%) had potential NHP-NHP interaction(s). Twenty-seven (32.9%) patients had one potential interaction, eight (9.8%) had two potential interactions and 18 (21.9%) had ≥3 potential interactions.
DISCUSSION
In the present study, 77% of paediatric patients receiving respite care had taken at least one NHP at admission. Adams et al (60) found 37% of the paediatric population they studied, which included 979 surveys from the Stollery Children’s Hospital (Edmonton, Alberta) and the Children’s Hospital of Eastern Ontario (Ottawa), currently used NHPs. We found a higher frequency of NHP use in children admitted to hospice, which may be explained by several factors. In the present study, we investigated patient’s medical records, while Adams et al (60) gathered the information through surveys. Investigating using medical records can avoid recall bias, ie, the defective recall of events by interview or questionnaire respondents. Moreover, surveyed respondents may not be able to judge whether a product is an NHP, which may lower the reported frequency of NHP use. The population studied by Adams et al (60) and the children in the present study have chronic conditions; however, our patients had more serious pathology and limited life expectancy. The reasons behind the higher use of NHPs by patients with serious chronic medical conditions may include the lack of hope of achieving full success with conventional medications, or higher rates of side effects experienced due to conventional medications. Often, patients will turn to NHPs, either hoping for better success or to reduce side effects/deficiencies caused by drugs. Finally, the hospice is not only a medical setting; it is a ‘home away from home’.
In patients taking NHPs, the frequency of use of vitamins/minerals and herbs was similar between Adams et al (60) and the present study (85% versus 73.2%, and 15.6% versus 14.6%, respectively). The use of homeopathic remedies was lower in our study group (1.2%) than in Adams et al (60) (11.5%). This may be explained by the fact that homeopathic medicine implies complex dosage forms and schedules; it is possible that the parents would not want to impose this on the hospice.
The use of miscellaneous NHPs (probiotics, fish oil, melatonin, etc) was higher in our study than in the study by Adams et al (60) (54.9% versus 29.8%), respectively. This could be the result of emerging evidence of efficacy of these products and a higher acceptance by the medical community.
We found a considerable number of patients who presented with at least one potential NHP-medication and/or NHP-NHP interaction (65.8% of all patients). This rate is higher than that observed by Goldman et al (2), who surveyed children arriving in emergency departments, 16% of which had potential NHP-NHP or NHP-medication interactions. This may be explained by a lower number of patients (28%) with chronic conditions arriving at the emergency department than at our hospice (100%).
The high number of pharmacokinetic interactions between NHPs and conventional medications was due to the frequent use of proton pump inhibitors and H2-receptor antagonists (77.4% of children), which decrease the absorption of different vitamins and minerals, leading to deficiencies (17). Additionally, some drugs, such as levothyroxine and several antibiotics, are sensitive to other elements (such as calcium and magnesium) taken at the same time. Regular monitoring or staggered dosing between NHPs and medications or other NHPs can easily avoid many potential interactions.
In spite of the potential, most of these interactions do not present with clinical signs or symptoms. There were no reports of adverse events attributed to interactions during the study period.
The number of well-controlled published NHP clinical trials is limited. However, the integration of supplements into current pharmacotherapeutic approaches to patient care can be considered in selected cases. Some NHPs may decrease the risk of side effects from conventional drugs such as iron’s reduction of dry cough due to angiotensin-conversing-enzyme inhibitor use (35,36). Other NHPs could reduce the need for nonsteroidal antinflammatory drugs and acetaminophen, and indirectly decrease their side effects. For example, omega-3 fatty acids may have anti-inflammatory properties and, thus, reduce the need for higher doses of nonsteroidal antinflammatory drugs and acetaminophen (30). Several NHPs and conventional drugs have a synergistic action. Melatonin has GABAergic properties and may, thus, potentiate the effect of some anticonvulsants, and reduce the oxidative stress and subsequent damage caused by anticonvulsant therapy (15,16). However, caution is advised because melatonin has been reported to cause seizures in certain patients with severe neurological disorders, and treatment should be individualized based on patients’ response (61). No such pharmacodynamic combinations are advised or available in the drug market. The only beneficial pharmacokinetic associations recognized in the present study and approved by Health Canada are calcium-vitamin D (62) and iron-vitamin C (63). More and better-quality research on NHPs is needed to determine whether these agents have any significant therapeutic potential and whether they can be used in conjunction with prescription medications that will have clinical benefits.
Study limitations
Our study had several limitations. First, we included all potential interactions for conventional drugs and NHPs prescribed, even if they were not always administered to the children. Second, we did not include topical preparations. Third, it is possible that parents did not bring all of the NHPs to the hospice in fear of judgement from health care personnel on the use of NHPs. All of these factors could explain why we found only a low incidence of herbal and homeopathic remedies. Additionally, the composition of some multivitamins was unknown; therefore, it was not possible to evaluate the potential interactions for these products.
Not all potential interactions between medications and NHPs or between NHPs and other NHPs have been studied in full. Some interactions may exist but have not been studied or become apparent over time; others have been studied, but the results are unclear. This subject remains controversial, and additional well-designed studies are needed to clarify this issue. Some interactions during the absorption phase could have been avoided if more time was allowed between administrations. Finally, most of the interactions do not show clinical manifestations and would only be apparent with blood sampling or other means, which were not evaluated in the present study.
CONCLUSION
A high proportion of the patients in respite care in the present study use NHPs. Most use NHPs and medications that have potential interactions, although in the present study they did not lead to adverse clinical manifestations. However, it is important to educate health care professionals on NHPs, the evidence available and lack thereof. This could reduce the most serious interactions, and improve the alliance between parents and health care providers to balance the potential risks and benefits of NHPs.
Acknowledgments
The authors thank the team from Roger’s House for their assistance and support in this study. This work originated at the Children’s Hospital of Eastern Ontario, and was approved by the Research Ethics Board at the Children’s Hospital of Eastern Ontario.
REFERENCES
- 1.Ipsos Reid Public Affairs. Natural Health Product Tracking Survey – 2010 Final Report. Jan 1, 2011. < http://epe.lac-bac.gc.ca/100/200/301/pwgsc-tpsgc/por-ef/health/2011/135-09/report.pdf> (Accessed June 4, 2014).
- 2.Goldman RD, Rogivik AL, Lai D, Vohda S. Potential interactions of drug-natural health products and natural health products-natural health product among children. J Pediatr. 2008;152:521–6. doi: 10.1016/j.jpeds.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Kemper KJ, Vohra S, Walls R. The use of complementary and alternative medicine in pediatrics. Pediatrics. 2008;122:1374. doi: 10.1542/peds.2008-2173. [DOI] [PubMed] [Google Scholar]
- 4.Dany J, Cyr C. Use of complementary and alternative medicine in a general pediatric clinic. Pediatrics. 2007;120:e138–41. doi: 10.1542/peds.2006-3105. [DOI] [PubMed] [Google Scholar]
- 5.Loman DG. The use of complementary and alternative health care practices among children. J Pediatr Health Care. 2003;17:58–63. doi: 10.1067/mph.2003.29. [DOI] [PubMed] [Google Scholar]
- 6.Vohra S, Cvijovic K, Boon H, Foster B, Jaeger W, LeGatt D, et al. Study of Natural Product Adverse Reactions (SONAR): Active surveillance of adverse events following concurrent natural health products and prescription drug use in community pharmacies. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson CR, De Broe ME. Kidney injury from alternative medicines. Adv Chronic Kidney Dis. 2005;12:261–75. doi: 10.1016/j.ackd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Raynor DK, Dickinson R, Knapp P, Long AF, Nicolson DJ. Buyer beware? Does the information provided with herbal products available over the counter enable safe use? BMC Med. 2011;9:94. doi: 10.1186/1741-7015-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Canada. Drugs and Natural Health Products (updated June 6, 2012). What are natural health products? < http://hc-sc.gc.ca/dhp-mps/prodnatur/about-apropos/cons-eng.php> (Acccessed June 4, 2014).
- 10.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2014 (updated December 19, 2013) < www.whocc.no/atc_ddd_index/> (Accessed June 4, 2014).
- 11.Natural Standard. The Authority on Integrative Medicine. Databases: Food, Herbs & Supplements. < https://naturalmedicinestherapeuticresearch-com.myaccess.library.utoronto.ca/databases/food,-herbs-supplements.aspx> (Accessed June 4, 2014).
- 12.U.S. National Library Medicine. MedlinePlus: Herbs and Supplements. < www.nlm.nih.gov/medlineplus/druginfo/herb_All.html> (Accessed June 4, 2014).
- 13.UMeSH Database. National Library of Medicine National Institutes of Health, PubMed. < www.ncbi.nlm.nih.gov/mesh> (Accessed June 4, 2014).
- 14.Leslie Dan Faculty of Pharmacy. University of Toronto libraries. < http://onesearch.library.utoronto.ca/?q=onesearch/search/> (Accessed June 4, 2014).
- 15.Gupta M, Gupta YK, Agarwal S, Aneja S, Kohli K. A randomized, double-blind, placebo controlled trial of melatonin add on therapy in epileptic children on valproate monotherapy: Effect on glutathione peroxidase and glutathione reductase enzymes. Br J Clin Pharmacol. 2004;58:542–7. doi: 10.1111/j.1365-2125.2004.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina-Carballo A, Munoz-Hoyos A, Reiter RJ, et al. Utility of high doses of melatonin as adjunctive anticonvulsant therapy in a child with severe myoclonic epilepsy: Two years’ experience. J Pineal Res. 1997;23:97–105. doi: 10.1111/j.1600-079x.1997.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 17.Heidelbaugh J. Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–33. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott M, Boppana P, Toguri J, DeSantis A. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia. 2006;47:510–5. doi: 10.1111/j.1528-1167.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 19.Baek JH, Seo YH, Kim GH, Kim MK, Eun BL. Vitamin D levels in children and adolescents with antiepileptic drug treatment. Yonsei Med J. 2014;55:417–21. doi: 10.3349/ymj.2014.55.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettekoven S, Strohle A, Trunz B, et al. Effects of antiepileptic drug therapy on vitamin D status and biochemical markers of bone turnover in children with epilepsy. Eur J Pediatr. 2008;167:1369–77. doi: 10.1007/s00431-008-0672-7. [DOI] [PubMed] [Google Scholar]
- 21.Robien K, Oppeneer SJ, Kelly JA, Hamilton-Reeves JM. Drug-Vitamin D interactions: A systematic review of the literature. Nutr Clin Pract. 2013;28:194–208. doi: 10.1177/0884533612467824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: S randomized crossover trial. Am J Med. 2005;118:778–81. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Kopic S, Geibel J. Gastric acid, calcium absorption, and their impact on bone heath. Physical Rev. 2013;93:189–268. doi: 10.1152/physrev.00015.2012. [DOI] [PubMed] [Google Scholar]
- 24.Henry EB, Carswell A, Wirz A, Fyffe V, McColl KE. Proton pump inhibitors reduce the bioavailability of dietary vitamin C. Aliment Pharmacol Ther. 2005;22:539–45. doi: 10.1111/j.1365-2036.2005.02568.x. [DOI] [PubMed] [Google Scholar]
- 25.McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol. 2009;104:S5–S9. doi: 10.1038/ajg.2009.45. [DOI] [PubMed] [Google Scholar]
- 26.Houston JB, Levy G. Drug biotransformation interactions in man VI: Acetaminophen and ascorbic acid. J Pharmacol Sci. 1976;65:1218–21. doi: 10.1002/jps.2600650822. [DOI] [PubMed] [Google Scholar]
- 27.Famularo G, Gasbarrone L, Minisola G. Hypomagnesemia and proton-pump inhibitors. Expert Opin Drug Saf. 2013;12:709–16. doi: 10.1517/14740338.2013.809062. [DOI] [PubMed] [Google Scholar]
- 28.Cundy T, Mackay J. Proton pump inhibitors and severe hypomagnesemia. Curr Opin Gatroenterol. 2011;27:180–5. doi: 10.1097/MOG.0b013e32833ff5d6. [DOI] [PubMed] [Google Scholar]
- 29.Sheen E, Triadafilopoulous G. Adverse effects of long-term proton inhibitor therapy. Dig Dis Sci. 2011;56:931–50. doi: 10.1007/s10620-010-1560-3. [DOI] [PubMed] [Google Scholar]
- 30.Tur J, Bibilone M, Sureda A, Pons A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Brit J Nutr. 2012;107:S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- 31.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 32.Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001;10:467–76. doi: 10.1517/13543784.10.3.467. [DOI] [PubMed] [Google Scholar]
- 33.Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483–6. doi: 10.1089/thy.2010.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822–5. doi: 10.1001/jama.283.21.2822. [DOI] [PubMed] [Google Scholar]
- 35.Lee SC, Park SW, Kim DK, Lee SH, Hong KP. Iron supplementation inhibits cough associated with ACE inhibitors. Hypertension. 2001;38:166–70. doi: 10.1161/01.hyp.38.2.166. [DOI] [PubMed] [Google Scholar]
- 36.Bhalla P, Singh NP, Ravi K. Attenuation of angiotensin converting enzyme inhibitor induced cough by iron supplementation: Role of nitric oxide. J Renin-Angio-Aldo. 2011;12:491–7. doi: 10.1177/1470320311399604. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Hoyos A, Femandez-Garcia JM, Molina-Carballo A, et al. Effect of clonidine on plasma ACTH, cortisol and melatonin in children. J Pineal Res. 2000;29:48–53. doi: 10.1034/j.1600-079x.2000.290107.x. [DOI] [PubMed] [Google Scholar]
- 38.Lheureux PE, Penaloza A, Zahir S, Gris M. Science review: Carnitine in the treatment of valproic acid-induced toxicity – what is the evidence? Critical Care. 2005;9:431–40. doi: 10.1186/cc3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro-Gago M, Eirís-Puñal J, Novo-Rodríguez MI, et al. Serum carnitine levels in epileptic children before and during treatment with valproic acid, carbamazepine, and phenobarbital. J Child Neurol. 1998;13:546–9. doi: 10.1177/088307389801301104. [DOI] [PubMed] [Google Scholar]
- 40.Hall M, Trojian T. Creatine supplementation. Curr Sports Med Rep. 2013;12:240–4. doi: 10.1249/JSR.0b013e31829cdff2. [DOI] [PubMed] [Google Scholar]
- 41.Miller PE, Elswyk MV, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885–96. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueshima H, Stamler J, Elliot P, et al. Food omega-3 fatty acid intake of individuals and their blood pressure: INTERMAP study. Hypertens. 2007;50:313–9. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Brit J Nutr. 2012;107(S2):S195–S200. doi: 10.1017/S0007114512001584. [DOI] [PubMed] [Google Scholar]
- 44.Itagaki S, Ochiai A, Kobayashi M, Sugawara M, Hirano T, Iseki K. Interaction of coenzyme Q10 with the intestinal drug transporter P-Glycoprotein. J. Agric Fodd Chem. 2008;56:6923–7. doi: 10.1021/jf800992p. [DOI] [PubMed] [Google Scholar]
- 45.Ozutemiz AO, Aydin HH, Isler M, Celik HA, Batur Y. Effect of omeprazole on plasma zinc levels after oral zinc administration. Indian J Gastroenterol. 2001;21:216–8. [PubMed] [Google Scholar]
- 46.Neuvonen P. The effect of magnesium hydroxide on the oral absorption of ibuprofen, ketoprofen and diclofenac. Br J Clin Pharmacol. 1991;31:263–6. doi: 10.1111/j.1365-2125.1991.tb05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maenpaa J, Tarpila A, Jouhikainen T, et al. Magnesium hydroxide in ibuprofen tablet reduces the gastric mucosal tolerability of ibuprofen. J Clin Gastroenterol. 2004;38:41–5. doi: 10.1097/00004836-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride. J Am Acad Dermatol. 1985;12:308–12. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 49.Palm R, Hallmans G. Zinc and copper metabolism in phenytoin therapy. Epilepsia. 1982;23:453–61. doi: 10.1111/j.1528-1157.1982.tb05433.x. [DOI] [PubMed] [Google Scholar]
- 50.Zenuck C, Healey J, Donnelly J, Vaillancourt R, Almalki Y, Smith S. Thiamine deficiency in congestive heart failure patients receivng long term furosemide therapy. Can J Clin Pharmacol. 2002;10:184–8. [PubMed] [Google Scholar]
- 51.Suter PM, Vetter W. Diuretics and Vitamin B1: Are diuretics a risk factor for thiamin malnutrition? Nutr Rev. 2000;58:319–23. doi: 10.1111/j.1753-4887.2000.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 52.Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347:25–9. doi: 10.1016/j.mce.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saris NEL, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium, an update on physiological, clinical and analytical aspects. Clinica Chimica Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 54.Harwick LL, Jones MR, Brautbar N, Lee DB. Magnesium absorption: Mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991;121:13–23. doi: 10.1093/jn/121.1.13. [DOI] [PubMed] [Google Scholar]
- 55.Wood RJ, Zheng JJ. High dietay calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr. 1997;65:1803–9. doi: 10.1093/ajcn/65.6.1803. [DOI] [PubMed] [Google Scholar]
- 56.Fishman SM, Christian P, West KP. The role of vitamins in the prevention and control of anaemia. Public Heath Nutr. 2000;3:125–50. doi: 10.1017/s1368980000000173. [DOI] [PubMed] [Google Scholar]
- 57.Spencer H, Norris C, Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J Am Coll Nutr. 1994;13:479–84. doi: 10.1080/07315724.1994.10718438. [DOI] [PubMed] [Google Scholar]
- 58.Nielsen FH, Milne DB. A moderately high intake compared to a low intake of zinc depresses magnesium balance and alters indices of bone turnover in postmenopausal women. Eur J Nutr. 2004;58:703–10. doi: 10.1038/sj.ejcn.1601867. [DOI] [PubMed] [Google Scholar]
- 59.Hallberg L. Does calcium interfere with iron absorption? Am J Clin Nutr. 1998;68:3–4. doi: 10.1093/ajcn/68.1.3. [DOI] [PubMed] [Google Scholar]
- 60.Adams D, Dagenais S, Clifford T, et al. Complementary and alternative medicine use by padreatric subspeciality outpatients. Pediatrics. 2013;131:225–32. doi: 10.1542/peds.2012-1220. [DOI] [PubMed] [Google Scholar]
- 61.Sheldon SH. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet. 1998;351:1254. doi: 10.1016/S0140-6736(05)79321-1. [DOI] [PubMed] [Google Scholar]
- 62.Health Canada. Licensed Natural Health Products Database: Caltrate 600 with Vitamin D. Updated February 2, 2014. < http://webprod5.hc-sc.gc.ca/lnhpd-bdpsnh/info.do?licence=02231948&lang=eng> (Accessed June 4, 2014).
- 63.Health Canada. Licensed Natural Health Products Database: Vitron-C. Updated February 2 2014. < http://webprod5.hc-sc.gc.ca/lnhpd-bdpsnh/info.do?licence=80027636&lang=eng> (Accessed June 4, 2014).


