Abstract
Introduction
Delayed gastric emptying (DGE) is a common complication after a pylorus-preserving pancreatoduodenectomy (PPPD) and is associated with significant morbidity. This study determines whether DGE is affected by antecolic (AC) or retrocolic (RC) reconstruction after a PPPD.
Method
An electronic search was performed of the MEDLINE, EMBASE and PubMed databases to identify all articles related to this topic. Pooled risk ratios (RR) were calculated for categorical outcomes, and mean differences (MD) for secondary continuous outcomes using the fixed-effects and random-effects models for meta-analysis.
Results
Nine studies including 878 patients met the inclusion criteria. DGE was lower with an AC reconstruction RR 0.31 [0.12, 0.78] Z = 2.47 (P = 0.010). Length of stay (LOS) MD −4 days [−7.63, −1.14] Z = 2.65 (P = 0.008) and days to commence a solid diet MD −5 days [−6.63, −3.15] Z = 5.50 (P ≤ 0.000) were also significantly in favour of the AC group. There was no difference in the incidence of pancreatic fistula, intra-abdominal collection/bile leak or mortality between the two groups.
Conclusion
AC reconstruction after PPPD is associated with a lower incidence of DGE. Time to oral intake was significantly shorter with AC reconstruction, with a reduced hospital stay.
Introduction
A pancreatoduodenectomy (PD) is an established operation offering a potential chance of a cure to patients with peri-ampullary malignancies as well as other benign and malignant conditions.1 Morbidity associated with this procedure is high with delayed gastric emptying (DGE) being a common complication with an incidence of between 5% and 80%.2–5
The most widely accepted and validated definition of DGE is that by the International Study Group on Pancreatic Surgery (ISGPS).6 Although DGE is not usually a life-threatening complication it leads to patient symptoms, prolonged hospital stay and increased cost.7 There are a number of factors which might influence the return of normal stomach emptying after a pylorus-preserving pancreatoduodenectomy (PPPD), including pancreatic leak, diabetic gastroparesis, roux limb versus loop and anterior or posterior anastamosis.8
Another factor that has been suggested is the route of reconstruction, either antecolic (AC) or retrocolic (RC). The incidence of DGE ranges from 5% to 80% between studies2–4,9,10 and generally favours AC over RC reconstruction. But there have been other studies that have failed to demonstrate any advantage.7,11 The aim of this systematic review and meta-analysis was to compare AC and RC reconstruction after PPPD for the relative risk of DGE and other secondary measures.
Method
Randomized and case-controlled studies, irrespective of language, country of origin, hospital, blinding, sample size or publication status, that compared AC and RC gastroenteric reconstruction for PPPD were included in this review. The Cochrane Colorectal Cancer Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials in the Cochrane Library, MEDLINE, Embase and Science Citation Index Expanded were searched for articles published up to January 2014 using the medical subject headings (MeSH) terms ‘AC, RC, gastroenteric reconstruction and pylorus-preserving pancreatoduodenectomy’. Equivalent free-text search terms, such as ‘AC and RC’ were used in combination with ‘pylorus-preserving pancreatoduodenectomy’. The references from the included studies were searched to identify additional studies comparing the two techniques.
All patients who underwent PPPD for both benign and malignant conditions were included. Inclusion criteria for searching were: randomized and non-randomized studies evaluating the use of AC gastroenteric reconstruction and RC gastroenteric reconstruction for PPPD. The search strategy is illustrated in Fig. 1.
Figure 1.
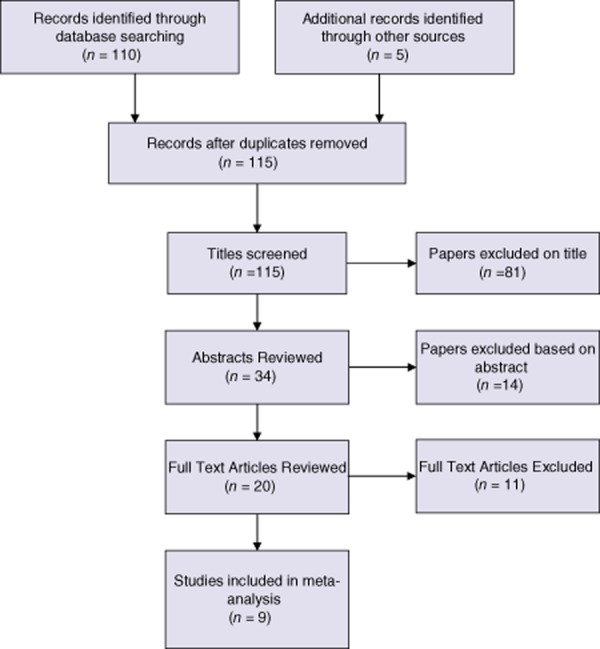
PRISMA flow diagram
Outcome measures
The primary outcome measure was the incidence of DGE. Secondary outcome measures were pancreatic fistula, intra-abdominal collection and bile leak, days to start liquid food, days to start solid food, length of stay (LOS) and 90-day mortality.
Definitions
DGE was defined on the basis of the requirement for prolonged gastric drainage and delayed return to a solid diet as per the ISGPS6 definition.
The definition of intra-abdominal collection and bile leak was any fluid collection requiring drainage.
A pancreatic fistula was defined as drainage of fluid with an amylase concentration three times the upper limit of normal serum as per the ISGPF definition.12
Data extraction and quality assessment
Studies were identified and data were extracted by two authors independently (R.B. and S.P.). The accuracy of the extracted data was further adjudicated by a third author.
Statistical analysis
Statistical analysis was performed using Review Manager Version 5.2 software (Cochrane Collaboration). The risk ratio (RR) with 95% confidence interval (CI) was calculated for categorical data, and the mean difference with 95% CI for continuous variables. When median and range were reported instead of mean and variance, the latter was calculated using the methods described by Hozo et al.13 Random and fixed-effects models were used to calculate the combined outcomes of both binary and continuous data.14,15 In cases of heterogeneity, only the results of the random-effects model were reported. Heterogeneity was explored using the χ2 test, with significance set at P < 0.050. Low heterogeneity was defined as an I2 value of 33% or less.16 If the standard deviation was not available, it was calculated according to the guidelines of the Cochrane Collaboration.17 This process involved assumptions that both groups had the same variance, which may not have been true, and variance was estimated either from the range or from the P-value. Forest plots were used for graphical display of the results. The quality of included studies was assessed using the Jadad score18 for randomized controlled trials and the Newcastle–Ottawa score19 for case–control studies.
Results
Nine studies met the inclusion criteria and formed the basis of this meta-analysis.2–4,7,9–11,20,21 Five studies were randomized controlled trials, one was a prospective observational study and there were three retrospective studies. There were a total of 878 patients, including 451 in the AC group and 427 in the RC group. The characteristics and quality of the studies are given in Table 1. Pooled data were analysed by combining the results of the nine studies.
Table 1.
Characteristics of included studies
| Study | Year | Country | n per Group | Design | Reconstruction | % DGE | Quality Score (Jadad – RCT/Newcastle Ottawa – Non RCT) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | RC | AC | RC | PD | AC (%) | RC (%) | |||||
| Chijiiwa et al. | 2005–2007 | Japan | 17 | 18 | RCT | Antecolic duodenojejunostomy | Vertical end to side duodenojejunostomy. Caudal side. | PJ | 6 | 22 | 2 |
| Eshuis et al. | 2009–2011 | The Netherlands | 121 | 125 | RCT | Antecolic duodenojejunostomy | End to side duodenojejunostomy. Stomach fixed to transverse mesocolon | PJ | 61 | 60 | 3 |
| Hartel et al. | 1996–2001 | Germany | 100 | 100 | Retro | Antecolic duodenojejunostomy. On same limb as HJ and PJ | Retrocolic duodenojejunostomy. On same limb as HJ and PJ. | PJ | 5 | 24 | 9 |
| Imamura et al. | 2005–2011 | Japan | 58 | 58 | RCT | End to side antecolic duodenojejunostomy | Vertical end to side duodenojejunostomy. Caudal side. | PJ | 12.1 | 20.7 | 3 |
| Murakami et al. | 1994–2006 | Japan | 78 | 54 | Retro | Antecolic roux-en-y reconstruction | Retrocolic Bilroth I reconstruction | PG | 10 | 81 | 9 |
| Sugiyama et al. | NS | Japan | 12 | 18 | Retro | Jejunal loop with antecolic duodenojejunostomy on same limb as HJ and PJ | Retrocolic duodenojejunostomy Same limb as HJ and PJ. | PJ | 8 | 72 | 9 |
| Tamandl et al. | 2007–2009 | Austria | 34 | 26 | RCT | Antecolic duodenojejunostomy on same limb as HJ and PJ | Retrocolic Duodenojejunostomy on same limb as HJ and PJ | PJ | 18 | 23 | 1 |
| Tanabe et al. | 2001–2006 | Japan | 11 | 8 | Prosp | Antecolic duodenojejunostomy | Retrolic duodenojejunostomy | PJ | NS | NS | 8 |
| Tani et al. | 2002–2004 | Japan | 20 | 20 | RCT | Antecolic duodenjejunostomy on same proximal jejunal loop as HJ and PJ | Retrocolic duodenojejunostomy on same proximal jejunal loop as HJ and PJ | PJ | 5 | 50 | 3 |
AC, antecolic; RC, retrocolic; PD, pancreatoduodenectomy; DGE, delayed gastric emptying; RCT, randomized controlled trial; NS, not significant.
Primary outcome measure
Delayed gastric emptying
Eight studies were included in this analysis. There was marked heterogeneity amongst the included studies [τ2 = 1.41, χ2 = 68.38, d.f. = 7 (P ≤ 0.000); I2 = 90%]. In a random effects model, there was a significant difference in the incidence of DGE between AC and RC reconstruction in favour of AC reconstruction RR 0.31 [0.12, 0.78] Z = 2.47 (P = 0.010) (see Fig. 2). No difference was seen in grade A DGE (P = 0.790) or grade B and C DGE (P = 0.500) between AC and RC reconstruction.
Figure 2.
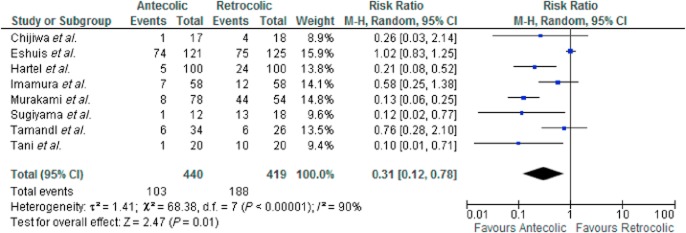
Delayed gastric emptying (DGE) – all studies
A subset analysis was performed using only the five randomized controlled trials (RCTs). There was some heterogeneity amongst the included studies [τ2 = 0.43, χ2 = 9.00, d.f. = 4 (P = 0.060); I2 = 56%]. There was no significant difference in DGE amongst the included RCTs (OR 0.5 [0.23, 1.17] Z = 1.59 (P = 0.110) (see Fig. 3).
Figure 3.
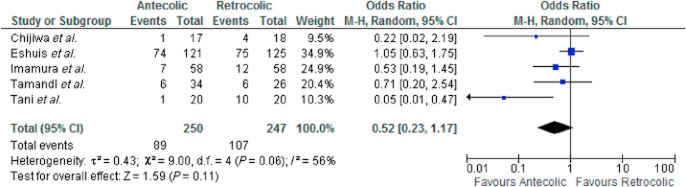
Delayed gastric emptying (DGE) – randomized controlled trails (RCTs)
Secondary outcome measures
Pancreatic leak
Nine studies were included in the analysis. There was no heterogeneity amongst the included studies [χ2 = 3.33, d.f. = 8 (P = 0.910); I2 = 0%]. In a fixed effects model there was no significant difference in the frequency of pancreatic leak between the AC and RC groups OR 1.06 [0.71, 1.58] Z = 0.29 (P = 0.770) (see Fig. 4).
Figure 4.
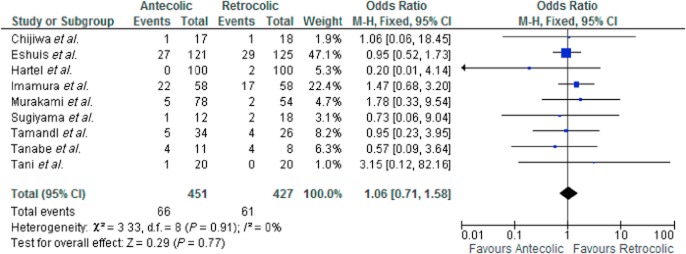
Pancreatic leak
Intra-abdominal collection and bile leak
Eight studies were included in the analysis. There was no heterogeneity amongst the included studies [χ2 = 4.64, d.f. = 7 (P = 0.700); I2 = 0%]. In a fixed effects model there was no significant difference in the frequency of intra-abdominal collection/abscess between the AC and RC groups OR 0.91 [0.57, 1.45] Z = 0.42 (P = 0.680).
Time to start liquid diet
Five studies were included in the analysis. There was marked heterogeneity amongst the included studies [τ2 = 42.97, χ2 = 178.63, d.f. = 4 (P ≤ 0.000); I2 = 98%]. In a random effects model there was a significant difference in the days taken to restart a liquid diet in favour of the AC group MD −7 days [−13.23, −1.33] Z = 2.40 (P = 0.020).
Time to start solid food
Eight studies were included in the analysis. There was marked heterogeneity amongst the included studies [τ2 = 4.14, χ2 = 288.70, d.f. = 7 (P ≤ 0.000); I2 = 98%]. In a random effects model there was a significant difference in the days taken to restart a solid diet in favour of AC reconstruction MD −5 days [−6.63, −3.15] Z = 5.5 (P ≤ 0.000). See Fig. 5.
Figure 5.
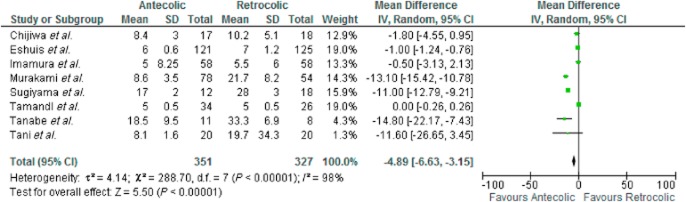
Days to commence solid diet
LOS
Nine studies were included in the analysis. There was marked heterogeneity amongst the included studies [τ2 = 16.68, χ2 = 490.02, d.f. = 8 (P ≤ 0.000); I2 = 98%]. In a random effects model there was a significant difference in the LOS after surgery in favour of the AC group MD −4 days [−7.63, −1.14] Z = 2.65 (P = 0.008). See Fig. 6.
Figure 6.
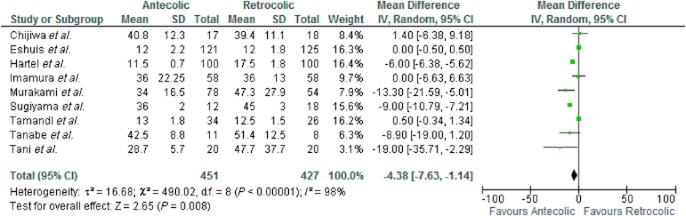
Length of stay
Mortality
Five studies were included in the analysis. There was no heterogeneity amongst the included studies [χ2 = 0.18, d.f. = 2 (P = 0.910); I2 = 0%]. In a fixed effects model there was no significant difference between mortality with AC or RC reconstruction RR 0.61 [0.24, 1.60] Z = 1.00 (P = 0.300).
Discussion
This meta-analysis shows that AC reconstruction after PPPD is associated with a lower incidence of DGE, reduced LOS and a more rapid resumption of normal oral intake, and this occurred without any increase in pancreatic leak or intra-abdominal collection or mortality. The overall incidence of DGE using the ISGPS definitions in this meta-analysis was 23% with AC reconstruction and 45% with RC reconstruction. The absolute risk reduction with AC reconstruction was 0.21 and the number needed to treat in this way to avoid one case of DGE is 5.
Although well described, the aetiology of DGE after PPPD in the absence of other post-operative complications remains unclear. There are a number of potential causes for ‘primary’ DGE including local ischaemia of the pylorus and antrum,22 reduced levels of motilin leading to gastric atony,23 gastric atony as a result of vagotomy23 and torsion or angulation of the gastroenteric reconstruction.24 This last point may help explain the superiority of the AC reconstruction and it has been hypothesized that one reason that no difference has been identified in some studies is the use of the vertical RC duodenojejunostomy. It was suggested that the vertical nature of the RC reconstruction avoided flexion and angulation of the stomach which in turn contributed to flow of gastric contents.11 The authors felt this may be a reason why no difference was seen between the two groups.
The subgroup analysis of the five RCTs that compared AC and RC reconstructions gave variable results with one trial favouring an AC anastomosis and four showing no difference. The pooled data of just these RCT's seemed to favour AC reconstruction but did not show a statistically significant difference (P = 0.110). Interestingly, no difference was noted in the grade of DGE amongst the included studies. Eshuis et al. noted in a RCT that one possible explanation for the difference between their study and previous studies was that their RC reconstruction was performed by bringing the duodenal stump through a separate opening in the transverse mesocolon so that the gastroenteric anastamosis is in a different abdominal compartment to the other two anastamoses. It has been speculated that this may help to reduce inflammation around the gastroenteric anastamosis in the event of small pancreatic or biliary leaks. In addition the duodenal stump is sutured to the transverse mesocolon to prevent angulation.7 Indeed Tani et al. had to suspend their RCT at the first interim analysis owing to the disparity between rates of DGE between the AC and RC group with this trial strongly favouring AC reconstruction.3
The quality of the studies was variable and is demonstrated in Table 1. Retrospective studies were of generally good quality and RCTs were generally of intermediate quality. There are several limitations to this review. The definitions of DGE varied between most of the studies. The ISGPS definition was used in two of the nine studies7,21 although variations of the criteria used in this definition were used in the other studies.2–4,9,11,12,20 Another limitation of the meta-analysis was inclusion of both randomized and non-randomized studies. This was considered necessary because of the small number of potential studies.
In conclusion, this meta-analysis supports the use of AC as opposed to RC gastroenterostomy and is associated with shorter LOS and early oral intake and is routinely recommended during PPPD.
Conflicts of interest
None declared.
References
- Gouma DJ, Nieveen van Dijkum EJ, Obertop H. The standard diagnostic work-up and surgical treatment of pancreatic head tumours. Eur J Surg Oncol. 1999;25:113–123. doi: 10.1053/ejso.1998.0612. [DOI] [PubMed] [Google Scholar]
- Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Müller MW, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pyloruspreserving Whipple procedure. Arch Surg. 2005;140:1094–1099. doi: 10.1001/archsurg.140.11.1094. [DOI] [PubMed] [Google Scholar]
- Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243:316–320. doi: 10.1097/01.sla.0000201479.84934.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Abe N, Ueki H, Masaki T, Mori T, Atomi Y. A new reconstruction method for preventing delayed gastric emptying after pylorus preserving pancreatoduodenectomy. Am J Surg. 2006;187:743–746. doi: 10.1016/j.amjsurg.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Eshuis WJ, van Dalen JW, Busch OR, van Gulik TM, Gouma DJ. Route of gastroenteric reconstruction in pancreatoduodenectomy and delayed gastric emptying. HPB. 2012;14:54–59. doi: 10.1111/j.1477-2574.2011.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Hwang HK, Kim JK, Cho SI, Yoon DS, Lee WJ, et al. Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) classification. Surgery. 2009;146:882–887. doi: 10.1016/j.surg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Eshuis WJ, van Eijck CHJ, Gerhards MF, Coene PP, de Hingh IH, Karsten TM, et al. Antecolic versus retrocolic route of the gastroenteric anastamosis after pancreatoduodenectomy. Ann Surg. 2014;259:45–51. doi: 10.1097/SLA.0b013e3182a6f529. [DOI] [PubMed] [Google Scholar]
- Parmar AD, Sheffield KM, Vargas GM, Pitt HA, Kilbane EM, Hall BL, et al. Factors associated with delayed gastric emptying after pancreaticodeuodenectomy. HPB. 2013;15:763–772. doi: 10.1111/hpb.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa K, Imamura N, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. Prospective randomized controlled study of gastric emptying assessed by 13C-acetate breath test after pylorus-preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. J Hepatobiliary Pancreat Surg. 2009;16:49–55. doi: 10.1007/s00534-008-0004-3. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakagawa N, et al. An antecolic Roux-en Y type reconstruction decreased delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Gastrointest Surg. 2008;12:1081–1086. doi: 10.1007/s11605-008-0483-1. [DOI] [PubMed] [Google Scholar]
- Tamandl D, Sahora K, Prucker J, Schmid R, Holst JJ, Miholic J, et al. Impact of the reconstruction method on delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: a prospective randomized study. World J Surg. 2014;38:465–475. doi: 10.1007/s00268-013-2274-4. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Demets D. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–350. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green SE. Handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). Available at http://www.handbook.cochrane.org (last accessed 2 August 2014)
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- Tanabe R, Ohtsuka T, Miyatake E, Kawamoto M, Nakamura M, Takahata S, et al. Manometric evidence of earlier recovery of fasting gastric motility after antecolic duodenojejunostomy than after retrocolic duodenojejunostomy following PPPD. Hepatogastroenterology. 2012;59:1981–1985. doi: 10.5754/hge10725. [DOI] [PubMed] [Google Scholar]
- Imamura N, Chijiiwa K, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. Prospective randomized clinical trial of a change in gastric emptying and nutritional status after a pylorus-preserving pancreaticoduodenectomy: comparison between an antecolic and a vertical retrocolic duodenojejunostomy. HPB. 2014;16:384–394. doi: 10.1111/hpb.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreaticoduodenectomy. A clinical and physiological appraisal. Ann Surg. 1986;204:655–664. doi: 10.1097/00000658-198612000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying following pancreaticoduodenectomy: a prospective, randomized placebo-controlled trial. Ann Surg. 1993;218:229–238. doi: 10.1097/00000658-199309000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch JW, Deziel DJ, Rossi RL, Watkins E, Jr, Winter PF. Pyloric and gastric preserving pancreatic resection: experience with 87 patients. Ann Surg. 1986;204:411–418. doi: 10.1097/00000658-198610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


