Abstract
Objective
The aim of this study was to compare the effectiveness of percutaneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) in the management of liver abscess.
Methods
Electronic searches (Cochrane Library, MEDLINE, EMBASE, SCIE) were conducted to identify randomized controlled trials (RCTs) comparing PNA and PCD. A meta-analysis was subsequently performed.
Results
A total of five RCTs covering 306 patients were included. The meta-analysis showed that outcomes in patients treated with PCD were superior to those in patients treated with PNA in terms of success rate [relative risk (RR): 0.81, 95% confidence interval (CI) 0.66–0.99; P = 0.04], clinical improvement [standardized mean difference (SMD): −0.73, 95% CI 0.36–1.11; P = 0.0001] and days to achieve a 50% reduction in abscess cavity size (SMD: −1.08, 95% CI 0.64–1.53; P < 0.00001). No significant differences were found in duration of hospitalization (mean difference: −0.17, 95% CI −2.10 to 1.75; P = 0.86) or procedure-related complications (RR: 0.50, 95% CI 0.10–2.63; P = 0.41). Days to achieve the total or near total resolution of the abscess cavity and mortality were not calculated because data in the RCTs in the meta-analysis were insufficient.
Conclusions
Both PNA and PCD are safe methods of draining liver abscesses. However, PCD is more effective than PNA because it facilitates a higher success rate, reduces the time required to achieve clinical relief and supports a 50% reduction in abscess cavity size. However, among successfully treated patients, the outcomes of PNA are comparable with those of PCD.
Introduction
Liver abscess is a common clinical problem in tropical countries and is most commonly caused by pyogenic, amoebic or mixed infections.1 Less commonly, the infection causing a liver abscess may be fungal in origin.
For amoebic liver abscesses (ALAs), the primary treatment is medical; however, 15% of amoebic abscesses may be refractory to medical therapy and 20% of ALAs may be complicated by secondary bacterial infection.2,3 In the past, surgical drainage was the traditional mode of treatment in such patients and in patients with pyogenic liver abscesses (PLAs).4 However, this type of drainage was associated with remarkably high (10–47%) morbidity and mortality rates.5
Over the last two decades, outcomes in patients presenting with liver abscesses have improved as a result of advances in radiological diagnosis and percutaneous treatment options.6–8 Currently, patients are treated with antibiotics along with percutaneous needle aspiration (PNA) or percutaneous catheter drainage (PCD), and surgical drainage is used only in patients who fail to respond to such treatment.9,10
Previous studies have shown both PNA and PCD to be effective and safe,11,12 although the optimal treatment remains unclear.13,14 The aim of this study was to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing the effectiveness of PNA and PCD in the management of liver abscesses.
Materials and methods
Search strategy and study selection
A systematic literature search was performed as shown in Table 1. No language restriction was imposed. The citations within the reference lists of the articles identified were subsequently searched manually to identify additional eligible studies.
Table 1.
Search strategies used to identify studies comparing the outcomes of percutaneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) in the management of liver abscess
| Databases | Period of search | Search strategies |
|---|---|---|
| Cochrane Library | To 30 April 2014 |
|
| MEDLINE (PubMed) | To 30 April 2014 |
|
| EMBASE (OvidSP) | To 30 April 2014 |
|
| SCIE | To 30 April 2014 |
|
| CBM | To 30 April 2014 | Search strategy was performed in Chinese using search terms similar to those used in MEDLINE |
CBM, Chinese Biomedical Literature Database; EMBASE, Excerpta Medica Database; MEDLINE, Medical Literature Analysis and Retrieval System Online; MeSH, Medical Subject Heading; SCIE, Science Citation Index Expanded.
Screenings of titles, abstracts and full-text articles were completed by two authors (YC and CY). Any discrepancies were resolved by consultation with a third author (XX).
Inclusion and exclusion criteria
Studies were required to meet the following criteria: (i) the study design must be that of an RCT; (ii) outcomes of PNA and PCD in the management of liver abscesses must be compared, and (iii) at least one outcome must be reported. The type of abscess was not restricted and thus amoebic, pyogenic, mixed and indeterminate abscesses were included. If two studies were found to overlap, the publication which provided data on more types of abscess was selected. Articles were excluded if they failed to fulfil any of these criteria.
Data extraction
Two authors (JL and JZ) independently extracted and confirmed the data and entered them into an electronic data collection form. Any disagreement in the two reviewers' data collection and quality assessment was discussed until a consensus was reached; otherwise, a third reviewer (XX) joined the discussion as a referee. For the validity assessment, another two authors (YC and YL) independently assessed the methodological quality of the included trials using the quality checklist recommended by the Cochrane Handbook for Systematic Reviews of Interventions.15 The assessment referred to six domains: (i) random sequence generation; (ii) allocation concealment; (iii) blinding of participants and personnel; (iv) blinding of outcome assessment; (v) addressing of incomplete outcome data, and (vi) selective reporting. Following the evaluation of these domains, an included trial was judged as being at low risk for bias if it was evaluated as ‘low’ in all domains. If the risk for bias was judged to be ‘unclear’ or ‘high’, the trial was listed under the group of trials with ‘high risk for bias’.
Outcomes and definitions
In this report, data for the following outcomes were extracted: success rate; duration of hospital stay; procedure-related complications; days to achieve clinical improvement; days to achieve a 50% reduction in the size of the abscess cavity; days to achieve total or near total resolution of the abscess cavity, and mortality. The criteria for a successful percutaneous intervention were defined as the adequate drainage of the abscess to achieve the resolution of infection without the need for surgical drainage and with the subsequent discharge of the patient from hospital. Procedure-related complications included haemorrhage, pleural effusion/empyema, persistent bile drainage, catheter displacement, and sepsis, etc. Clinical improvement was defined as the subsidence of fever, a normal leukocyte count and the resolution of local signs and symptoms after successful PCD or PNA.
Statistical analysis
All statistical analyses were performed using RevMan Version 5.2 (Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). For dichotomous data and continuous data, respectively, the risk ratio (RR) and mean difference (MD) with 95% confidence intervals (CIs) for both were calculated. For continuous outcomes with different measurement scales in different RCTs, the standardized mean difference (SMD) with 95% CI was calculated. Heterogeneity was described with the chi-squared test. A P-value of < 0.1 was considered to indicate a difference of statistical significance. The I2 statistic was used to measure the quantity of heterogeneity. If significant heterogeneity existed, a random-effects model was used. In the absence of significant heterogeneity, a fixed-effects model was adopted.15
In cases of missing data, the original investigators were contacted to request further information. If there was no reply, the analysis was performed on an intention-to-treat principle, if applicable. Otherwise, the available-case analysis, also known as the per-protocol (PP) analysis, was adopted. A few published clinical trials reported data as the median and range or interquartile range (IQR) rather than as the mean and standard deviation (SD). According to the Cochrane Handbook, it was assumed that the median was equal to the mean, and the SD represented a ratio of the range or IQR of 1/4 or 1/1.23, respectively. Funnel plots were used to determine reporting biases. The meta-analysis and systematic review were conducted according to the Cochrane Handbook for Systematic Reviews of Interventions15 and PRISMA (preferred reporting items for systematic reviews and meta-analyses) criteria.16
Results
Literature research and selection of studies
A detailed outline of the process of selecting studies for the meta-analysis is shown in Fig. 1.
Figure 1.
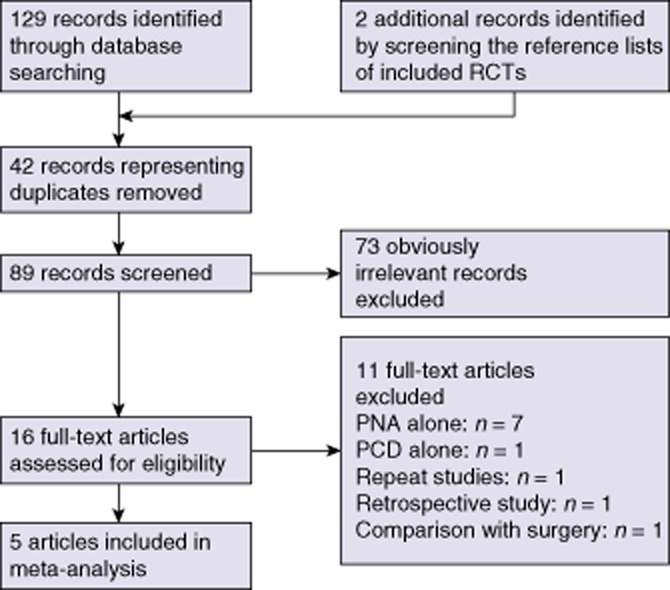
Process of selection of studies comparing the outcomes of percutaneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) in the management of liver abscess for inclusion in a meta-analysis. RCT, randomized controlled trial
Five studies17–21 including a total of 306 patients, which fulfilled all of the inclusion criteria, were considered for the analysis.
Study description and study quality
These five RCTs17–21 were published between 1998 and 2013. Trial design is shown in Table 2.
Table 2.
Characteristics of studies comparing the outcomes of percutaneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) in the management of liver abscess
| Authors | Year of study | Type of abscess included | Size of abscess included | Participants (PNA/PCD) | Initial i.v. antibiotic treatment | Risk for bias |
|---|---|---|---|---|---|---|
| Rajak et al.17 | 1998 | ALA and PLA | All sizes | 50 (25/25) | Cloxacillin, gentamicin, metronidazole | High |
| Yu et al.18 | 2004 | PLA only | All sizes | 64 (32/32) | Ampicillin, cefuroxime, metronidazole | Low |
| Zerem & Hadzic19 | 2007 | PLA only | All sizes | 60 (30/30) | Cefazolin, gentamicin | High |
| Singh et al.20 | 2009 | ALA and PLA | ≥ 10 cm | 72 (36/36) | Ceftriaxone, gentamicin, metronidazole | High |
| Singh et al.21 | 2013 | ALA and PLA | All sizes | 60 (30/30) | Cefazolin, gentamicin, metronidazole | High |
ALA, amoebic liver abscess; PLA, pyogenic liver abscess.
In these five RCTs, the percutaneous treatment procedures were performed under continuous real-time sonographic guidance using freehand ultrasound. For PNA, a 16-G or 18-G trocar needle was advanced into the abscess cavity and the contents were aspirated in an attempt to completely evacuate the cavity. Aspiration was repeated if there was either no clinical improvement or no reduction in the size of the abscess cavity. For PCD, an appropriately sized catheter (8-F to 14-F pigtail or drainage catheter) was introduced into the abscess cavity using the Seldinger technique or a single-step trocar technique.
The risk for bias is summarized in Table 3. Four RCTs had a high risk for bias17,19–21 and one RCT had a low risk for bias.18
Table 3.
Risk for bias in studies comparing the outcomes of percutaneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) in the management of liver abscess
| Studies | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Rajak et al.17 | Uncertain | High risk | Uncertain | Uncertain | High risk | High risk |
| Yu et al.18 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zerem & Hadzic19 | Low risk | Low risk | Uncertain | Uncertain | High risk | High risk |
| Singh et al.20 | Low risk | Low risk | Uncertain | Uncertain | Low risk | Low risk |
| Singh et al.21 | Low risk | Low risk | Uncertain | Uncertain | Low risk | High risk |
Risk for bias was classified as low, uncertain or high. The detail of each grade is referred to in the Cochrane Handbook for Systematic Reviews of Interventions.15
Outcomes
Liver abscess was found more commonly in men (male : female ratios in PNA and PCD were 101:52 and 105:48, respectively). The reported mean age of patients within the RCTs varied between 35 years and 50 years. In total, 84.0% of abscesses (125 treated with PNA and 132 treated with PCD) were solitary and three RCTs reported that 67.1% of abscesses (114/170) were located in the right lobe of the liver. The most common symptoms were pain in the right upper quadrant of the abdomen and fever, recorded in 219 of 306 (71.6%) patients (PNA, n = 111; PCD, n = 108) and 255 of 306 (83.3%) patients (PNA, n = 131; PCD, n = 126), respectively. Of the coexisting diseases reported in three of the RCTs,18–20 diabetes was the most common (23.7%, 44 of 186 patients). Among the five RCTs, there were no statistically significant differences between the two treatment groups with regard to any of the present data, other clinical characteristics or laboratory results.
Success rate
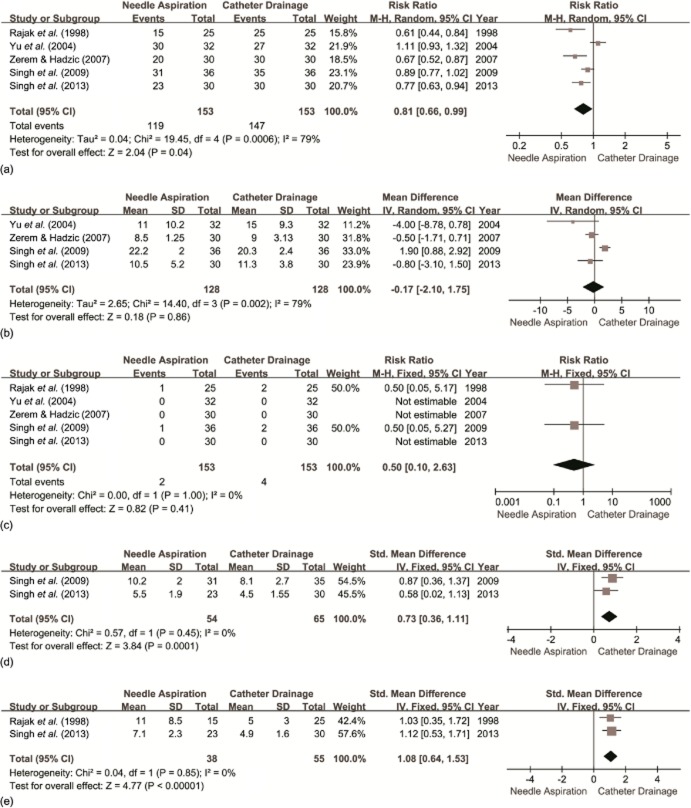
All five RCTs17–21 reported the success rate (Fig. 2a). Success rates were 77.8% (119 of 153 patients) and 96.1% (147 of 153 patients) in the PNA and PCD groups, respectively (P = 0.041).
Figure 2.
Forest plots resulting from the meta-analysis comparing the outcomes of percutaneous needle aspiration (PNA) with those of percutaneous catheter drainage (PCD) for: (a) success rates; (b) duration of hospital stay; (c) procedure-related complications; (d) time to clinical improvement (days), and (e) time to achieve a 50% reduction in abscess cavity size (days). SD, standard deviation; 95% CI, 95% confidence interval; M-H, Mantel–Haenszel; IV, inverse variance
Duration of hospital stay
Four of the trials18–21 provided data on hospital stay (Fig. 2b). Rajak et al.17 did not report the mean hospital stay, but suggested no meaningful difference for the duration of hospitalization among patients successfully treated with either technique. Two studies reported the data as the mean ± SD20,21 and two studies as the median (range).18,19 There was no significant difference between the two groups.
Procedure-related complications
All five trials17–21 reported procedure-related complications (Fig. 2c). Two studies17,19 reported six patients with minimal complications. The remaining studies18,20,21 stated that no complications occurred. No significant difference in procedure-related complications was observed following analysis.
Clinical improvement
The days to achieve clinical improvement were evaluated in three RCTs17,20,21 (Fig. 2d). Rajak et al.17 reported that in patients treated successfully, the average time required to achieve clinical relief was similar in the two treatment groups (P > 0.05), but did not provide the actual data. Therefore, only two studies were included for analysis.20,21 Time to clinical improvement was significantly longer in patients treated with PNA than in those treated with PCD.
Days to achieve a 50% reduction in abscess cavity size
Two studies reported the time required to achieve a 50% reduction in cavity size17,21 (Fig. 2e). The time required to achieve a 50% reduction in cavity size was significantly greater in those undergoing PNA.
Days to achieve the total or near total resolution of the abscess cavity
Two of the included RCTs17,21 reported the time required to achieve the total or near total resolution of the abscess cavity. Singh et al.21 found no significant difference between the two groups (PNA, 10.1 weeks; PCD, 10.9 weeks; P = 0.454). Similar observations were recorded by Rajak et al.17 (P > 0.05), although the latter group did not give the specific data. Thus, only one study provided the mean ± SD and thus these values were not calculated in this analysis.
Mortality
Yu et al.18 reported five deaths (four in the PCD group and one in the PNA group). All four of the patients who died in the PNA group had an underlying malignancy and the remaining patient in the PCD group had chronic obstructive pulmonary disease. Singh et al.20 reported one patient, in the PCD group, who suffered abscess rupture and died. No deaths were reported in the three remaining RCTs.
Discussion
In the modern era of minimal invasiveness, percutaneous treatment (either needle aspiration or catheter drainage) has become the preferred method for the management of liver abscess. Previous investigations have shown that the combination of parenteral antibiotics and image-guided percutaneous treatment is also successful.8,14,22,23 The aim of this study was to determine which approach is superior. The conclusions of the five RCTs differ. Yu et al.18 recommend PNA as a first-line approach because the procedure is simple, facilitates patient comfort, and is of low cost. Rajak et al.17 conclude that PCD is more effective than PNA. Zerem and Hadzic19 recommend PNA only in patients with liver abscess cavities of < 5 cm in diameter. Singh et al.20 and Singh et al.21 hold the view that PCD represents a better treatment option than PNA for large liver abscesses (≥10 cm in diameter). Therefore, in the setting of diametric conclusions meta-analytical techniques may provide evidence as to which treatment option is superior.
Regarding the effectiveness of treatment, the current meta-analysis showed a higher rate of success in the PCD group. This may be a convincing argument in support of the PCD method. Two reasons were identified to explain the lower rate of success in the PNA group. The first concerns the number of aspiration attempts. In the study by Rajak et al.,17 which reported the lowest success rate (PNA: 60%) of the five RCTs, aspiration attempts were restricted to two. However, Yu et al.18 did not limit the number of attempts made and achieved the highest rate of success (PNA: 97%). The remaining three trials19–21 limited attempts to three and reported success rates of 67–86%. In addition, PCD was associated with the highest success rates (97.2–100%) except in the study by Yu et al.18 (84.7%), in which the deaths of four (12.5%) patients with underlying malignancies decreased the success rate. Thus, even with repeat aspirations, the success rate of PNA remains inferior to that achieved with PCD. In addition, a recent retrospective study revealed that decreases in success rates are associated with subsequent aspiration attempts.24 This finding confirms the conclusions of three of the RCTs.19–21 The second reason for the low rate of success achieved by PNA relates to the size of the liver cavity or the volume of the abscess. In smaller abscesses, the amount of pus produced per day may be small and can be completely evacuated by PNA. However, a larger abscess cavity produces a larger quantity of pus, which needs to be drained continuously and is not suitable for PNA. In the study by Zerem and Hadzic,19 the mean ± SD longest diameter of the abscess cavity in the PNA group was significantly greater in patients in whom PNA was unsuccessful (97 ± 42 mm) than in patients in which it was successful (62 ± 35 mm). Rajak et al.17 also reported a larger mean volume of abscesses (425 ml) in patients in whom PNA failed in comparison with that in patients in whom it was successful (178 ml) (P < 0.05). Other authors have drawn similar conclusions.20,21,24,25 Thick viscous pus and the rapid accumulation of pus are important causes of failure in both PNA and PCD.26 However, these factors may affect the success of PNA more because PNA cannot provide continuous drainage. Four of the RCTs17–19,21 included in the current meta-analysis involved abscesses of all sizes, but the meta-analysis still found a higher rate of success in the PCD group.
Baek et al. 13 and Giorgio et al.14 initially reported a much lower incidence of complications with PNA than with PCD as one of the major advantages of PNA over PCD. These results are inconsistent with findings in the current analysis, which indicate no significant difference between PNA and PCD. Major procedure-related complications were rare in either group. Recent studies report a low incidence of minor complications.21,24
Other advantages of PCD over PNA refer to the fact that the former requires less time to achieve clinical improvement and a 50% reduction in the cavity size, as the current meta-analysis shows. Percutaneous catheter drainage has the obvious advantage of providing continuous catheterization by the placement of an indwelling drainage catheter. Because of this, pus can be evacuated more frequently and the abscess cavity shows a faster rate of collapse during the initial period in patients treated with PCD. However, the extent of this evidence is insufficient because only two RCTs report these data.17,21 In addition, i.v. antibiotics may play an important role in these outcomes. Unfortunately, the antibiotics used differed among all five RCTs.17–21 To eliminate this confounding variable, more data from RCTs in which the use of antibiotics is controlled are required.
Conclusions
In summary, this meta-analysis and systematic review indicates that PCD is more effective than PNA. Further high-quality RCTs, controlling for other factors such as the aetiology and size of abscesses, and the use of antibiotics and other therapeutic interventions, are required.
Acknowledgments
The authors would like to acknowledge the support of the Department of Bile Duct Surgery, West China Hospital, Sichuan University.
Conflicts of interest
None declared.
References
- Naveed S, Gupta VB, Kapoor M, Quari H, Altaf A, Para M. Liver abscess in the tropics: an experience from Jammu. Scott Med J. 2014;59:167–171. doi: 10.1177/0036933014543049. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Jr, Forlenza S, Verma R. Amoebic liver abscess: a therapeutic approach. Rev Infect Dis. 1985;7:171–179. doi: 10.1093/clinids/7.2.171. [DOI] [PubMed] [Google Scholar]
- Sherlock S, Dooley J. Diseases of the Liver and Biliary System. 9th edn. Oxford: Blackwell Science Publishing; 1993. pp. 471–502. [Google Scholar]
- Theron P. Surgical aspects of amoebiasis. Br Med J. 1947;2:123–126. doi: 10.1136/bmj.2.4516.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satiani B, Davidson ED. Hepatic abscesses: improvement in mortality with early diagnosis and treatment. Am J Surg. 1978;135:647–650. doi: 10.1016/0002-9610(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Mohsen AH, Green ST, Read RC, McKendrick MW. Liver abscess in adults: ten years experiences in a UK centre. QJM. 2002;95:797–802. doi: 10.1093/qjmed/95.12.797. [DOI] [PubMed] [Google Scholar]
- Branum GD, Tyson GS, Branum MA, Meyers WC. Hepatic abscess: changes in aetiology, diagnosis, and management. Ann Surg. 1990;212:655–662. doi: 10.1097/00000658-199012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Pitt HA, Lipsett PA, Osterman FA, Jr, Lillemoe KD, Cameron JL, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg. 1996;223:600–607. doi: 10.1097/00000658-199605000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeto RK, Rockey DC. Pyogenic liver abscess: changes in aetiology, management and outcome. Medicine (Baltimore) 1996;75:99–113. doi: 10.1097/00005792-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Wong KP. Percutaneous drainage of pyogenic liver abscess. World J Surg. 1990;14:492–497. doi: 10.1007/BF01658674. [DOI] [PubMed] [Google Scholar]
- Saraswat VA, Agarwal DK, Baijal SS, Roy S, Choudhuri G, Dhiman RK, et al. Percutaneous catheter drainage of amoebic liver abscesses. Clin Radiol. 1992;45:187–189. doi: 10.1016/s0009-9260(05)80639-7. [DOI] [PubMed] [Google Scholar]
- Van Sonnenberg E, D'Agostino HB, Casola G, Halasz NA, Sanchez RB, Goodacre BW. Percutaneous abscess drainage: current concepts. Radiology. 1991;181:617–626. doi: 10.1148/radiology.181.3.1947068. [DOI] [PubMed] [Google Scholar]
- Baek SY, Lee MG, Cho KS, Lee SC, Sung KB, Auh YH. Therapeutic percutaneous aspiration of hepatic abscesses: effectiveness in 25 patients. AJR Am J Roentgenol. 1993;160:799–802. doi: 10.2214/ajr.160.4.8456667. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Tarantino L, Mariniello N, Francica G, Scala E, Amoroso P, et al. Pyogenic liver abscesses: 13 years of experience in percutaneous needle aspiration with US guidance. Radiology. 1995;l95:122–124. doi: 10.1148/radiology.195.1.7892451. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Available at http://www.cochrane.org/resources/handbook/ (last accessed 7 March 2014)
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajak CL, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR Am J Roentgenol. 1998;170:1035–1039. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- Yu SC, Ho SS, Lau WY, Yeung DT, Yuen EH, Lee PS, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932–938. doi: 10.1002/hep.20133. [DOI] [PubMed] [Google Scholar]
- Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. AJR Am J Roentgenol. 2007;189:W138–W142. doi: 10.2214/AJR.07.2173. [DOI] [PubMed] [Google Scholar]
- Singh O, Gupta S, Moses S, Jain DK. Comparative study of catheter drainage and needle aspiration in management of large liver abscesses. Indian J Gastroenterol. 2009;28:88–92. doi: 10.1007/s12664-009-0032-1. [DOI] [PubMed] [Google Scholar]
- Singh S, Chaudhary P, Saxena N, Khandelwal S, Poddar DD, Biswal UC. Treatment of liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Ann Gastroenterol. 2013;26:332–339. [PMC free article] [PubMed] [Google Scholar]
- Barakate MS, Stephen MS, Waugh RC, Gallagher PJ, Solomon MJ, Storey DW, et al. Pyogenic liver abscess: a review of 10 years experiences in management. Aust N Z J Surg. 1999;69:205–209. doi: 10.1046/j.1440-1622.1999.01523.x. [DOI] [PubMed] [Google Scholar]
- Mohan S, Talwar N, Chaudhary A, Andley M, Ravi B, Kumar A. Liver abscess: a clinicopathological analysis of 82 cases. Int Surg. 2006;91:228–233. [PubMed] [Google Scholar]
- Abusedera MA, Ashraf ME. Percutaneous treatment of large pyogenic liver abscess. Egypt J Radiol Nucl Med. 2014;45:109–115. [Google Scholar]
- Gupta SS, Singh O, Sabharwal G, Hastir A. Catheter drainage versus needle aspiration in management of large (>10 cm diameter) amoebic liver abscesses. Aust N Z J Surg. 2011;81:547–551. doi: 10.1111/j.1445-2197.2010.05494.x. [DOI] [PubMed] [Google Scholar]
- Dietrick RB. Experience with liver abscess. Am J Surg. 1984;147:288–291. doi: 10.1016/0002-9610(84)90109-0. [DOI] [PubMed] [Google Scholar]