Abstract
The nuclear envelope in eukaryotic cells has important roles in chromatin organization. The inner nuclear membrane contains over 60 transmembrane proteins. LAP2-emerin-MAN1 (LEM)-domain containing proteins of the inner nuclear membrane are involved in tethering chromatin to the nuclear envelope and affect gene expression. They contain a common structural, bi-helical motif, the so-called LEM domain, which mediates binding to a conserved chromatin protein, Barrier-to-Autointegration Factor (BAF). Interestingly, this domain is highly related to other bi-helical motifs, termed helix-extension-helix (HeH) - and SAF-Acinus-PIAS (SAP) motifs, which are directly linked to DNA. Here we summarize evidence that the LEM motif evolved from the HeH and SAP domains concomitantly with BAF. In addition we discuss the potential evolution of HeH/SAP and LEM domain containing proteins and their role in chromatin tethering and gene regulation from unicellular eukaryotes to mammals.
Keywords: LEM, SAP, HeH, nuclear envelope, chromatin organization, gene expression
The nucleus of eukaryotic cells houses the genomic DNA and is physically and functionally separated from the cytoplasm by the nuclear envelope (NE) [1, 2]. The NE consists of two concentric lipid bilayers, the inner and outer nuclear membrane (INM and ONM), which are connected by the pore membrane at the sites of nuclear pore complexes (NPCs). The ONM is continuous with the endoplasmic reticulum (ER) membrane and many membrane proteins of the ER are also found in the ONM. The INM, in contrast includes over 60 putative transmembrane proteins specifically targeted and/or retained at the INM [3, 4]. In metazoa, the lamina - a protein meshwork of type V intermediate filament proteins called lamins - is attached to the nuclear side of the INM. It provides mechanical stability for the nucleus and serves as an interaction platform for chromatin and various signaling molecules and transcription factors [5]. Lamins and several proteins of the INM directly interact with DNA and/or chromatin proteins and have active roles in chromatin organization and in the regulation of gene expression. In this review, we focus on the role of a specific group of INM proteins, termed LEM-proteins, in chromatin tethering to the NE, and highlight some aspects of the evolution of LEM-proteins in particular the evolvement of the domains mediating chromatin binding.
Chromatin organization in the 3D space of the nucleus regulates gene expression
Interphase chromosomes occupy distinct territories within the nucleoplasm [6-8]. There is increasing evidence that the localization of a gene within the nucleus affects its epigenetic regulation and gene expression [9-12]. Chromosome and gene positioning in the nucleus are tightly regulated during differentiation. In fact, specific (re-) positioning of gene clusters during development and during differentiation is increasingly recognized as means to control differentiation-specific gene expression. For instance, testis specific gene clusters are repressed in Drosophila somatic cells by lamin-mediated tethering at the nuclear periphery [13]. In C. elegans, ectopic DNA arrays containing reporters driven by myogenic promoters have been shown to translocate away from the nuclear periphery during muscle differentiation - concomitant with the activation of muscle specific promoters [9]. In addition, the IgH loci in B-cells were found to change their subnuclear position before and after V(D)J recombination, indicating that NE tethering can also limit the availability of certain loci to recombination factors [14, 15].
In metazoan cells the nuclear periphery has mostly been described as a gene-silencing compartment [16]: Gene-rich, transcriptionally active euchromatin is mostly found in the nuclear interior, whereas gene-poor and transcriptionally silent heterochromatin is concentrated near the nuclear envelope (NE) [17, 18]. A DamID approach using a Lamin B1-adenine methyltransferase (Dam) fusion protein revealed preferential tethering of intergenic regions with repressive histone marks at the NE [19]. This led to the concept that lamina-associated domains (LADs), whose molecular properties remain elusive, mediate anchoring of genomic regions to the periphery and keep genes in a transcriptionally “locked” state in response to differentiation cues [20-22]. In addition, several groups have shown that artificial tethering of ectopic and endogenous genes to the nuclear periphery can repress transcription [11, 15] although this was not seen in all studies [23] and may also be influenced by the respective experimental setup, such as the promoters used [24]. Although lamins and several INM proteins have been shown to bind DNA or chromatin in vitro [3, 25], the role of these proteins in chromatin tethering at cellular level remains unclear. In view of previous proteomic studies showing that the NE of rat liver cells contains over 60 different potential integral membrane proteins [4], most of which are uncharacterized at the moment [26], it is plausible that functional redundancy of these proteins assures a tight regulation of position-mediated gene expression. The picture may become even more complex, as evidence is emerging that different cell types express different sets of INM proteins [27-29]. The overlap of INM protein expression in different cells/tissues and their general and tissue-specific functions have yet to be determined, but LEM proteins are good candidates for having fundamental roles in chromatin tethering and gene positioning.
Evolution of the LEM domain as a chromatin-binding motif in INM proteins
Members of the LEM-protein family are among the best characterized INM proteins to date [30]. LEM proteins are defined by the presence of a common structural bi-helical motif called LAP2-Emerin-MAN1 (LEM) domain (Fig. 1) [31, 32]. The canonical LEM-domain was shown by several means to bind Barrier-to-Autointegration Factor (BAF) [33-35], an essential 10 kDa chromatin-associated protein found in all metazoa [36-38] (Fig. 2B). Thereby, LEM-proteins are thought to dynamically associate with chromatin [39], likely regulated by phosphorylation of BAF [40-42] and other so far unknown mechanisms.
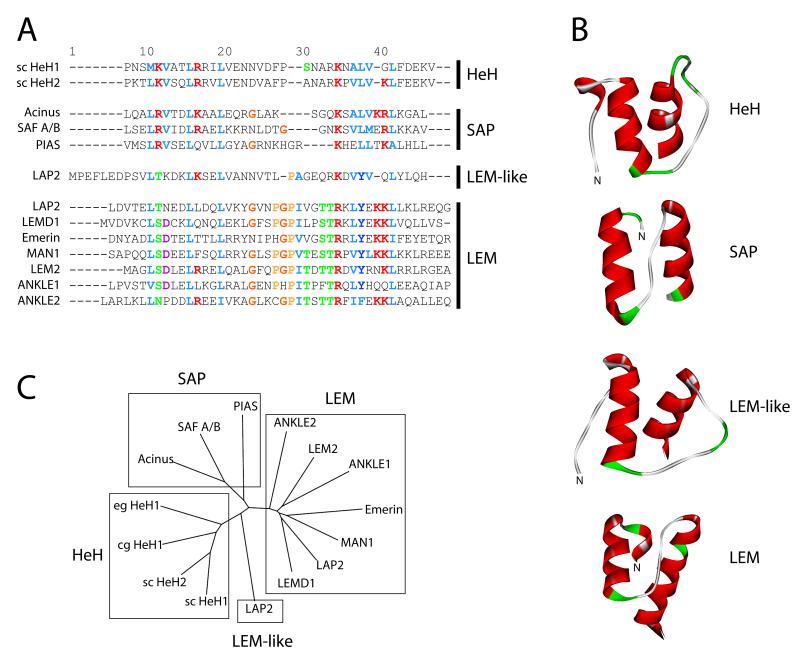
Figure 1. The LEM-, LEM-like, SAP and HeH motifs are conserved at sequence and structural levels.
(A) Alignment of yeast HeH-, and human SAP-, LEM-like and LEM-domain sequences. Colors indicate conservation of biochemically similar residues according to the ClustalX color scheme (http://www.clustal.org/). (B) In silico secondary structure predictions (http://www.cbs.dtu.dk/services/CPHmodels/) of yeast HeH1 and the human SAP-motif of PIAS1, LEM-like motif of LAP2 and LEM-motif of emerin. (C) Phylogenetic tree calculated from sequence alignments using ClustalX.
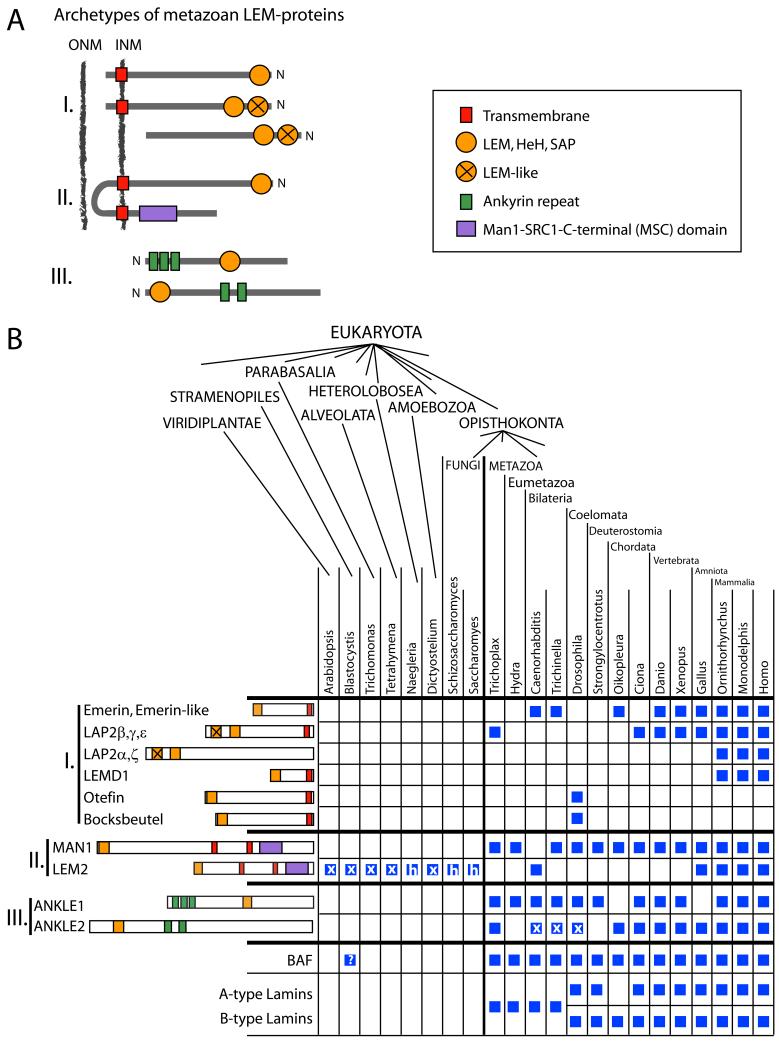
Figure 2. The evolution of the LEM domain-containing protein family.
(A) Schematic drawing of the domain organization of LEM-proteins, defining three different groups. (B) Phylogenetic analysis of predicted and experimentally identified LEM-protein sequences obtained from NCBI and ENSEMBL databases. The presence of homologous LEM-proteins in selected species representing major lineages of the eukaryotic evolution are indicated by a blue square, “h” indicates the presence of a predicted HeH/SAP motif instead of a canonical LEM-motif. Squares are marked with “X” if the bi-helical motif was not detectable in an otherwise homologous sequence. Note that incomplete genomic sequence information may account for the absence of LEM-proteins in the scheme (e.g. Oikopleura Ankle1). Accession numbers can be found in supplemental table 1. Polypeptides predicted from genomic sequence using Genscan software (http://genes.mit.edu/GENSCAN.html) are marked with a “*”.
Interestingly, lamina-associated-polypeptide 2 (LAP2) one of the “founding” members of the LEM-protein family, contains an additional LEM-related motif, termed LEM-like domain, which is 30% similar to the canonical LEM motif in primary sequence and forms a similar bi-helical structure (Fig. 1 A, B). However, in contrast to the canonical LEM motif, the LEM-like domain has been found to interact directly with ds DNA [34].
Intriguingly, the LEM- and LEM-like domains are also highly related to the SAF-A/B, Acinus and PIAS (SAP) - and helix-extension-helix (HeH)-domain superfamilies [43, 44]. All four motifs share conserved residues on primary sequence level (Fig. 1A) and a highly similar structural organization, consisting of two parallel alpha helices connected by an extended loop (Fig. 1B and compare fold #63450 in SCOP database http://scop.mrc-lmb.cam.ac.uk/scop/). Similar to the LEM-like motif, the SAP and very likely also the HeH motifs interact directly with DNA [45]. Consistently, orthologs of metazoan LEM-proteins in unicellular eukaryotes, which lack BAF, contain a SAP or HeH instead of a LEM motif (Fig. 2B, squares with “h”), suggesting that the LEM-domain evolved from an ancestral SAP/HeH domain in chromosome tethering proteins, concomitant with the emergence of BAF.
The evolution of LEM-proteins and their role as essential chromatin-tethering factors
In mammals three major types of LEM proteins can be distinguished based on their domain organization (Fig. 2A). Lamina-associated polypeptide 2 (LAP2), emerin and LEMD1 are transmembrane proteins of the INM (Fig. 2A, group I), with a long nucleoplasmic domain including the LEM motif, a single transmembrane spanning domain and a short luminal domain between INM and ONM [46, 47]. Interestingly, LAP2 is expressed as six alternatively spliced isoforms, all of which contain a LEM-like motif upstream of the LEM motif [48]. Two of the LAP2 isoforms, LAP2α and LAP2ζ, lack a transmembrane domain and localize in the nucleoplasm [48, 49]. The second group of LEM proteins (II) in the INM includes LEM2 and MAN1. They contain an N-terminal LEM domain in the nucleoplasm, and two transmembrane domains [32, 50]. Group III LEM proteins include two so far uncharacterized LEM-proteins, Ankyrin and LEM domain containing protein (Ankle) 1 and 2, which lack a transmembrane domain and may localize in the nucleoplasm and/or cytoplasm.
The number of genes encoding LEM domain-containing proteins increased during evolution (Fig. 2B). In metazoa, at least one group I, one group II- and both group III proteins seem to form a minimal set of LEM-proteins. In unicellular eukaryotes, however, only one type of LEM-protein is conserved, representing an ortholog of the metazoan group II LEM2/MAN1 gene (Fig. 2B). According to in silico predictions, all representatives of group II LEM-proteins containing an N-terminal LEM-, SAP- or HeH domain, also contain a C-terminal MAN1-SRC1p-C-terminal (MSC) motif downstream of the second transmembrane region. The structure of the MSC motif was solved for human MAN1 and revealed a winged-helix fold that interacts directly with DNA [51]. The MSC motif likely is evolutionary older than the bi-helical SAP / HeH domain, as it is also found in related transmembrane proteins in early eukaryotic lineages (unicellular organisms and plants), which otherwise lack a bi-helical motif at their N-terminus (Fig. 2B, squares with X). Based on the high conservation of the MSC domain we hypothesize that the LEM domain (initially the HeH/SAP domain) evolved in MSC-domain containing, chromatin-binding proteins, to generate a specific “clamp-like” DNA-interacting conformation. Comparing the two mammalian group II LEM-proteins, MAN1 and LEM2, MAN1 contains an additional C-terminal domain downstream of the MSC domain, which is predicted to serve as an RNA recognition motif (RRM). Experimentally, however, RNA binding could not be detected so far. Instead, this motif was reported to interact with R-Smad proteins, downstream components of the transforming growth factor (TGF)ß-/bone morphogenic protein (BMP) signaling pathway [52]. This interaction is thought to recruit and deactivate Smad-complexes [53], thus antagonizing the TGFß- and BMP signaling. The findings that (1) unlike LEM2, MAN1 is present in Placozoa (Trichoplax adherens) and most other early metazoan lineages, and that (2) both, LEM2 and MAN1, are co-expressed only in amniota (Fig. 2B), suggests that LEM2 in birds and mammals originated from a MAN1 gene duplication and a concomitant loss of the RRM motif. Noteworthy, database searches showed, that among all pseudocoelomata analyzed, Caenorhabditis was the only genus that lacks a MAN1-type protein, but possess LEM2 (lacking the RRM motif). In species, where both genes are present the proteins may share redundant functions in chromatin organization, mediated by their common LEM-and MSC domains. Both proteins, however, seem to be essential, as lack of either LEM2 (see homepage Larry Gerace, http://www.scripps.edu/cb/gerace/research.html) or MAN1 [54] causes embryonic lethality in mice.
LEM-proteins acquired multiple functions throughout evolution
Four excellent recent studies on the LEM2 homologs in yeast, HeH1 or SRC1p, and in C.elegans, ceLEM-2, shed more light on their role in tethering chromosomes to the NE. HeH1 was shown to associate predominantly with repetitive and transcriptionally silent DNA, such as telomeric and subtelomeric DNA and rDNA loci [12, 55-57]. HeH1-mediated tethering of these chromatin regions causes transcriptional silencing [56] and represses deleterious recombination events at rDNA loci [12, 55, 57], thereby maintaining genomic stability. Also in C.elegans, LEM2 was shown experimentally to tether chromosome arms at the NE [58]. ChIP-on-chip and ChIP-Seq analyses identified distal ends of chromosomes as LEM2-associated chromatin subdomains, which contain increased levels of the silencing epigenetic marks, H3K9me3 and H3K27me3. In contrast, the genomic regions located between LEM2-associated subdomains contained highly transcribed genes, which may loop out into the nuclear interior. It remains to be tested, whether the LEM2-associated regions in C.elegans correlate with the lamina-associated domains (LADs) described in mammalian cells [19]. Overall LEM-proteins clearly seem to be involved in tethering distinct chromatin subdomains to the nuclear periphery, thereby regulating gene expression and maintaining genomic integrity. Very little is known about the DNA sequence motifs, chromatin-associated factors or epigenetic marks in these genomic regions that mediate LEM-protein binding.
HeH/LEM-proteins have acquired additional functions during evolution, some of which are redundant. In yeast, heh1Δ and heh2Δ strains exhibited mislocalization of key components of the NPC (nucleoporins), suggestion functions in NPC assembly and/or integrity [59]. In addition, HeH1 is also part of the THO-transcription export (TREX) pathway, implying a potential function in mRNA export [56]. Whether any of these additional functions might be conserved in multicellular organisms remains elusive at present. To date, other, partially redundant roles have been identified in metazoan LEM-proteins. For instance, emerin and LEM2 can functionally compensate each other in several important activities, as shown in C.elegans [60] and mammalian cells [61]. While the phenotype of individual emerin or Lem-2 knockdown in worm strains was mild, the combined depletion of both genes revealed crucial functions in chromosome segregation and mitosis [60]. In human cells, LEM2 knockdown caused nuclear envelope deformations and mitotic defects [62]. Various LEM-proteins have also been found to regulate signaling pathways: Mouse LEM2 modulates ERK signaling during myogenic differentiation [61]. Otefin, a group I LEM-protein present in Drosophila and other insect species is required for Dpp signaling-mediated germline stem cell fate regulation [63]. Emerin mediates nuclear export of ß-catenin, a component of the wnt signaling pathway and represses the expression of wnt targets [64]. Both, emerin and MAN1 also interact with the germ cell-less (GCL) repressor protein and the transcriptional regulator Btf [65, 66]. In addition, the identification of emerin binding partners on a proteome wide basis suggested a multitude of additional functions of emerin in gene regulation, mRNA splicing, signaling, mechanosensing and nuclear architecture [67, 68]. The INM LAP2 isoform, LAP2ß, functions in the initiation of replication and as a transcriptional repressor via interaction with HA95 and GCL, respectively [69, 70].
Although not experimentally proven yet, it is tempting to speculate that many of these functions of the LEM proteins require the chromatin-binding activity of their LEM motif.
Supplementary Material
Acknowledgements
Work in the authors’ laboratory was supported by a grant from the Austrian Science Research Fund (FWF P17871) to RF.
References
- 1.D’Angelo MA, Hetzer MW. The role of the nuclear envelope in cellular organization. Cell Mol Life Sci. 2006;63:316–332. doi: 10.1007/s00018-005-5361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hetzer MW, Walther TC, Mattaj IW. Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu Rev Cell Dev Biol. 2005;21:347–380. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 3.Wilson KL, Foisner R. Lamin-binding Proteins. Cold Spring Harbor perspectives in biology. 2010;2:a000554. doi: 10.1101/cshperspect.a000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 5.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider R, Grosschedl R. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 2007;21:3027–3043. doi: 10.1101/gad.1604607. [DOI] [PubMed] [Google Scholar]
- 7.Cremer T, Cremer M, Dietzel S, Muller S, Solovei I, Fakan S. Chromosome territories--a functional nuclear landscape. Curr Opin Cell Biol. 2006;18:307–316. doi: 10.1016/j.ceb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Bickmore WA, Zaret KS. Altered states: how gene expression is changed during differentiation. Curr Opin Genet Dev. 2010;20:467–469. doi: 10.1016/j.gde.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Meister P, Towbin BD, Pike BL, Ponti A, Gasser SM. The spatial dynamics of tissue-specific promoters during C. elegans development. Genes Dev. 2010;24:766–782. doi: 10.1101/gad.559610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4:e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mekhail K, Moazed D. The nuclear envelope in genome organization, expression and stability. Nat Rev Mol Cell Biol. 2010;11:317–328. doi: 10.1038/nrm2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevelyov YY, Lavrov SA, Mikhaylova LM, Nurminsky ID, Kulathinal RJ, Egorova KS, Rozovsky YM, Nurminsky DI. The B-type lamin is required for somatic repression of testis-specific gene clusters. Proc Natl Acad Sci U S A. 2009;106:3282–3287. doi: 10.1073/pnas.0811933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q, Riblet R, Schildkraut CL. Sites that direct nuclear compartmentalization are near the 5′ end of the mouse immunoglobulin heavy-chain locus. Mol Cell Biol. 2005;25:6021–6030. doi: 10.1128/MCB.25.14.6021-6030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 16.Towbin BD, Meister P, Gasser SM. The nuclear envelope--a scaffold for silencing? Curr Opin Genet Dev. 2009;19:180–186. doi: 10.1016/j.gde.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Chuang CH, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–831. doi: 10.1016/j.cub.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 18.Geyer PK, Vitalini MW, Wallrath LL. Nuclear organization: taking a position on gene expression. Curr Opin Cell Biol. 2011;23:354–359. doi: 10.1016/j.ceb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Graf S, Flicek P, Kerkhoven RM, van Lohuizen M, Reinders M, Wessels L, van Steensel B. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Steensel B. Chromatin: constructing the big picture. EMBO J. 2011;30:1885–1895. doi: 10.1038/emboj.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peric-Hupkes D, van Steensel B. Role of the nuclear lamina in genome organization and gene expression. Cold Spring Harbor symposia on quantitative biology. 2010;75:517–524. doi: 10.1101/sqb.2010.75.014. [DOI] [PubMed] [Google Scholar]
- 22.Kind J, van Steensel B. Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol. 2010;22:320–325. doi: 10.1016/j.ceb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol. 2008;180:51–65. doi: 10.1083/jcb.200706060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubben N, Voncken JW, Misteli T. Mapping of protein- and chromatin-interactions at the nuclear lamina. Nucleus. 2010;1:460–471. doi: 10.4161/nucl.1.6.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoeman RL, Traub P. The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J Biol Chem. 1990;265:9055–9061. [PubMed] [Google Scholar]
- 26.Batrakou DG, Kerr AR, Schirmer EC. Comparative proteomic analyses of the nuclear envelope and pore complex suggests a wide range of heretofore unexpected functions. J Proteomics. 2009;72:56–70. doi: 10.1016/j.jprot.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Korfali N, Wilkie GS, Swanson SK, Srsen V, Batrakou DG, Fairley EA, Malik P, Zuleger N, Goncharevich A, de Las Heras J, Kelly DA, Kerr AR, Florens L, Schirmer EC. The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol Cell Proteomics. 2010;9:2571–2585. doi: 10.1074/mcp.M110.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik P, Korfali N, Srsen V, Lazou V, Batrakou DG, Zuleger N, Kavanagh DM, Wilkie GS, Goldberg MW, Schirmer EC. Cell-specific and lamin-dependent targeting of novel transmembrane proteins in the nuclear envelope. Cell Mol Life Sci. 2010;67:1353–1369. doi: 10.1007/s00018-010-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanagh DM, Powell WE, Malik P, Lazou V, Schirmer EC. Organelle proteome variation among different cell types: lessons from nuclear membrane proteins. Subcell Biochem. 2007;43:51–76. doi: 10.1007/978-1-4020-5943-8_5. [DOI] [PubMed] [Google Scholar]
- 30.Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int Rev Cytol. 2007;261:1–46. doi: 10.1016/S0074-7696(07)61001-8. [DOI] [PubMed] [Google Scholar]
- 31.Laguri C, Gilquin B, Wolff N, Romi-Lebrun R, Courchay K, Callebaut I, Worman HJ, Zinn-Justin S. Structural characterization of the LEM motif common to three human inner nuclear membrane proteins. Structure. 2001;9:503–511. doi: 10.1016/s0969-2126(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 32.Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- 33.Shumaker DK, Lee KK, Tanhehco YC, Craigie R, Wilson KL. LAP2 binds to BAF.DNA complexes: requirement for the LEM domain and modulation by variable regions. EMBO J. 2001;20:1754–1764. doi: 10.1093/emboj/20.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai M, Huang Y, Ghirlando R, Wilson KL, Craigie R, Clore GM. Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. EMBO J. 2001;20:4399–4407. doi: 10.1093/emboj/20.16.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai M, Huang Y, Suh JY, Louis JM, Ghirlando R, Craigie R, Clore GM. Solution NMR structure of the barrier-to-autointegration factor-Emerin complex. J Biol Chem. 2007;282:14525–14535. doi: 10.1074/jbc.M700576200. [DOI] [PubMed] [Google Scholar]
- 36.Zheng R, Ghirlando R, Lee MS, Mizuuchi K, Krause M, Craigie R. Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc Natl Acad Sci U S A. 2000;97:8997–9002. doi: 10.1073/pnas.150240197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margalit A, Segura-Totten M, Gruenbaum Y, Wilson KL. Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc Natl Acad Sci U S A. 2005;102:3290–3295. doi: 10.1073/pnas.0408364102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margalit A, Brachner A, Gotzmann J, Foisner R, Gruenbaum Y. Barrier-to-autointegration factor--a BAFfling little protein. Trends Cell Biol. 2007;17:202–208. doi: 10.1016/j.tcb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Shimi T, Koujin T, Segura-Totten M, Wilson KL, Haraguchi T, Hiraoka Y. Dynamic interaction between BAF and emerin revealed by FRAP, FLIP, and FRET analyses in living HeLa cells. Journal of structural biology. 2004;147:31–41. doi: 10.1016/j.jsb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Gorjanacz M, Klerkx EP, Galy V, Santarella R, Lopez-Iglesias C, Askjaer P, Mattaj IW. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J. 2007;26:132–143. doi: 10.1038/sj.emboj.7601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols RJ, Wiebe MS, Traktman P. The vaccinia-related kinases phosphorylate the N’ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol Biol Cell. 2006;17:2451–2464. doi: 10.1091/mbc.E05-12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bengtsson L, Wilson KL. Barrier-to-autointegration factor phosphorylation on Ser-4 regulates emerin binding to lamin A in vitro and emerin localization in vivo. Mol Biol Cell. 2006;17:1154–1163. doi: 10.1091/mbc.E05-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aravind L, Mazumder R, Vasudevan S, Koonin EV. Trends in protein evolution inferred from sequence and structure analysis. Curr Opin Struct Biol. 2002;12:392–399. doi: 10.1016/s0959-440x(02)00334-2. [DOI] [PubMed] [Google Scholar]
- 44.Aravind L, Koonin EV. SAP - a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci. 2000;25:112–114. doi: 10.1016/s0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki R, Shindo H, Tase A, Kikuchi Y, Shimizu M, Yamazaki T. Solution structures and DNA binding properties of the N-terminal SAP domains of SUMO E3 ligases from Saccharomyces cerevisiae and Oryza sativa. Proteins. 2009;75:336–347. doi: 10.1002/prot.22243. [DOI] [PubMed] [Google Scholar]
- 46.Yorifuji H, Tadano Y, Tsuchiya Y, Ogawa M, Goto K, Umetani A, Asaka Y, Arahata K. Emerin, deficiency of which causes Emery-Dreifuss muscular dystrophy, is localized at the inner nuclear membrane. Neurogenetics. 1997;1:135–140. doi: 10.1007/s100480050020. [DOI] [PubMed] [Google Scholar]
- 47.Furukawa K, Pante N, Aebi U, Gerace L. Cloning of a cDNA for lamina-associated polypeptide 2 (LAP2) and identification of regions that specify targeting to the nuclear envelope. EMBO J. 1995;14:1626–1636. doi: 10.1002/j.1460-2075.1995.tb07151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris CA, Andryuk PJ, Cline SW, Mathew S, Siekierka JJ, Goldstein G. Structure and mapping of the human thymopoietin (TMPO) gene and relationship of human TMPO beta to rat lamin-associated polypeptide 2. Genomics. 1995;28:198–205. doi: 10.1006/geno.1995.1131. [DOI] [PubMed] [Google Scholar]
- 49.Shaklai S, Somech R, Gal-Yam EN, Deshet-Unger N, Moshitch-Moshkovitz S, Hirschberg K, Amariglio N, Simon AJ, Rechavi G. LAP2zeta binds BAF and suppresses LAP2beta-mediated transcriptional repression. European journal of cell biology. 2008;87:267–278. doi: 10.1016/j.ejcb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Brachner A, Reipert S, Foisner R, Gotzmann J. LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J Cell Sci. 2005;118:5797–5810. doi: 10.1242/jcs.02701. [DOI] [PubMed] [Google Scholar]
- 51.Caputo S, Couprie J, Duband-Goulet I, Konde E, Lin F, Braud S, Gondry M, Gilquin B, Worman HJ, Zinn-Justin S. The carboxyl-terminal nucleoplasmic region of MAN1 exhibits a DNA binding winged helix domain. The Journal of biological chemistry. 2006;281:18208–18215. doi: 10.1074/jbc.M601980200. [DOI] [PubMed] [Google Scholar]
- 52.Pan D, Estevez-Salmeron LD, Stroschein SL, Zhu X, He J, Zhou S, Luo K. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J Biol Chem. 2005;280:15992–16001. doi: 10.1074/jbc.M411234200. [DOI] [PubMed] [Google Scholar]
- 53.Bengtsson L. What MAN1 does to the Smads. TGFbeta/BMP signaling and the nuclear envelope. FEBS J. 2007;274:1374–1382. doi: 10.1111/j.1742-4658.2007.05696.x. [DOI] [PubMed] [Google Scholar]
- 54.Cohen TV, Kosti O, Stewart CL. The nuclear envelope protein MAN1 regulates TGFbeta signaling and vasculogenesis in the embryonic yolk sac. Development. 2007;134:1385–1395. doi: 10.1242/dev.02816. [DOI] [PubMed] [Google Scholar]
- 55.Mekhail K, Seebacher J, Gygi SP, Moazed D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature. 2008;456:667–670. doi: 10.1038/nature07460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grund SE, Fischer T, Cabal GG, Antunez O, Perez-Ortin JE, Hurt E. The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. J Cell Biol. 2008;182:897–910. doi: 10.1083/jcb.200803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan JN, Poon BP, Salvi J, Olsen JB, Emili A, Mekhail K. Perinuclear Cohibin Complexes Maintain Replicative Life Span via Roles at Distinct Silent Chromatin Domains. Dev Cell. 2011;20:867–879. doi: 10.1016/j.devcel.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Ikegami K, Egelhofer TA, Strome S, Lieb JD. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 2010;11:R120. doi: 10.1186/gb-2010-11-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yewdell WT, Colombi P, Makhnevych T, Lusk CP. Lumenal interactions in nuclear pore complex assembly and stability. Mol Biol Cell. 2011;22:1375–1388. doi: 10.1091/mbc.E10-06-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Lee KK, Segura-Totten M, Neufeld E, Wilson KL, Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100:4598–4603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber MD, Guan T, Gerace L. Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol Cell Biol. 2009;29:5718–5728. doi: 10.1128/MCB.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulbert S, Antonin W, Platani M, Mattaj IW. The inner nuclear membrane protein Lem2 is critical for normal nuclear envelope morphology. FEBS Lett. 2006;580:6435–6441. doi: 10.1016/j.febslet.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 63.Jiang X, Xia L, Chen D, Yang Y, Huang H, Yang L, Zhao Q, Shen L, Wang J. Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Developmental cell. 2008;14:494–506. doi: 10.1016/j.devcel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, Salpingidou G, Wilson RG, Ellis JA, Hutchison CJ. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haraguchi T, Holaska JM, Yamane M, Koujin T, Hashiguchi N, Mori C, Wilson KL, Hiraoka Y. Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur J Biochem. 2004;271:1035–1045. doi: 10.1111/j.1432-1033.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 66.Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem. 2005;280:13863–13870. doi: 10.1074/jbc.M413020200. [DOI] [PubMed] [Google Scholar]
- 67.Bengtsson L, Wilson KL. Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr Opin Cell Biol. 2004;16:73–79. doi: 10.1016/j.ceb.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Holaska JM, Wilson KL. An emerin “proteome”: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007;46:8897–8908. doi: 10.1021/bi602636m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nili E, Cojocaru GS, Kalma Y, Ginsberg D, Copeland NG, Gilbert DJ, Jenkins NA, Berger R, Shaklai S, Amariglio N, Brok-Simoni F, Simon AJ, Rechavi G. Nuclear membrane protein LAP2beta mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less) J Cell Sci. 2001;114:3297–3307. doi: 10.1242/jcs.114.18.3297. [DOI] [PubMed] [Google Scholar]
- 70.Martins S, Eikvar S, Furukawa K, Collas P. HA95 and LAP2 beta mediate a novel chromatin-nuclear envelope interaction implicated in initiation of DNA replication. J Cell Biol. 2003;160:177–188. doi: 10.1083/jcb.200210026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.